Abstract
Chondroitin 6-sulfate (C6S), a glycosaminoglycan (GAG), is distributed mainly in the growth plates, aorta, and cornea; however, the physiological function of C6S is not fully understood. One of the limitations is that no rapid, accurate quantitative method to measure C6S has been established. Mucopolysaccharidosis IVA and VII (MPS IVA and VII) are caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase and β-d-glucuronidase, respectively, resulting in accumulation of C6S and other GAG(s). While levels of keratan sulfate (KS), heparan sulfate, and dermatan sulfate in samples from MPS patients are well described, this is the first report of quantitative analysis of C6S levels in samples from MPS IVA and VII patients.
We developed a method to digest polymeric C6S and measure resultant disaccharides using liquid chromatography–tandem mass spectrometry (LC-MS/MS). C6S levels were measured in the blood from control subjects and patients with MPS IVA and VII aged from 0 to 58 years of age. We also assayed KS levels in the same samples for comparison with C6S.
Levels of C6S in the blood decreased with age and were significantly elevated in patients with MPS IVA and VII, compared with age-matched controls. Levels of KS in patients with MPS IVA were also higher than those in age-matched controls, although differences were less pronounced than with C6S. Combining KS and C6S data, discriminated patients with MPS IVA from age-matched control subjects were better than either C6S or KS levels alone.
In conclusion, this first report showing that blood levels of C6S are quantitatively evaluated in patients with MPS IVA and VII indicates that C6S could be a useful biomarker for these metabolic disorders.
Introduction
Mucopolysaccharidoses (MPS) are a family of inheritable metabolic disorders caused by deficiency of lysosomal enzymes required for degradation of glycosaminoglycans (GAGs). Each known MPS type involves deficiency of a specific lysosomal enzyme required for the stepwise degradation of specific GAG(s) (chondroitin sulfate, CS; dermatan sulfate, DS; heparan sulfate, HS; and keratan sulfate, KS).
Mucopolysaccharidosis IVA (MPS IVA, Morquio A syndrome) is caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS), resulting in the accumulation of chondroitin 6-sulfate (C6S) and KS mainly in the cartilage and its extracellular matrix (ECM). MPS IVB is caused by deficiency of β-galactosidase, leading to the accumulation of KS but not C6S. Clinically, a classic (severe) form of MPS IVA is characterized by systemic skeletal dysplasia such as short trunk dwarfism, kyphoscoliosis, coxa valga, odontoid hypoplasia, abnormal gait, joint mobility problems, restriction of chest wall movement, and a life span of 20–30 years. Patients with an attenuated form can have a nearly normal life span, with mild involvement of the skeleton (Dũng et al. 2013; Yasuda et al. 2013; Tomatsu et al. 2011, 2012a, 2013a, b; Northover et al. 1996; Montaño et al. 2007, 2008; Suzuki et al. 2001; Hendriksz et al. 2013; Möllmann et al. 2013; Harmatz et al. 2013). In general, patients with MPS IVB have a milder phenotype of skeletal dysplasia. Patients with MPS VII have accumulation of HS, DS, chondroitin 4-sulfate (C4S), and C6S in various tissues and have coarse facial features, mental retardation, short stature, hepatomegaly, bony deformities, GAG excretion, and striking metachromatic granules in peripheral leukocytes (Sly et al. 1973; Tomatsu et al. 1991).
Elevation of total urinary GAG or KS in the blood and/or urine of MPS IVA patients has been detected by colorimetric analysis using dimethylmethylene blue (Melrose and Ghosh 1988), ELISA (Tomatsu et al. 2004, 2005a), and HPLC methods (Linhardt et al. 1989; Whitham et al. 1999). We developed a rapid, reproducible, sensitive, and specific assay system in which the disaccharides produced from DS, HS, and KS in the blood, urine, and dried blood spot (DBS) samples are analyzed simultaneously by using liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Oguma et al. 2007a, b; Tomatsu et al. 2010a, b, 2013a; Hintze et al. 2011). KS levels in the plasma and urine from patients with MPS IVA were associated with age and clinical severity and decreased with enzyme replacement therapy in MPS IVA mouse models (Tomatsu et al. 2008). These findings indicate that KS values in the blood and/or urine could be a suitable biomarker for early diagnosis and screening, assessment of disease severity, and monitoring therapeutic efficacy in MPS IVA (Tomatsu et al. 2008, 2010c, 2013b). It is noteworthy that there is some overlap of KS values from patients with MPS IVA and age-matched controls and that this is more pronounced in children over 10 years old, suggesting that better biomarkers are required to monitor all patients with MPS IVA (Tomatsu et al. 2010c).
CS levels have been determined by HPLC, LC-MS/MS, and capillary electrophoresis, based on the differences in enzymatic digestion using chondroitinase ABC and/or chondroitinase ACII (Imanari et al. 1996; Koshiishi et al. 1998; Oguma et al. 2001; Karamanos and Hjerpe 2001; Lamari et al. 2002). Although capillary electrophoresis studies provided subjective data that CS is elevated in the urine of MPS IVA (Hopwood and Harrison 1982; Hata and Nagai 1972), C4S and C6S were not separated. Thus, no quantitative analyses have yet been reported on specific levels of C6S in patients with MPS IVA and VII. Blood specimens (plasma, serum, or DBS) are appropriate and stable for enzymatic diagnosis, newborn screening, assessment of clinical severity, and monitoring therapeutic efficacy (Tomatsu et al. 2008, 2010b, c, 2013a, b; Wang et al. 2007; Blanchard et al. 2008; de Ru et al. 2013; de Ruijter et al. 2012). We are developing a pilot study in newborn screening for MPS by measuring GAGs (DS, HS, and KS) in blood specimens (Tomatsu et al. 2013a). Therefore, development of methods to measure C6S levels in blood specimens should provide an additional approach to have a significant impact on clinical practice in caring for MPS patients.
In this study, we evaluated C6S levels in the blood of control subjects and patients with MPS IV and VII by LC-MS/MS after digestion with chondroitinase ABC and keratanase II and show the potential of C6S as a biomarker for MPS IVA and VII. In addition, we investigated the correlation between C6S and KS levels in patients with MPS IVA.
Materials and Method
Subjects
Blood (plasma or serum) samples were obtained from 35 patients with MPS IVA (phenotype: severe, 32; undefined, 3), four patients with MPS IVB (attenuated, 4), and three patients with MPS VII. Informed consent was obtained from the patients and/or their guardians through the physicians who were in charge of the patients with MPS at each local institute approved by the Institutional Review Board (IRB). For all samples, the ages of the patients were identified. Blood samples were also obtained from 138 healthy controls. In previous experiments by LC-MS/MS, we confirmed that there is no difference of GAG value in specificity and sensitivity between plasma and serum (Oguma et al. 2007a, b; Tomatsu et al. 2010a, b, c). Diagnosis of MPS IVA, MPS IVB, and MPS VII was made on the basis of enzyme activity (GALNS, β-galactosidase, and β-d-glucuronidase, respectively) reduced to ≤5% the normal level in plasma, leukocytes or fibroblasts. We classified clinical severity for patients with MPS IVA based on growth charts, as described previously (Montaño et al. 2008; Tomatsu et al. 2012b). According to the isopleth upon which the patient falls, patients above the 90th centile on the growth chart for each gender are defined as attenuated (Tomatsu et al. 2012b). We classified the severity of the disease in MPS IVB in reference with the growth charts of MPS IVA since the growth chart of MPS IVB was not available.
Chemicals and Materials
To digest “polymer” C6S and KS to disaccharides, chondroitinase ABC and keratanase II were provided from Seikagaku Co. (Tokyo, Japan). Chondroitinase A produced disaccharides of C4S, while chondroitinase B and chondroitinase C produced disaccharides of DS and C6S, respectively. All of these disaccharides have identical molecular masses. Chondrosine (internal standard, IS), ΔDi-6S (C6S) [2-acetamido-2-deoxy-4-O-(4-deoxy-a-l-threo-hex-4-enopyranosyluronic acid)-6-O-sulfo-d-glucose], and ΔDi-4S (DS) [2-acetamido-2-deoxy-4-O-(4-deoxy-l-threo-hex-4-enopyranosyluronic acid)-4-O-sulfo-d-glucose] were provided from Seikagaku Co. Stock solutions of ΔDi-6S (100 μg/mL), ΔDi-4S (100 μg/mL), “polymer” KS (20 μg/mL), and IS (5 mg/mL) were prepared separately in ddH2O and stored at −80°C. Standard solutions of ΔDi-6S (10, 20, 100, 200, and 1,000 ng/mL) and KS (0.1, 0.2, 1.0, 2.0, and 10.0 μg/mL) and IS solution (500 ng/mL) were prepared freshly.
Sample Preparation
Blood specimens and standards were prepared as follows. Ten microliters of each serum or plasma sample and 90 μL of 50 mM Tris–hydrochloric acid buffer (pH 7.0) were placed in wells of AcroPrep™ Advance 96-Well Filter Plates that have Ultrafiltration Omega 10 K membrane filters (PALL Corporation, NY, USA). The filter plates were placed on a receiver and centrifuged at 2,000 g for 15 min to remove free disaccharides. The membrane plates were transferred to a fresh receiver plate. Standards were added to unused wells of the filter plate. Twenty microliters of IS solution (500 ng/mL), 60 μL of 50 mM Tris–hydrochloric acid buffer (pH 7), and 10 μL of chondroitinase ABC and keratanase II mixture solution (2 mU each per sample) were added to each filter well. The plate was incubated in a water bath at 37°C for 15 h and centrifuged at 2,000 g for 15 min. The receiver plate containing disaccharides was stored at −20°C until injection to LC-MS/MS.
Apparatus
The chromatographic system consisted of a 1,260 infinity (Agilent Technologies, Palo Alto, CA, USA) and a Hypercarb column (2.0 mm i.d. 50 mm, 5 μm, Thermo Electron, USA). The column temperature was kept at 50°C. The mobile phase was a gradient elution of 5 mM ammonium acetate in acetonitrile −5 mM ammonium acetate buffer (pH 11.0). The gradient condition was programmed as follows. The initial composition of 0% acetonitrile was kept for 0.1 min, linearly modified to 30% over 1.8 min, maintained at 30% for 0.3 min, modified to 0% over 0.01 min, and finally maintained at 0% for 2.5 min. The flow rate was 0.7 mL/min. The 6460 Triple Quad mass spectrometer (Agilent Technologies) was operated in the negative ion detection mode. In the multiple reaction monitoring (MRM) mode, the mass spectrometer detected ions by monitoring the decay of the m/z 462 precursor ion to the m/z 97 production for Galβ1 → 4GlcNAc(6S) disaccharides derived from KS, the decay of the m/z 458.4 precursor ion to the m/z 282.1 product ion for ΔDi-6S (C6S), the decay of the m/z 458.4 precursor ion to the m/z 300.2 product ion for ΔDi-4S (DS), and the decay of the m/z 354.29 precursor ion to the m/z 193.1 product ion for IS. Peak areas for all components were integrated automatically by using QQQ Quantitative Analysis Software (Agilent Technologies), and peak area ratios (area of analytes/area of IS) were plotted against level by weighted linear regression. Raw LC-MS/MS data were automatically preserved. The levels for each disaccharide were calculated using QQQ Quantitative Analysis Software.
Method Validation
Intraday precision evaluated as coefficient of variation (CV) was determined by replicate analyses (n = 5) of three different control serum. Inter-day precision was determined by replicate analyses (n = 5) of three different serum controls on three separate days.
The selectivity of the assay was investigated by processing and analyzing five independent samples by the procedure described above without enzymatic digestion. Calibration curves were constructed by plotting the peak area ratio of the analytes to IS against the level of the analytes. Each calibration curve consisted of seven calibration points (n = 1).
Statistical Analysis
Analysis was performed using SPSS for Windows (version 17.0, SPSS Inc., Chicago, IL, USA). For age-matched comparisons, patient and control samples were grouped in age ranges <3, 3–4, 5–9, 10–14, 15–35, and 36+. Data are shown as the mean ± SD.
Results
LC-MS/MS Conditions
The peaks of ΔDi-6S (C6S) and ΔDi-4S (DS and C4S) with the same molecular weight were separated by the LC component (Fig. 1). The retention time of ΔDi-6S and ΔDi-4S was 2.15 and 0.83 min, respectively.
Fig. 1.
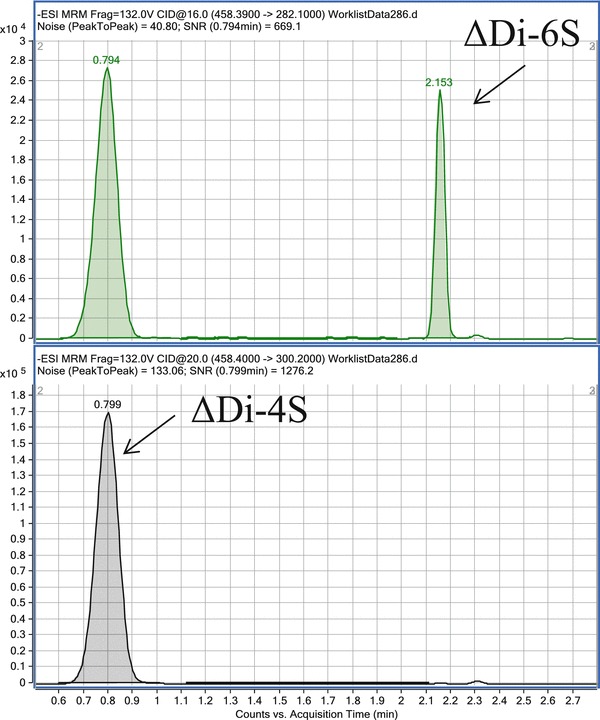
Representative ΔDi-4S and ΔDi-6S MRM chromatograms of extracts obtained from a patient with MPS IVA
Calibration Curves
Calibration curves for ΔDi-6S and Galβ1 → 4GlcNAc(6S) (KS) obtained on five separate days were linear over the level ranges of 10–1,000 ng/mL and 0.1–10 μg/mL, respectively. The correlation coefficients of determination (r) were not less than 0.99.
Precision and Accuracy
Results of intra- and inter-assay precision for ΔDi-6S and Galß1-4GlcNAc(6S) in control specimens are as follows. The intra-assay precision values/coefficient of variation (CV) determined from analysis of ΔDi-6S and Galß1-4GlcNAc(6S) for control serum are less than 11.9 and 6.8%, respectively. The inter-assay precision values/CVs for these disaccharides in control serum are less than 12.2 and 6.5%, respectively. These results demonstrate the reproducibility and accuracy of the method.
Chondroitin 6-Sulfate (C6S) Levels
The C6S values for the blood samples from 35 MPS IVA patients (average age 16.1 years, range 3.4–56 years), 4 MPS IVB patients (average age 15.2 years, range 12.7–17.7 years), 3 MPS VII patients (average age 15 years, range 0–30 years), and 138 control subjects (average age 4.1 years, range 0–32 years) are described in Table 1 and Fig. 2. Blood C6S levels in control subjects were found to vary with age. The level was the highest in newborns and rapidly decreased until 5 years of age. After 5 years of age, there was a gradual decline with age.
Table 1.
Levels of C6S (ng/ml) in patients with MPS IVA, IVB, and VII
| Age (year) | Control | MPS IVA | MPS IVB | MPS VII |
|---|---|---|---|---|
| <3 | 156.7 ± 116.9 (n = 81) | 940.7 (n = 1) | ||
| 3–4 | 58.7 ± 17.4 (n = 20) | 166.4 ± 83.8 (n = 2) | ||
| 5–9 | 70.5 ± 17.1 (n = 18) | 175.7 ± 87.2*** (n = 12) | ||
| 10–14 | 66.1 ± 25.9 (n = 13) | 141.7 ± 54.9*** (n = 8) | 94.9 ± 6.2 (n = 2) | |
| 15–35 | 33.6 ± 19.2 (n = 6) | 109.2 ± 45.0** (n = 11) | 33.2 ± 6.8 (n = 2) | 468.2 ± 533.8 (n = 2) |
| >36 | 103.7 ± 68.0 (n = 3) |
Data represent the mean ± SD values
** and ***; significantly different from the control at p < 0.005, and 0.001, respectively
Fig. 2.
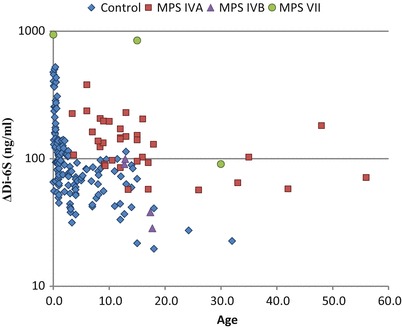
Level of blood ΔDi-6S of patients with MPS and control subjects. Results of all specimens from patients and control subjects were plotted on a semilogarithmic scale with respect to age (years)
The C6S levels in samples from patients with MPS IVA in age groups 5–9, 10–14, and 15–35 years were significantly higher than those in age-matched controls (p < 0.001, p < 0.001, and p < 0.005, respectively). Only two MPS IVA patients were younger than 5 years old, so although levels of C6S were high in these patients, significance could not be determined (Table 1, Fig. 2). Although in these group comparisons mean levels of C6S in MPS IVA samples are higher than controls in age ranges 5–35, there is no clear distinction between patients and controls over the age of ten.
The level of C6S was also compared between patients with MPS IVB and VII and the age-matched controls (Table 1, Fig. 2). Four patients with MPS IVB showed that the level of C6S were similar to those of age-matched controls (Fig. 2). Blood C6S levels in all three patients with MPS VII were more than 2 SD above the mean of age-matched controls (Tables 1 and 3).
Table 3.
Ratio of 2 SD above the mean of the age-matched controls in MPS IVA, IVB, and VII
| C6S | KS | Either C6S or KS | |
|---|---|---|---|
| MPS IVA | 75% (24 out of 32) | 71.9% (23 out of 32) | 90.6% (29 out of 32) |
| MPS IVB | 0% (0 out of 4) | 0% (0 out of 4) | 0% (0 out of 4) |
| MPS VII | 100% (3 out of 3) | 66.7% (2 out of 3) | 100% (3 out of 3) |
Keratan Sulfate (KS) Level
KS levels in blood samples from the same patients with MPS IVA, IVB, and VII and the same control subjects in C6S assay are shown in Table 2 and Fig. 3. Blood KS levels in control subjects were also age dependent. Blood KS levels were highest in children up to 10 years of age and then gradually declined in older children and adults. As seen for C6S, KS in the blood of patients with MPS IVA in age groups between 5–9, 10–14, and 15–35 years were significantly higher than those in age-matched control subjects (p < 0.001, p < 0.005, and p < 0.001, respectively) (Table 2, Fig. 3). KS levels in the blood from two MPS IVA patients younger than 5 were indistinguishable from controls, and there was significant overlap of KS values between control subjects and patients with MPS IVA in the entire age range (Fig. 3).
Table 2.
Levels of KS (μg/ml) in patients with MPS IVA, IVB, and VII
| KS (μg/mL) | Control | MPS IVA | MPS IVB | MPS VII |
|---|---|---|---|---|
| <3 | 3.2 ± 0.9 (n = 81) | 3.4 (n = 1) | ||
| 3–4 | 3.2 ± 0.9 (n = 20) | 3.0 ± 0.9 (n = 2) | ||
| 5–9 | 2.9 ± 0.4 (n = 18) | 4.3 ± 0.9*** (n = 12) | ||
| 10–14 | 2.5 ± 1.1 (n = 13) | 4.2 ± 1.2** (n = 8) | 3.1 ± 0.1 (n = 2) | |
| 15–35 | 0.9 ± 0.3 (n = 6) | 2.7 ± 0.9*** (n = 11) | 1.3 ± 0.2 (n = 2) | 2.4 ± 0.5 (n = 2) |
| >36 | 1.9 ± 1.0 (n = 3) |
Data represent the mean ± SD values
** and ***; significantly different from the control at p < 0.005, and 0.001, respectively
Fig. 3.
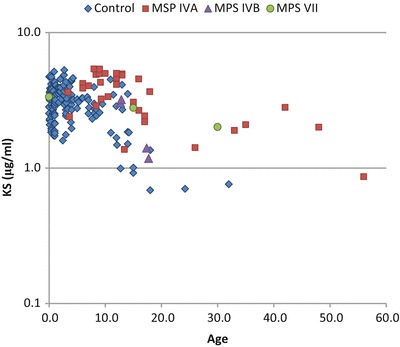
Level of blood KS of patients with MPS and control subjects. Results of all specimens from patients and control subjects were plotted on a semilogarithmic scale with respect to age (years)
The levels of KS were also compared between patients with MPS IVB and VII and the age-matched controls (Table 2, Fig. 3). All samples in patients with MPS IVB showed that blood KS level was within the level of the age-matched controls (Table 2). Blood KS values for two younger patients with MPS VII were more than 2 SD above the mean of the age-matched controls (Tables 2, 3).
Correlation Between KS and C6S Levels
Correlation between KS and C6S levels was not observed (r2 = 0.1136).
For C6S, 75% (24 out of 32) of the patients with MPS IVA were more than 2 SD above the mean of age-matched controls. For KS this proportion was 71.9% (23 out of 32). For either C6S or KS, 29 out of 32 (90.6%) patients were more than 2 SD above the mean for either C6S or KS level in the age-matched control subject (Table 3). The combined data of C6S and KS provided more difference between controls and patients with MPS IVA, compared with C6S or KS by alone.
Discussion
In this study, we have demonstrated (1) that the level of blood C6S in control subjects is age dependent at the peak in newborns and declines with age, (2) that C6S levels in patients with MPS IVA and VII are significantly higher than that in age-matched control subjects, (3) that combination of C6S and KS levels distinguishes patients with MPS IVA and control subjects more clearly compared with either C6S or KS level alone, and (4) that blood C6S and KS levels in patients with MPS IVB are overlapped with those in age-matched control subjects.
CS is involved in specific biological functions including cell adhesion, morphogenesis, neural network formation, and cell division (Sugahara et al. 2003). Historically, CS was divided into three major subtypes: chondroitin A (chondroitin 4-sulfate, C4S), chondroitin B (dermatan sulfate, DS), and chondroitin C (chondroitin 6-sulfate, C6S). Chondroitin B is no longer classified as CS. C6S is distributed in the growth plates (increasing from the proliferative zone to the hypertrophic zone, Ling et al. 1996), aorta (Yasuda et al. 2013), and cornea (Zhang et al. 2005). C6S has been identified in pathological states, including (1) contributing to arterial retention of cholesterol-rich, atherogenic lipoproteins (Mourão et al. 1981), a key event that initiates atherosclerosis (Williams and Tabas 1995), (2) accumulating in the connective tissue stroma of human colon carcinomas (Adany et al. 1990), and (3) excessive secretion of total CS in urinary excretions of MPS IVA and VII patients, although C4S and C6S are not clearly separate (semiquantitative) (Hopwood and Harrison 1982; Hata and Nagai 1972; Haskins et al. 1984). However, physiological and pathological roles and distributions of C6S are not well investigated because of the lack of accurate quantitative methods for measuring of C6S. We show here that the level of C6S in the blood is highest at birth and decreases with age. Total CS has also been shown to decrease with age in human and sheep cartilage (Dziewiatkowski et al. 1989; Elliott and Gardner 1979). Olczyk reported that progressive decrease in C6S in intervertebral discs is observed, most rapidly during the first two decades of life and then more slowly (Olczyk 1993). Age-dependent alterations of C6S level in cartilage most likely account for the age-dependent decrease of C6S levels in the blood in the current study. Levels of C6S in the blood of patients with MPS IVA are significantly higher than in age-matched control subjects, suggesting that C6S is a potential biomarker for MPS IVA, and may be better than KS. In all three patients with MPS VII, blood levels of C6S were markedly elevated compared to age-matched control subjects, showing that C6S level in the blood is likely to be a good biomarker for MPS VII. Two out of the three MPS VII patients also had elevated levels of KS in the blood; this secondary elevation could be related to underlying bone disease, especially of cartilage tissues (Tomatsu et al. 2010c). These MPS VII patients also had elevated levels of HS (data no shown), compatible with our previous report (Tomatsu et al. 2005b). Additional data from more patients with MPS VII will be required to validate these biomarkers.
Blood KS level in control subjects were high during the first 5 years of life and then steadily declined with age before stabilizing in late teenage years, in agreement with prior studies (Tomatsu et al. 2005a; Thonar et al. 1988). The level of blood KS in patients with MPS IVA was significantly higher than age-matched controls in older children and adults. KS in the blood is primarily a result of turnover of cartilage during development (Tomatsu et al. 2005a; Thonar et al. 1988). Elongation of the long bones during growth occurs through a process of endochondral ossification. Chondrocytes degrade the cartilage for replacement by the bone, releasing KS from the cartilage and secretion into the circulation. The decreased level of KS in the blood as healthy teenagers move towards adulthood is consistent with the fact that their growth rate begins to decline during this period. In previous studies we showed that blood KS level in severely affected MPS IVA patients less than 10 years old is markedly elevated and that KS level declines to near-normal or normal levels after 15 year of age, following closing of growth plate (Tomatsu et al. 2005a, 2010c). In the present study, KS levels are less dramatic and may reflect fewer young patients in this study and/or a cohort of patients with a less severe phenotype.
KS levels in the blood appears to be a suitable biomarker for early diagnosis, screening, assessment of disease severity, and monitoring therapeutic efficacy in young MPSIVA patients (Tomatsu et al. 2008, 2010c, 2013a); however, substantial overlaps of KS values between control subjects and MPS IVA patients especially, those older than 15 years of age, questions whether KS alone is a good biomarker for patients with MPS IVA at any age.
We did not see any elevation of KS in the blood of patients with MPS IVB in this study. All four of these patients were over 12 years of age and have the attenuated phenotype. Furthermore, patients with MPS IVB typically have a milder skeletal dysplasia compared with those with MPS IVA. Further study, especially of younger patients and those with a severe type with MPS IVB, is required to determine whether measurement of KS levels in these patients will be of value.
Levels of both C6S and KS in patients with MPS IVA and control overlapped after the age of five. However, over 93% of patients with MPS IVA showed elevation of either C6S or KS level more than 2 SD higher than age-matched control subjects, compared to only 75% for just one of these GAGs. This finding indicates that measurements of both GAGs could be more useful as a biomarker for early diagnosis, screening, assessment of disease severity, and monitoring therapeutic efficacy in patients with MPS IVA.
Our previous LC-MS/MS method determined levels of three GAGs, DS, HS, and KS, simultaneously after digestion with chondroitinase B, heparitinase, and keratanase II, respectively, but could not distinguish easily the peaks of ΔDi-4S (DS) and ΔDiHS-6S (HS) (Tomatsu et al. 2013b), since these products have the same molecular weight. Moreover, the method used chondroitinase B, heparitinase, and keratanase II that do not digest polymer CS to disaccharides, making it impossible to detect ΔDi-4S and ΔDi-6S derived from CS. This study and preliminary data showed that the peaks of ΔDi-4S (DS), ΔDi-6S (C6S), and ΔDiHS-6S (HS) can be separated with the current LC-MS/MS procedure. The peak of ΔDi-4S remains as a mixture of disaccharides derived from C4S and DS (stereoisomers with the same molecular weight). However, if we digest the samples with chondroitinase ABC or chondroitinase B separately and calculate the differences of the peaks for each disaccharide, all major disaccharides derived from KS, HS, CS, and DS can be determined.
By the current method, we could measure C6S level in the urine (human, rat, mouse), DBS (human), shark cartilage, bovine cornea (data not shown), as well as plasma or serum investigated in this study, suggesting that understanding of distribution pattern and physiological role of C6S can be explored more extensively. C6S was also found to be elevated in the urine of patients with MPS IVA and VII (data not shown). C6S levels in the urine and potential correlation with levels in the blood require further study. Preliminary data indicate that an alternative approach to measure C6S is to use chondroitinase C to digest polymer C6S to disaccharides. Digestion of polymer CS by chondroitinase C provided similar results as digestion of chondroitinase ABC for detection of disaccharides C6S. Thus, both chondroitinases can be used for detection of C6S, although chondroitinase ABC is more cost-effective (personal communication with Seikagaku Co.).
We discussed that measurement of C6S and/or KS is useful for the diagnosis of MPS IVA and VII patients. However, there was a limitation of data interpretation by a small number of patients, especially under 5 years of patients with MPS IV or MPS VII. Therefore, it is still unclear whether this assay could be used for diagnosis in young patients.
In conclusion, we have established the method to measure C6S levels by LC-MS/MS, leading to the finding of age-dependent alteration of C6S in control subjects and patients with MPS IVA. Significant difference in levels of C6S between control subjects and patients with MPS IVA and VII indicates that C6S may be a valuable biomarker for early diagnosis, screening of the disease, assessment of disease severity, and monitoring therapeutic efficacy in patients with MPS IVA and VII.
Acknowledgement
This work was supported by grants from the Austrian MPS Society and International Morquio Organization (Carol Ann Foundation). This work was also supported by the Japanese MPS Family Society. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P20GM103464.
S.T. was supported by the National Institutes of Health grant 1R01HD065767-02. The content of the article has not been influenced by the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Compliance with Ethics Guidelines
Conflict of Interest
All the authors contributed to this “Original Article” and have no conflict of interest with any other party.
Tsutomu Shimada, Eriko Yasuda, Robert W. Mason, William G. Mackenzie, Yuniko Shibata, Seiji Yamaguchi, Yasuyuki Suzuki, Tadao Orii, Francyne Kubaski, Roberto Giugliani, and Shunji Tomatsu declare that they have no conflict of interests.
Informed Consent
Informed consent was obtained from the patients and/or their guardians through the physicians who were in charge of the patients with MPS at each local institute approved by the Institutional Review Board (IRB).
Animal Rights
Not applicable
Contributions to the Project
Tsutomu Shimada: He has contributed to the concept of project, the planning, performance of experiments (LC-MS/MS), data analysis, and reporting of the work described in the article.
Eriko Yasuda: She has contributed to collecting samples, data analysis, and reporting of the work described in the article.
Robert W. Mason: He has contributed to the planning, performance of LC-MS/MS, data analysis, and reporting of the work described in the article.
William G. Mackenzie: He has contributed to collecting samples, data analysis, and reporting of the work described in the article.
Yuniko Shibata: She has contributed to analysis of standards, data analysis, and reporting of the work described in the article.
Seiji Yamaguchi: He has contributed to collecting samples and reporting of the work described in the article.
Yasuyuki Suzuki: He has contributed to collecting samples and reporting of the work described in the article.
Tadao Orii: He has contributed to collecting samples and reporting of the work described in the article.
Kenji E. Orii: He has contributed to collecting samples and reporting of the work described in the article.
Francyne Kubaski: She has contributed to collecting samples, data analysis, and reporting of the work described in the article.
Roberto Giugliani: He has contributed to collecting samples and reporting of the work described in the article.
Shunji Tomatsu: He is a Principal Investigator and is responsible for the entire project. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the article.
Footnotes
Competing interests: None declared
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint first authors.
Contributor Information
Shunji Tomatsu, Email: stomatsu@nemours.org.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990;265:11389–11396. [PubMed] [Google Scholar]
- Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–2070. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ru MH, van der Tol L, van Vlies N, et al. Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J Inherit Metab Dis. 2013;36:247–255. doi: 10.1007/s10545-012-9538-2. [DOI] [PubMed] [Google Scholar]
- de Ruijter J, de Ru MH, Wagemans T, et al. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol Genet Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Dũng VC, Tomatsu S, Montaño AM, et al. Mucopolysaccharidosis IVA: correlation between genotype, phenotype and keratan sulfate levels. Mol Genet Metab. 2013;110:129–138. doi: 10.1016/j.ymgme.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewiatkowski DD, LaValley J, Beaudoin AG. Age-related changes in the composition of proteoglycans in sheep cartilages. Connect Tissue Res. 1989;19:103–120. doi: 10.3109/03008208909043892. [DOI] [PubMed] [Google Scholar]
- Elliott RJ, Gardner DL. Changes with age in the glycosaminoglycans of human articular cartilage. Ann Rheum Dis. 1979;38:371–377. doi: 10.1136/ard.38.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmatz P, Mengel KE, Giugliani R, et al. The Morquio A clinical assessment program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109:54–61. doi: 10.1016/j.ymgme.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Hata R, Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972;45:462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Hendriksz CJ, Harmatz P, Beck M, Jones S, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze JP, Tomatsu S, Fujii T, et al. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- Imanari T, Toida T, Koshiishi I, Toyoda H. High-performance liquid chromatographic analysis of glycosaminoglycan-derived oligosaccharides. J Chromatogr A. 1996;720:275–293. doi: 10.1016/0021-9673(95)00338-X. [DOI] [PubMed] [Google Scholar]
- Karamanos NK, Hjerpe A. Disaccharide composition in glycosaminoglycans/proteoglycans analyzed by capillary zone electrophoresis. Methods Mol Biol. 2001;171:181–192. doi: 10.1385/1-59259-209-0:181. [DOI] [PubMed] [Google Scholar]
- Koshiishi I, Takenouchi M, Hasegawa T, Imanari T. Enzymatic method for the simultaneous determination of hyaluronan and chondroitin sulfates using high-performance liquid chromatography. Anal Biochem. 1998;265:49–54. doi: 10.1006/abio.1998.2883. [DOI] [PubMed] [Google Scholar]
- Lamari FN, Militsopoulou M, Mitropoulou TN, Hjerpe A, Karamanos NK. Analysis of glycosaminoglycan-derived disaccharides in biologic samples by capillary electrophoresis and protocol for sequencing glycosaminoglycans. Biomed Chromatogr. 2002;16:95–102. doi: 10.1002/bmc.144. [DOI] [PubMed] [Google Scholar]
- Ling J, Kincaid SA, McDaniel GR, Bartels JE, Johnstone B. Immunohistochemical study of a chondroitin-6-sulfate in growth plates of broiler chickens with high and low genetic predispositions to tibial dyschondroplasia. Avian Dis. 1996;40:88–98. doi: 10.2307/1592376. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, Gu KN, Loganathan D, Carter SR. Analysis of glycosaminoglycan-derived oligosaccharides using reversed-phase ion-pairing and ion-exchange chromatography with suppressed conductivity detection. Anal Biochem. 1989;181:288–296. doi: 10.1016/0003-2697(89)90245-5. [DOI] [PubMed] [Google Scholar]
- Melrose J, Ghosh P. The quantitative discrimination of corneal type I, but not skeletal type II, keratan sulfate in glycosaminoglycan mixtures by using a combination of dimethylmethylene blue and endo-beta-D-galactosidase digestion. Anal Biochem. 1988;170:293–300. doi: 10.1016/0003-2697(88)90634-3. [DOI] [PubMed] [Google Scholar]
- Möllmann C, Lampe CG, Müller-Forell W, et al. Development of a scoring system to evaluate the severity of craniocervical spinal cord compression in patients with mucopolysaccharidosis IVA (Morquio A syndrome) JIMD Rep. 2013;11:65–72. doi: 10.1007/8904_2013_223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- Mourão PA, Pillai S, Di Ferrante N. The binding of chondroitin 6-sulfate to plasma low density lipoprotein. Biochim Biophys Acta. 1981;674:178–187. doi: 10.1016/0304-4165(81)90376-7. [DOI] [PubMed] [Google Scholar]
- Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19:357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ionspray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides. Biomed Chromatogr. 2001;15:356–362. doi: 10.1002/bmc.74. [DOI] [PubMed] [Google Scholar]
- Oguma T, Tomatsu S, Montano AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- Olczyk K. Age-related changes in glycosaminoglycans of human intervertebral discs. Folia Histochem Cytobiol. 1993;31:215–220. [PubMed] [Google Scholar]
- Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/S0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Oshima A, Namba E. β-Galactosidase deficiency (β-galactosidosis) GM1 gangliosidosis and Morquio B disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3775–3809. [Google Scholar]
- Thonar EJ, Pachman LM, Lenz ME, Hayford J, Lynch P, Kuettner KE. Age related changes in the concentration of serum keratan sulphate in children. J Clin Chem Clin Biochem. 1988;26:57–63. doi: 10.1515/cclm.1988.26.2.57. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Sukegawa K, et al. Mucopolysaccharidosis type VII: characterization of mutations and molecular heterogeneity. Am J Hum Genet. 1991;48:89–96. [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Taketani T, et al. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Gutierrez MA, Ishimaru T, et al. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis. 2005;28:743–757. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Okamura K, Maeda H, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oguma T, et al. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol Genet Metab. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oguma T, et al. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J Inherit Metab Dis. 2010;33:S35–S42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res Rep Endocr Disord. 2012;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oikawa H et al (2012b) Impairment of body growth in mucopolysaccharidoses. In: Preedy VR (ed) Handbook of growth and growth monitoring in health and disease. Springer, LLC, pp 2091–2116
- Tomatsu S, Alméciga-Díaz CJ, Barbosa H, et al. Therapies of mucopolysaccharidosis IVA (Morquio A syndrome) Expert Opin Orphan Drugs. 2013;1:805–818. doi: 10.1517/21678707.2013.846853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wood T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for mucopolysaccharidosis II (Hunter disease) Clin Chem. 2007;53:137–140. doi: 10.1373/clinchem.2006.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham KM, Hadley JL, Morris HG, Andrew SM, Nieduszynski IA, Brown GM. An improved method for the structural profiling of keratan sulfates: analysis of keratan sulfates from brain and ovarian tumors. Glycobiology. 1999;9:285–291. doi: 10.1093/glycob/9.3.285. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.ATV.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol Genet Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Conrad AH, Tasheva ES, et al. Detection and quantification of sulfated disaccharides from keratan sulfate and chondroitin/dermatan sulfate during chick corneal development by ESI-MS/MS. Invest Ophthalmol Vis Sci. 2005;46:1604–1614. doi: 10.1167/iovs.04-1453. [DOI] [PubMed] [Google Scholar]


