Abstract
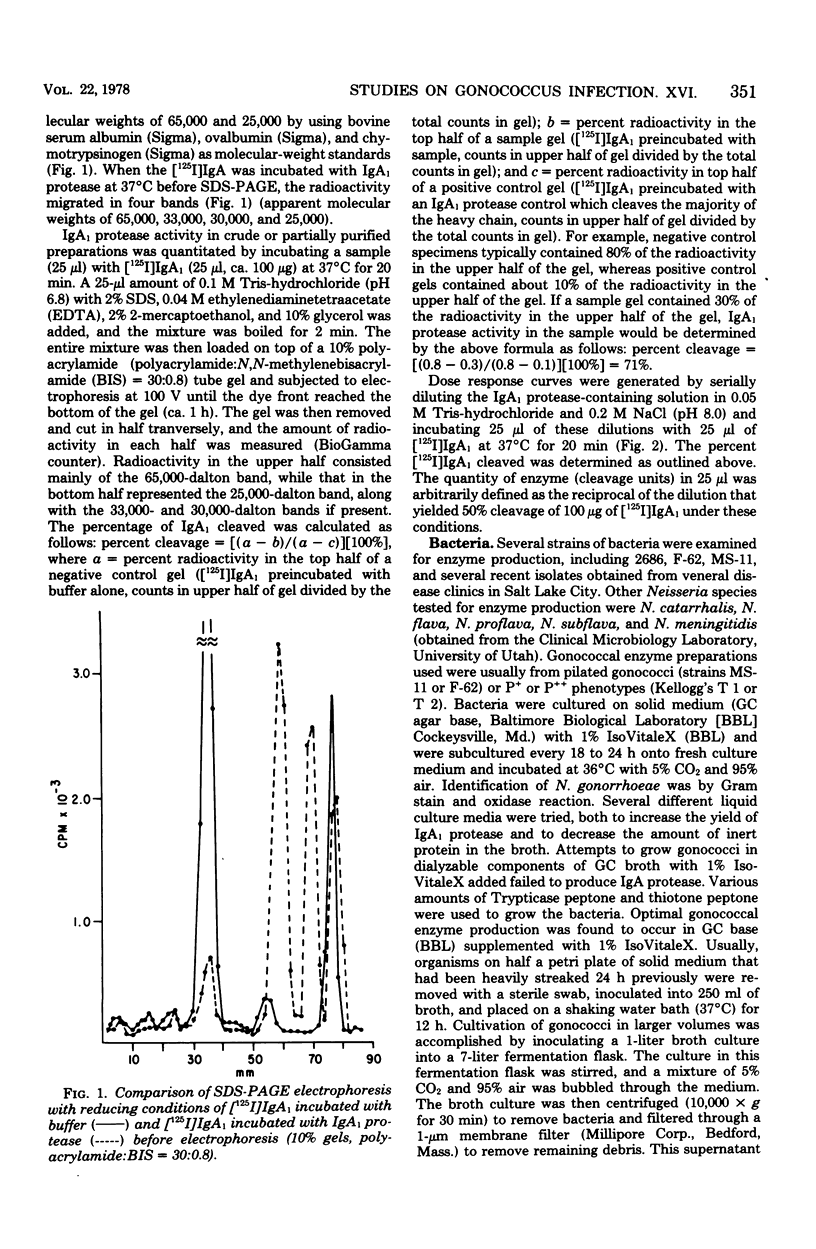
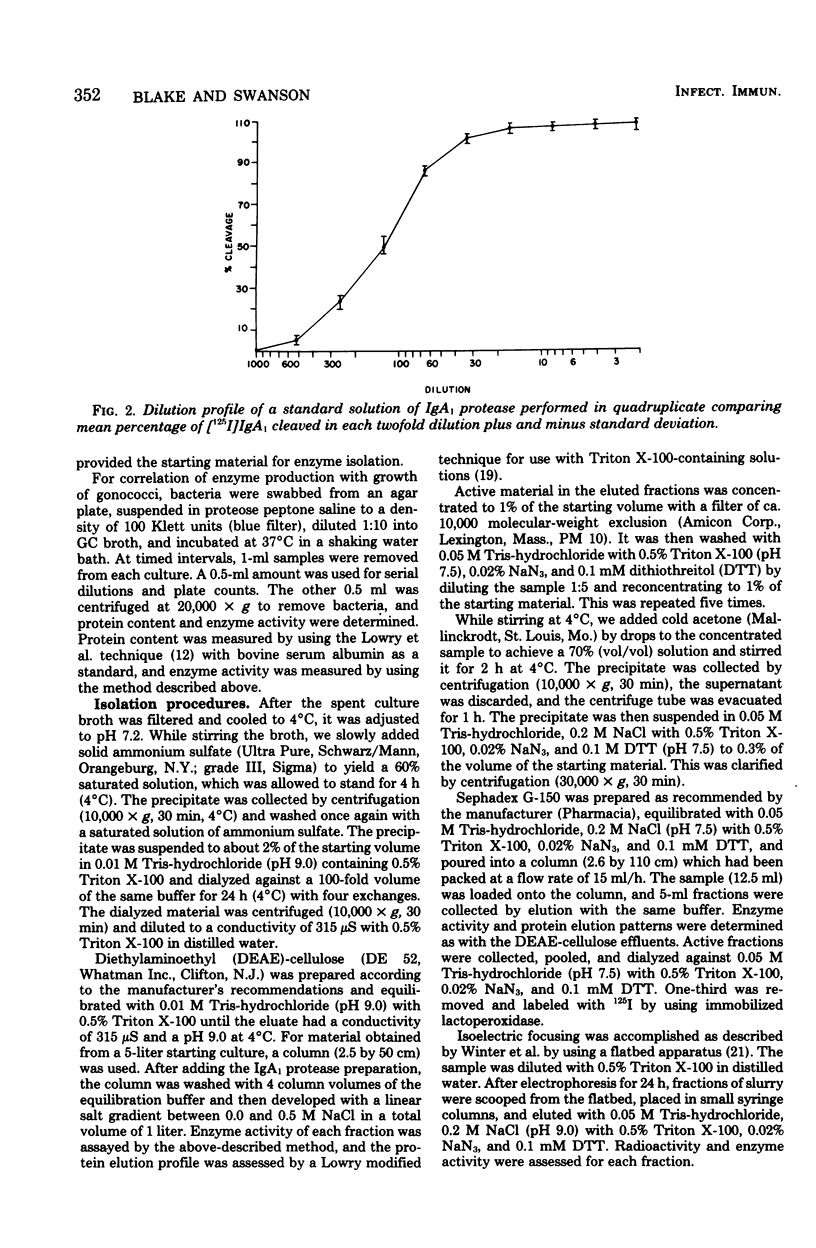
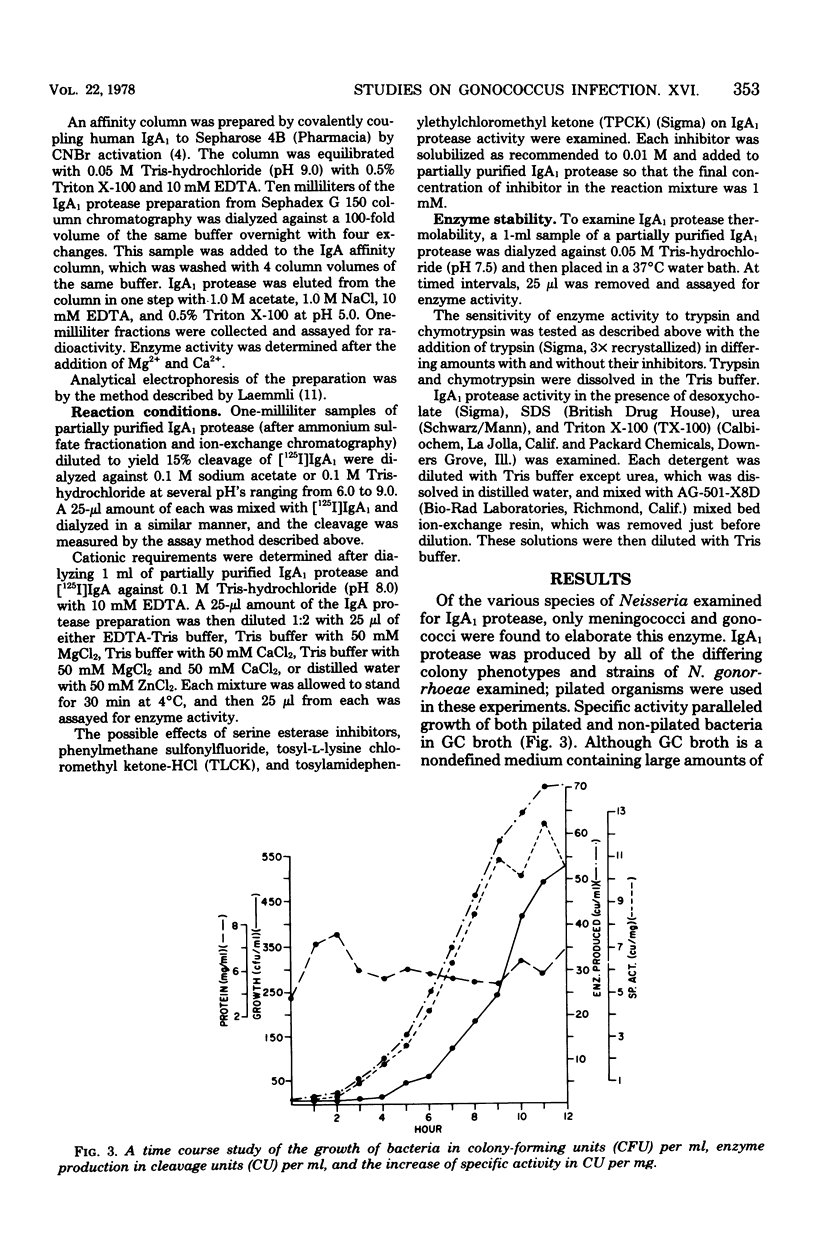
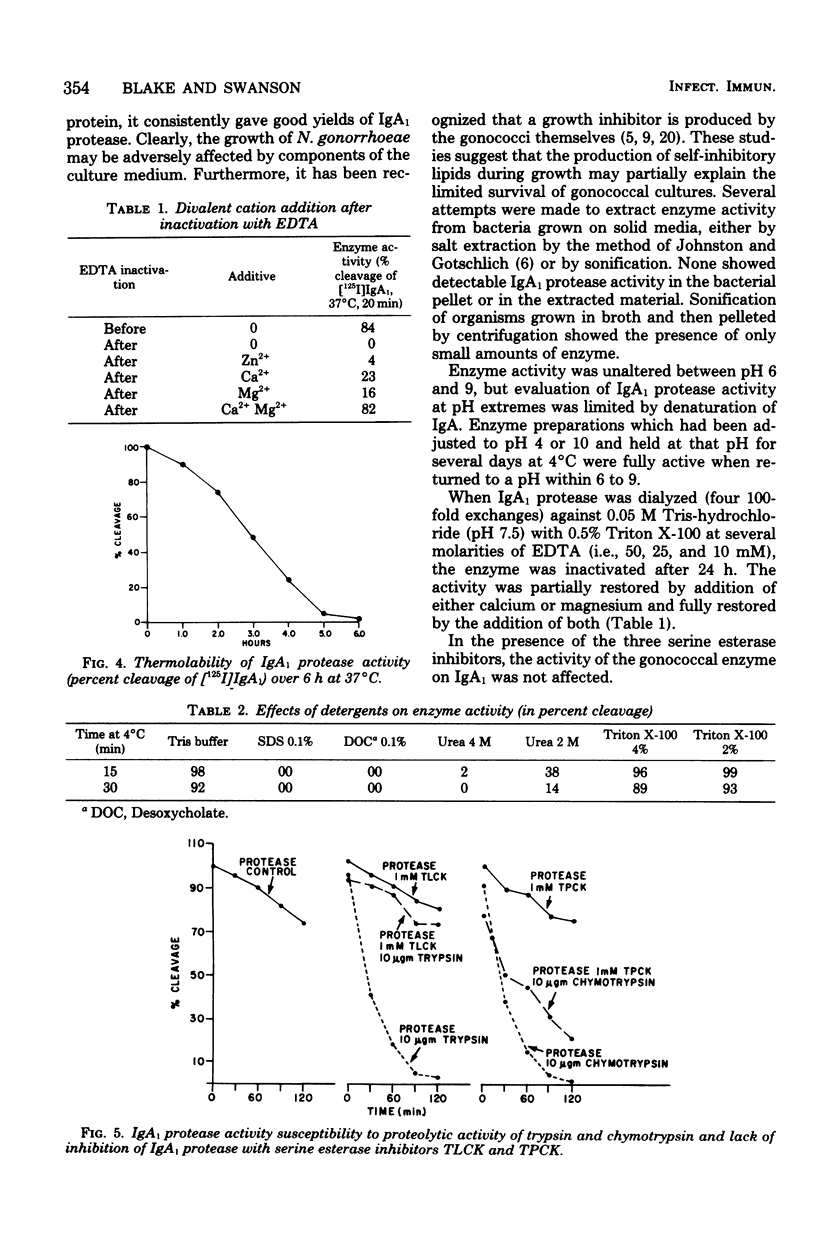
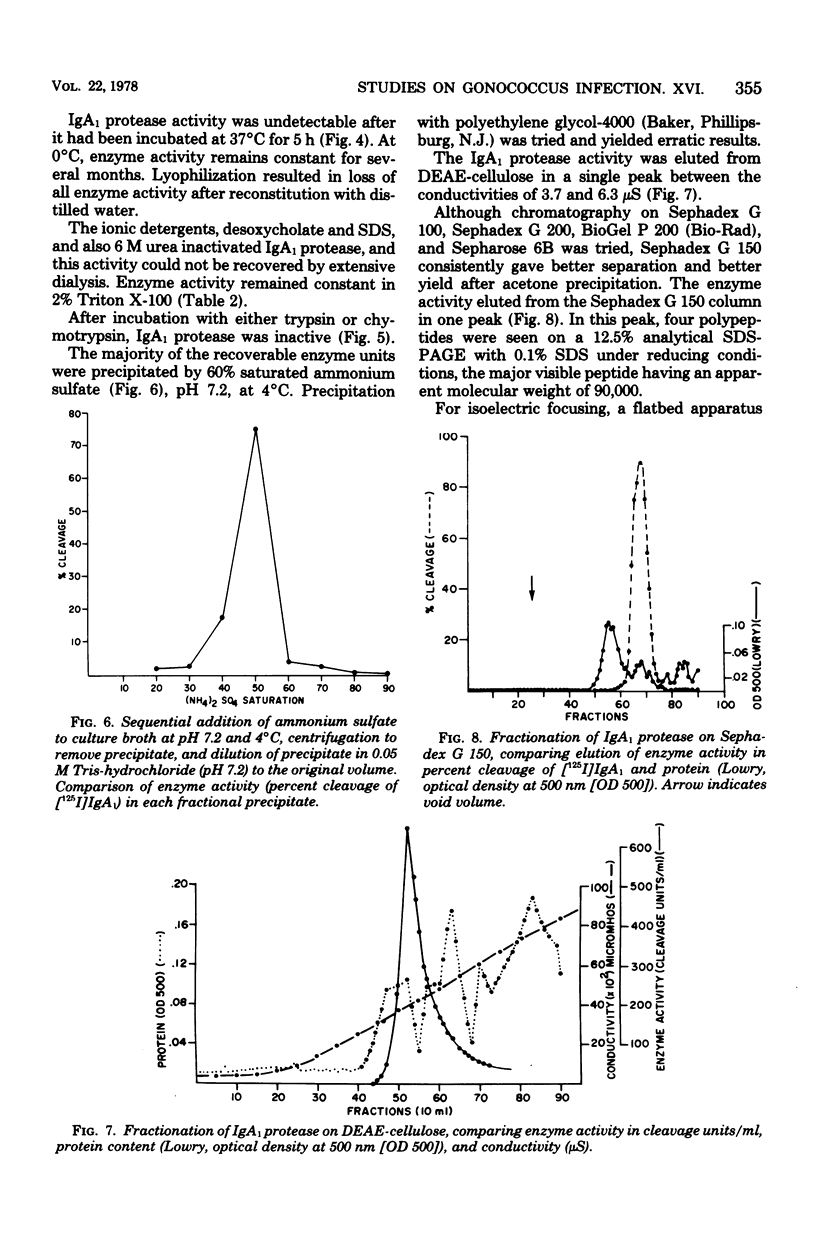
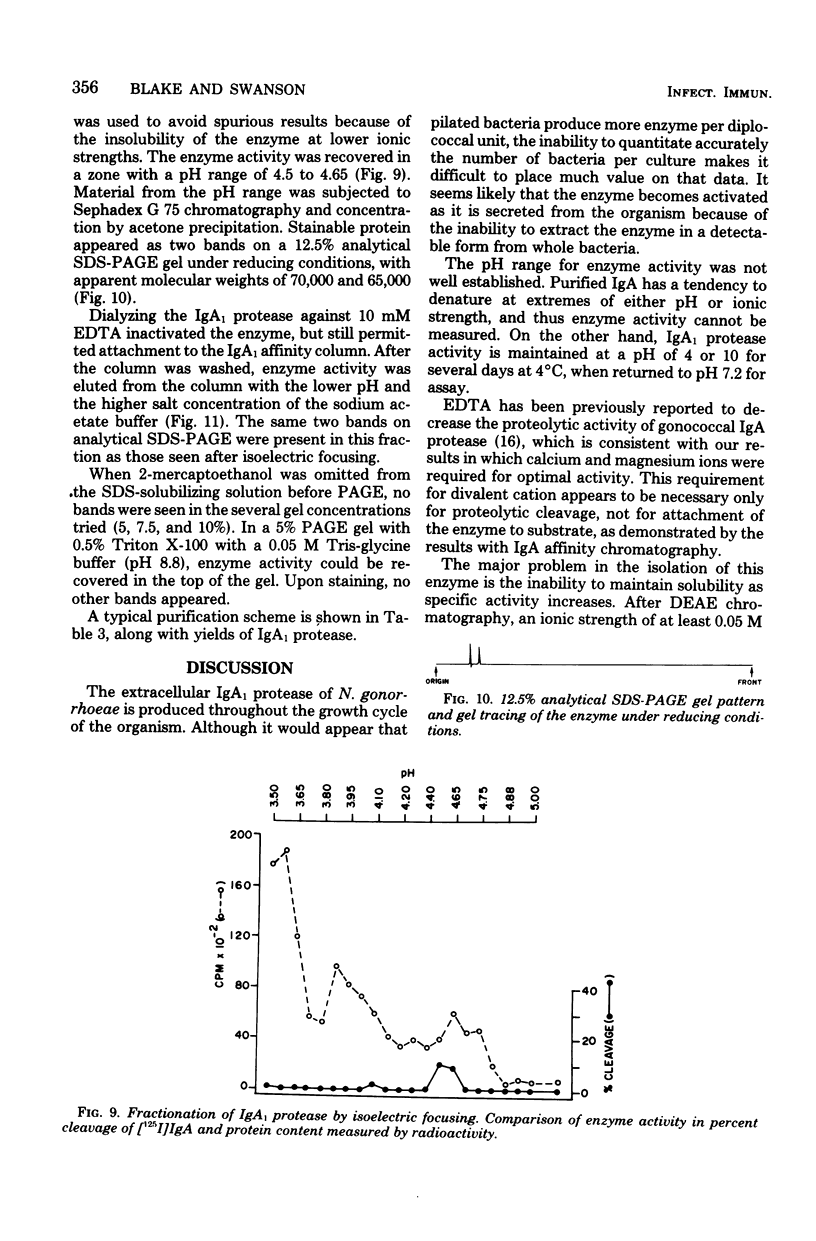
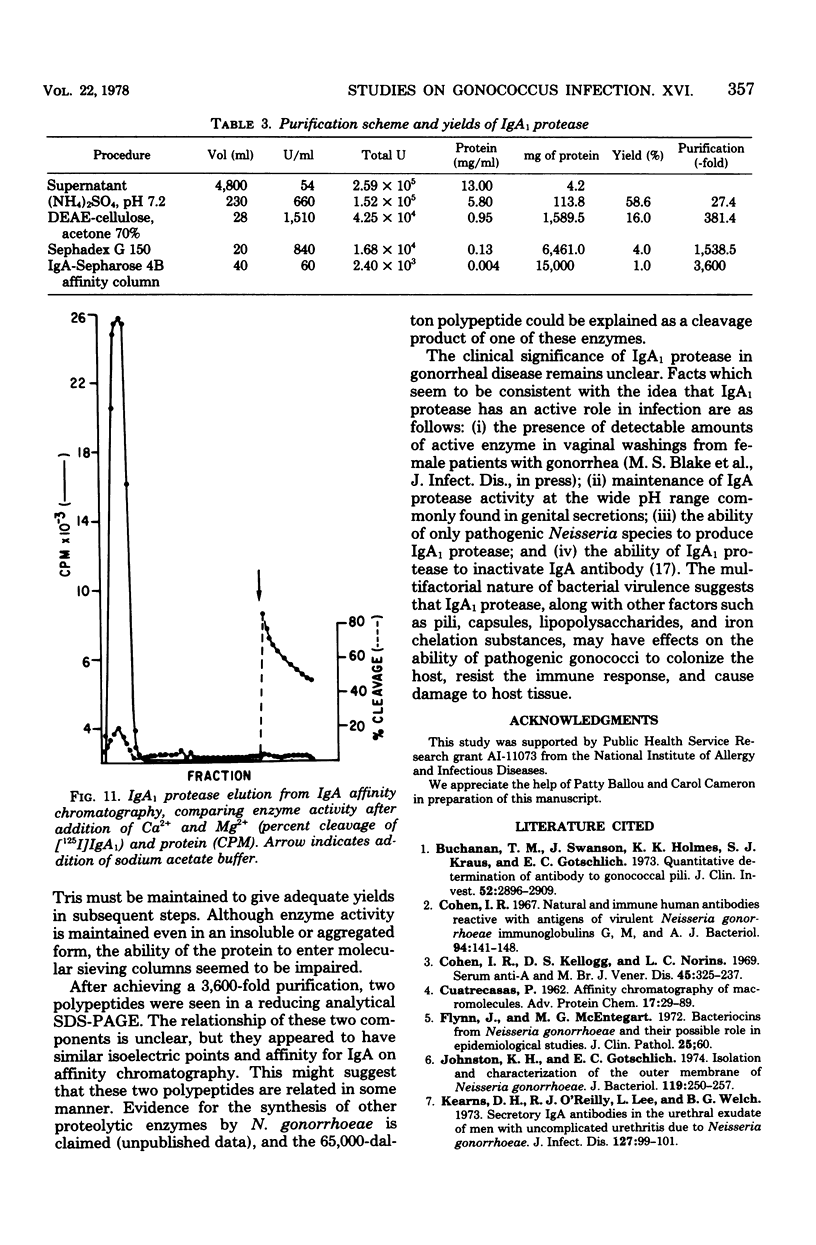
A protease which cleaves human immunoglobulin A1 (IgA1) has been purified from broth cultures of Neisseria gonorrhoeae. This IgA1 protease is produced by pilated and nonpilated gonococci throughout their growth cycles. A combination of ammonium sulfate precipitation, column chromatography, and either isoelectric focusing or affinity chromatography was utilized to obtain an enzyme preparation that showed approximately 3,800-fold purification and exhibited two bands (65,000 and 70,000 daltons) by analytical polyacrylamide electrophoresis in the presence of sodium dodecyl sulfate and reducing conditions. IgA1 protease activity is dependent on divalent cations and is heat labile. Detection and quantitation of IgA protease activity utilized an assay in which [125I]IgA1 is incubated with protease preparations and the cleavage products are analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Swanson J., Holmes K. K., Kraus S. J., Gotschlich E. C. Quantitative determination of antibody to gonococcal pili. Changes in antibody levels with gonococcal infection. J Clin Invest. 1973 Nov;52(11):2896–2909. doi: 10.1172/JCI107486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrarajan J., Klein L. Lowry assay of dilute protein solutions containing high concentrations of Triton X-100. Anal Biochem. 1975 Dec;69(2):632–636. doi: 10.1016/0003-2697(75)90169-4. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Kellogg D. S., Jr, Norins L. C. Serum antibody response in experimental human gonorrhoea. Immunoglobulins G, A, and M. Br J Vener Dis. 1969 Dec;45(4):325–327. doi: 10.1136/sti.45.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae: immunoglobulins G, M, And A. J Bacteriol. 1967 Jul;94(1):141–148. doi: 10.1128/jb.94.1.141-148.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J., McEntegart M. G. Bacteriocins from Neisseria gonorrhoeae and their possible role in epidemiological studies. J Clin Pathol. 1972 Jan;25(1):60–61. doi: 10.1136/jcp.25.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. H., O'Reilly R. J., Lee L., Welch B. G. Secretory IgA antibodies in the urethral exudate of men with uncomplicated urethritis due to Neisseria gonorrhoeae. J Infect Dis. 1973 Jan;127(1):99–101. doi: 10.1093/infdis/127.1.99. [DOI] [PubMed] [Google Scholar]
- Kearns D. H., Seibert G. B., O'Reilly R., Lee L., Logan L. Paradox of the immune response to uncomplicated gonococcal urethritis. N Engl J Med. 1973 Nov 29;289(22):1170–1174. doi: 10.1056/NEJM197311292892205. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Falkow S., Holmes K. K. Reevaluation of bacteriocinogeny in Neisseria gonorrhoeae. J Clin Pathol. 1975 Apr;28(4):274–278. doi: 10.1136/jcp.28.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Lee L., Welch B. G. Secretory IgA antibody responses to Neisseria gonorrhoeae in the genital secretions of infected females. J Infect Dis. 1976 Feb;133(2):113–125. doi: 10.1093/infdis/133.2.113. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Wistar R., Jr Loss of antibody activity in human immunoglobulin A exposed extracellular immunoglobulin A proteases of Neisseria gonorrhoeae and Streptococcus sanguis. Infect Immun. 1977 Jul;17(1):130–135. doi: 10.1128/iai.17.1.130-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Low T., Liu V., Huser H., Raff E., Wong F. C., Clamp J. R. Isolation, properties, and structure of human IgA myeloma globulins. Adv Exp Med Biol. 1974;45(0):177–189. doi: 10.1007/978-1-4613-4550-3_20. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistreich G. A., Baker R. F. The presence of fimbriae (pili) in three species of Neisseria. J Gen Microbiol. 1971 Feb;65(2):167–173. doi: 10.1099/00221287-65-2-167. [DOI] [PubMed] [Google Scholar]



