Abstract
The NADPH oxidase Nox2, a multi-subunit enzyme complex comprising membrane and cytosolic proteins, catalyzes a very intense production of superoxide ions O2•−, which are transformed into other reactive oxygen species (ROS). In vitro, it has to be activated by addition of amphiphiles like arachidonic acid (AA). It has been shown that the membrane part of phagocyte NADPH oxidase is present in lipid rafts rich in cholesterol. Cholesterol plays a significant role in the development of cardio-vascular diseases that are always accompanied by oxidative stress. Our aim was to investigate the influence of cholesterol on the activation process of NADPH oxidase. Our results clearly show that, in a cell-free system, cholesterol is not an efficient activator of NADPH oxidase like arachidonic acid (AA), however it triggers a basal low superoxide production at concentrations similar to what found in neutrophile. A higher concentration, if present during the assembly process of the enzyme, has an inhibitory role on the production of O2•−. Added cholesterol acts on both cytosolic and membrane components, leading to imperfect assembly and decreasing the affinity of cytosolic subunits to the membrane ones. Added to the cytosolic proteins, it retains their conformations but still allows some conformational change induced by AA addition, indispensable to activation of NADPH oxidase.
Keywords: NADPH oxidase, Cholesterol, Cell-free system, Arachidonic acid activation, Superoxide production
Abbreviations: AA, arachidonic acid; PBS, phosphate buffer saline; Cyt b558, cytochrome b558; Cytc, cytochrome c; DTT, dithiotreitol; EDTA, ethylenediaminetetraacetic acid; FAD, flavin adenine dinucleotide; FMLP, formyl-methionyl-leucyl-phenylalanine; GTP, guanosine-5′-triphosphate; HEPES, [4-(2-hydroxyethyl)piperazine-1-yl]ethanesulfonic acid; IPTG, isopropylthiogalatoside; LB, Luria Bertoni; LDL, low density lipoprotein; LR, lipid raft; MF, membrane fractions; MβCD, methyl-β-cyclodextrin; NADPH, reduced β-nicotinamide adenine dinucleotide phosphate; PMSF, phenylmethanesulfonyl fluoride; PtdIns(3)P, phosphatidyl-inositol3-phosphate; PtdIns(3,4)P2, phosphatidylinositol(3,4)-bisphosphate; PX, phox homology domain; ROS, reactive oxygen species; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
Highlights
-
•
Natural cholesterol is important for the NADPH oxidase function.
-
•
Added cholesterol alone activates slightly the NADPH oxidase.
-
•
Cholesterol addition lowers the AA dependent activity of NADPH oxidase.
-
•
Added cholesterol acts on both cytosolic and membrane components.
Introduction
The damaging role of reactive oxygen species (ROS) in cardiovascular diseases (atherosclerosis, vascular inflammation, and endothelial dysfunction) leading to coronary heart disease, stroke or angina pectoris has been known for some decades. NADPH oxidase Nox2 is one of the major actors at the origin of oxidative stress. Actually Nox2 is one of the main isomers involved in the cardiovascular field [1]. On the other hand, high cholesterol level is linked to an elevated risk of cardiovascular disease [2], through high concentration of LDL-cholesterol in blood [3,4]. High cholesterol has also been associated with diabetes and high blood pressure [5,6]. Interestingly, NADPH oxidases have been found in lipid rafts (LR), which are dynamic, detergent-resistant plasma membrane microdomains highly enriched in cholesterol and sphingolipids [7,8]. Cytoplasmic proteins are efficiently recruited to these raft-associated flavocytochrome b558 upon activation to reconstitute the active complex [9]. Moreover, distribution and regulation of NADPH oxidase by LRs were reported in murine microglial cells and bovine aortic endothelial and coronary arterial endothelial cells [10–14]. These facts prompted us to study the effect of cholesterol on the functioning of NADPH oxidase. In this paper, we investigated the consequences of the presence of cholesterol on the production of reactive oxygen species.
The NADPH oxidase catalyzes the formation of superoxide anion (O2•−) by a single-electron reduction of the molecular oxygen using NADPH as the electron donor [15–17]. O2•− is considered to be the starting point for the generation of a vast assortment of reactive oxidants since it is subsequently transformed into hydrogen peroxide, hypochlorous acid, hydroxyl radical and peroxynitrite [18,19]. Deregulation of NADPH-oxidase activity is linked with a large panel of pathologies in addition to cardiovascular ones, involving inflammatory processes, renal damage, central nervous system diseases, immune system disorders, induction of apoptosis after irradiation by low doses of ionizing radiations etc.,[20–31].
The functionally competent oxidase complex consists of a membrane-bound flavocytochrome b558 (Cyt b558), comprising two subunits (Nox2 also known as gp91phox, and p22phox) and four cytosolic components. Nox2 harbors the redox carriers (bound FAD and two hemes) and the NADPH binding site. The cytosolic components include p47phox, p67phox, p40phox, and a small GTPase Rac1 or Rac2 [32]. Because of the high toxicity of the reactive oxygen species (ROS), the NADPH-oxidase activity is tightly regulated spatially and temporally. In resting phagocytes, the components of the complex exist as separated entities but upon cell activation by pro-inflammatory mediators, the cytosolic subunits undergo posttranslational modifications such as phosphorylation [33,34] and migrate to the membrane bound Cyt b558 to constitute the activated NADPH–oxidase complex [35]. Actually this process involves a complicated set of protein–protein and protein–lipid interactions to conduct to oxidase assembly [36–39].
Studies on binding between the different soluble subunits p47phox, p67phox, p40phox performed in vitro suggested that these three cytosolic subunits are preassembled [40–42]. Recently, different constructions of chimeras were designed, in which individual cytosolic subunits were fused [p47phox–p67phox] [43] or [p67phox–Rac1] [44–48] and supplemented by the missing third subunit (Rac1 and p47phox, respectively). Another strategy was to construct a trimera which consisted of the following domains [p47phox (aa 1–286), p67phox (aa 1–212) and a full length Rac1 (aa 1–192)] in which interactions among cytosolic subunits were replaced by fusion. This trimera was found to act as potent amphiphile-dependent oxidase protein activator upon assembly to native phagocyte membrane or purified Cyt b558[49]. The subsequent change from this construction was performed by adding isoprenyl group to the C-terminus of Rac1 part, mimicking in vivo reality, where Rac is found exclusively in the prenylated form. Further modification was the introduction of Q61L mutation in the Rac part of the trimera, making Rac constitutively in the GTP-bound form. It ensures that in the trimera an intramolecular bond was built between Rac1 and p67phox which is essential for oxidase activity ability of trimera [50,51].
The development of a cell-free oxidase activation system was a great help in the understanding of the mechanism of NADPH oxidase activation. This system was designed to mimic in vivo oxidase activity under in vitro conditions. In cell-free systems, the activation process is bypassed by the introduction of an activator, an anionic amphiphile such as arachidonic or other fatty acids or surfactants [52–57]. We took advantage of this system, which permits strict quantification of the components of interest, and modifications of membrane composition. In addition, for simplicity, we have replaced the cytosolic subunits by the trimera [49,50]. A precondition for using the trimera was to ascertain that it is functionally comparable with the separated cytosolic subunits. We have verified that the rates of production of superoxide anions were similar (supplementary material) and that the dependences of the activity in function of AA concentration were also comparable with the cytosolic fractions and the trimera [58]. In addition, the presence of two states in the activation process, a sensitive one followed by a resistant one against ROS damages, observed with the separated cytosolic subunits [59] was also found with the trimera (data not shown). Consequently, we have chosen the trimera instead of the separated subunits in order to diminish the number of independent parameters to consider and to facilitate the interpretation.
Material and methods
Materials
Equine heart cytochrome c (cyt c), arachidonic acid (cis-AA), phenylmethanesulfonyl fluoride (PMSF), isopropylthiogalatoside (IPTG), cholesterol, Dulbecco PBS and methyl-β-cyclodextrin (MβCD) were from Sigma (Saint-Quentin Fallavier, France). Reduced β-nicotinamide adenine dinucleotide phosphate (NADPH) was from Acros. Ni-sepharose, superdex 75 and Ficoll-Paque Plus was from GE Healthcare, France.
Neutrophil membrane preparation
The neutrophils were prepared from human blood from healthy donors (ESF, Paris, France) as described in [60]. Briefly, 500 mL of blood was sedimented in 2% dextran solution for 40 min. PBS was added to the pellets, and then the neutrophils were separated from lymphocytes and the red cells by centrifugation for 30 min at 400g on Ficoll. The red cells were further eliminated after their lysis by centrifugation for 8 min, 400g, 4 °C. The pellet resuspended in PBS pH 7.4 containing 340 mM sucrose, 7 mM magnesium sulfate, 1 mM PMSF, 0.5 mM leupeptin was sonicated in the 30% pulse mode at power pulses (6) in an ice-cooled beaker 6 times during 10 s with interval of 1 min between the sonications (sonicator XL, Misonix Inc.). Neutrophil membranes and cytosol were separated by centrifugation for 1 h 30 min at 200,000g at 4 °C. The membrane fractions were resolubilized, aliquoted and stored at −80 °C for further experiments.
Expression of the trimera
The plasmid of the trimera was a generous gift from Prof. E. Pick. Trimera (p47phox aa 1–286, p67phox aa 1–212, and RacQ61L full length) was expressed and isolated from Escherichia coli BL21-DE3-plys. A stock culture of E. coli (glycerated, stored at −80 °C) expressing the trimera was used to inoculate a Petri dish of Luria Bertani (LB) agar, supplemented with kanamycin and chloramphenicol and incubated at 37 °C for 16 h. A colony was then cultured in 60 mL of LB medium supplemented with 50 mg/L of kanamycin and 34 mg/L of chloramphenicol, incubated at 37 °C for about 16 h. 20 mL of this culture were added to 1.5 L of Terrific Broth medium (TB) supplemented with 50 mg/L of kanamycin and 34 mg/L of chloramphenicol. The flask was incubated in shaking condition at 37 °C until it reached an absorbance of 0.9 at 600 nm, then 0.5 mM IPTG were added to induce the synthesis of protein and the culture was incubated overnight at 30 °C. The culture was pelleted and placed in the freezer at −20 °C until use.
Extraction of the trimera from bacteria
The bacterial pellet, containing the trimera obtained previously, was dissolved in a buffer containing 50 mM HEPES (pH 7.5), 200 mM NaCl and 1 mM EDTA to which was added 1 mg of DNase, 1 mM PMSF, 1 mM DTT and 1 mM benzamidine. The bacteria were sonicated during 4 times 2 min in a 50% pulse mood at power pulses (6) in an ice-cooled beaker with pauses of 2 min. The bacterial lysate was centrifuged at 160,000g for 1 h 30 min at 6 °C. The cleared cell-free supernatant was filtered to remove all traces of debris and bacteria.
Purification of the trimera
The trimera was expressed as fusion protein. Thus it was purified by metal chelate affinity chromatography. The above supernatant was applied to nickel affinity column after being diluted twice with buffer (0.5 M NaCl, 30 mM Na2HPO4, 20 mM imidazole and 1 mM PMSF, pH 7.4). The mixture was loaded for 1 h 30 min so that the proteins of interest effectively cling to the nickel resin. Then the column was washed with the same buffer to remove unwanted bound proteins. The proteins bound to the beads were eluted from the resin with elution buffer (0.1 M NaCl, 30 mM Na2HPO4 and 300 mM imidazole, pH 7.4). Then size exclusion chromatography was carried out to better purify the trimera. Proteins concentration was determined using a NanoDrop2000 spectrophotometer (Thermo scientific, France) and the extinction coefficient of 1.5 mg−1/mL cm. The purities of all proteins were checked by migration on 10% BisTris-NuPAGE SDS gels (Invitrogen), stained with Coomassie Brilliant Blue (Fig. S1 in Supplementary material).
Fig. 1.
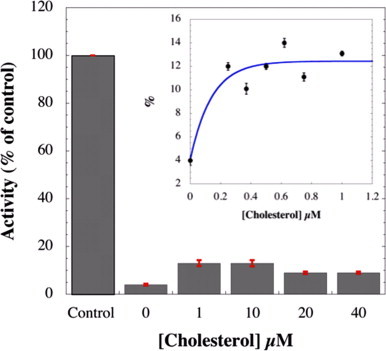
Dependence of NADPH oxidase activity as a function of cholesterol concentration in the absence of arachidonic acid. Membrane fractions (2 nM Cyt b558) with trimera 200 nM were incubated 4 min in presence of different concentrations of cholesterol. Control experiment representing 100% (68 mol O2•−/s/mol Cyt b558) of the activity was realized in presence of 40 µM AA and in absence of cholesterol. The rates of superoxide production were measured as described in Materials and methods. The data are the average of 3 independent measurements±SEM.
Dialysis and storage of the trimera
Trimera fractions were pooled in a dialysis tube whose membrane was of 10 kDa porosity. The tube was placed in 2 L dialysis buffer (100 mM NaCl and 30 mM Na2HPO4, pH 7.5) at 4 °C overnight. The dialyzate was recovered and trimera was stored at −80 °C.
Quantification of intrinsic cholesterol
Intrinsic cholesterol concentration in human neutrophil membrane fractions was measured by the Amplex Red Cholesterol Assay Kit purchased from Invitrogen [61,62]. The intrinsic cholesterol concentration was estimated by three independent measurements on different blood donors.
Depletion of cholesterol from neutrophil membrane
Methyl-β-cyclodextrin (MβCD) is a well established cholesterol depleting reagent of phospholipidic membrane without affecting their permeability and it is the most commonly used reagent [63–66]. The neutrophil membranes were incubated for 1 h, at 4 °C, in presence of 10 mM MβCD. The mixture was centrifuged at 148,000g for 1 h 30 min at 4 °C. Neutrophil membranes were found in the pellet and the cyclodextrin–cholesterol complexes were in the supernatant. The neutrophil membranes were resolubilized in PBS.
Measurement of superoxide production in cell-free assays
Superoxide anion production rates were quantified by the initial rate of cytochrome c (Cytc) reduction, as described before [67]. The reaction is as follows:
| Cyt cox + O2•− → O2 + Cyt cred |
Unless indicated, the components of the cell-free system were added as follows: (2–5 nM Cyt b558) membrane fractions, (100–200 nM) trimera and (40 µM) arachidonic acid in 500 µL Phosphate Buffer Saline supplemented with 10 mM MgSO4 for 4 min of incubation at 25 °C in order to allow the Nox complex assembly. The production was initiated by addition of (250 µM) NADPH and the rate of O2−• was quantified by the reduction of cytochrome c (50 µM). The rate was measured at 550 nm in a Thermo evolution500 Spectrophotometer. The amount of superoxide was calculated using a molar extinction coefficient (Δε of the reduced minus oxidized form of Cytc) of 21 mM−1 cm−1. 20 mM stock solution of cholesterol was prepared in ethanol. Further dilutions have been done in ethanol to get a concentration range of cholesterol (0.1–16 mM), which was mixed with AA 65 mM. 3 µL of each mixture was introduced in the cell free system to finally have cholesterol 0.25–40 µM in a mixture with 40 µM AA in the final reaction volume. This allowed keeping the volume constant.
2.10. Determination of enzymatic parameters and curve plotting
The enzymatic parameters EC50 and Vmax were calculated by non-linear least square fitting of the curves of superoxide rate of production vs. protein concentration using the following expression.
| (1) |
where [P] is the concentration of the considered protein (trimera). Plotting and calculation were performed using Graph Pad Prism Version 6.
Intrinsic fluorescence assays
Steady-state fluorescence spectra were performed on a Photon Technology International scanning fluorimeter at 25 °C. Various concentrations of AA and/or cholesterol were added as indicated to a final volume of 3 mL of buffer (phosphate buffer saline supplemented with 10 mM MgSO4) containing trimera (60 nM) in a quartz cuvette. The tryptophan fluorescence spectra of trimera were obtained by exciting the samples at 290 nm (2 nm bandwidth) and recorded between 300 and 400 nm (5 nm bandwidth). The excitation wavelength was chosen at 290 nm to optimize the signal to noise ratio and to reduce the contribution of tyrosine residues to the signal [68].
Results
Intrinsic cholesterol concentration and effect of cholesterol depletion by methyl-β-cyclodextrin on superoxide production rate
We first measured the intrinsic cholesterol concentration in human neutrophil membrane fractions. We found 3±1 µM cholesterol in the final reaction volume of cell free system assay (2 nM Cyt b558, 40 µg/mL membrane proteins) in three independent measurements. Then to investigate the role of cholesterol that is naturally present in the neutrophil membrane on NADPH oxidase, 10 µM MβCD was used to disrupt lipid rafts by removing cholesterol from membranes [66,69,70]. The rate was measured as described in Material and methods. We found that cholesterol depletion decreased superoxide production rate relative to the non-treated membrane neutrophil to (44±7)%.
In the following studies, the level of cholesterol was increased to a range corresponding to hypercholesterolemia (up to ca. 33% of the normal concentration) and above, to enlighten the effects of this addition.
Cholesterol as an activating molecule?
Having established the most propitious conditions for activation (see Materials and methods), we aimed at determining whether cholesterol could have an activator effect on NADPH oxidase. The results expressed as percentages of NADPH oxidase activity as a function of cholesterol are displayed in Fig. 1. Addition of cholesterol in the range 0.2–1 µM (ca. 7–33% increase compared to the intrinsic value) provoked slight but significant activation of NADPH oxidase complex, but not at an equivalent level to AA. The rate of production of O2•− stayed at around 15% of the rate value obtained with AA for concentrations above 1 µM. Comparable results were obtained using the separated subunits where a maximum activity of (20±2)% of AA-dependent activity was reached (data not shown).
Superoxide production in the presence of AA plus added cholesterol
NADPH oxidase activity was inhibited by the addition of cholesterol
Since cholesterol alone does not activate NADPH oxidase, we have tested the cholesterol effect on activation by AA.The AA induced NADPH-oxidase activity was followed upon increasing concentrations of cholesterol (Fig. 2). To avoid an increase of solvent volume, a mixture of cholesterol added with AA for each cholesterol concentration was prepared. The rate of production with AA alone was considered as 100%. Surprisingly, when membrane fractions and trimera were incubated together with 0.25 µM cholesterol and 40 µM AA, the activity dropped to about 86±5%. The AA-induced NADPH oxidase activity was thus reduced by addition of less than 10% of the intrinsic cholesterol amount. The decrease of the activity could be fitted by a two inhibitory sites equation (Fig. 2) and the parameters of the fit are given in Table 1.
Fig. 2.
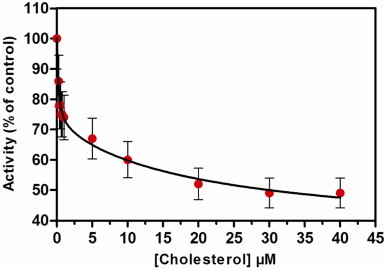
NADPH-oxidase activity inhibition by cholesterol. Neutrophil membrane fractions and trimera were incubated together in the presence of a mixture of 40 µM AA plus cholesterol. The oxidase activity was expressed as the percent of activity measured in the absence of cholesterol (79 mol O2•−/s/mol Cyt b558) as described in Materials and methods. The values are an average of 3 independent measurements±SEM. Table 1 shows the kinetic parameters of the fit.
Table 1.
⁎Parameters of the fits of Fig. 2. Y=Ymin
| Value | Error | |
|---|---|---|
| Ymin (%) | 35 | 9.6 |
| Y1 (%) | 30 | 4.6 |
| (µM) | 0.19 | 0.09 |
| Y2 (%) | 35 | 7.3 |
| (µM) | 21.2 | 16.7 |
Effect of cholesterol on AA activation profile
To probe the effect of cholesterol on the NADPH activation profile by AA, we performed titrations of the activity vs. AA concentration in the absence and in the presence of two concentrations of cholesterol (0.5 and 20 µM). In this experiment cholesterol was added at the same time as Arachidonic acid (Fig. 3).
Fig. 3.
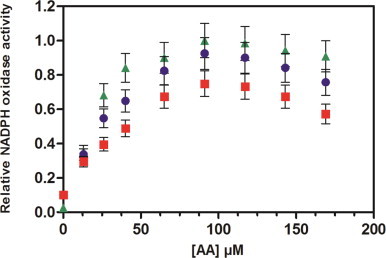
Effect of cholesterol on the AA-dependent activation profile. Neutrophil membrane fractions and trimera were incubated together in the presence of a mixture of varying amounts of AA plus cholesterol. The cholesterol concentration was as follow, ▲: no cholesterol; ●: 0.5 µM cholesterol; ■: 20 µM cholesterol. Oxidase activities were expressed relative to the maximum activity (119±12 mol O2•−/s/mol Cyt b558). The rate of O2•− production was achieved as described in Materials and methods.
In the presence of cholesterol, the O2•− production was lower on the full range of concentrations of AA, which confirms the inhibitory effect of cholesterol in a concentration-dependent manner. However the maximum activity was achieved with the same AA concentration and the bell-shape curve was kept.
Modification of kinetic parameters in the presence of added cholesterol
The absence of effect of cholesterol on the AA activation profile described above raised the question of the mechanism by which addition of cholesterol decreases the activity of the complex. In that purpose, the rate of superoxide production for increasing concentrations of trimera with and without cholesterol was determined. This dependence could always be fitted using a Michaelis–Menten-like equation (see materials and methods) and from each curve one could determine EC50 and Vmax values. There was a marked loss in the oxidase activity in the presence of cholesterol for the whole range of concentrations of trimera, with important variations for both the EC50 and Vmax values (Fig. 4, Table 2). In the presence of cholesterol, the complex exhibited 1.5 times lower Vmax value and the EC50 was 1.6 times higher than its values measured in absence of cholesterol, which might reflect a decrease of affinity of trimera for the Cyt b558.
Fig. 4.
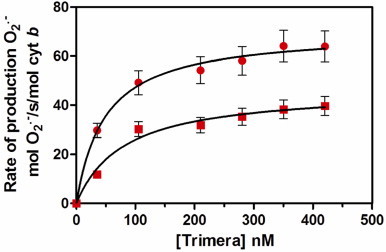
Effect of cholesterol on the trimera dependence NADPH oxidase activity. The assays mixtures consisted of trimera at varying concentrations (from 0 to 420 nM) and membrane fractions of human neutrophils (3 nM Cyt b558) to which were added ● 40 µM AA or ■ a mixture of 40 µM AA and 10 µM cholesterol. The superoxide formation was measured as indicated in Materials and methods. Each curve was fitted by Michaelis–Menten like equation leading to the determination of the EC50 and Vmax values related to the trimera. The values of these kinetic parameters in the presence of AA alone or AA plus cholesterol are in Table 2. The values are an average of three independent measurements±SEM.
Table 2.
Kinetic parameters of NADPH oxidase activation by trimera.
| Assay enriched with |
Vmax (mol O2•−/s/mol cytb) |
EC50 (nM trimera) |
|---|---|---|
| AA 40 µM | 70.3±2 | 48.6±3 |
| AA 40 µM, cholesterol 10 µM | 46.6±6 | 79.8±19 |
Effect of cholesterol addition during assembly phase
To examine whether cholesterol had an effect on the oxidase assembly process, 10 µM of cholesterol were added at different times during the 4-min phase of assembly (Fig. 5). Depending on the time at which cholesterol was added, various levels of inhibition were noticed. O2•− production was drastically lowered to ~66% of the control when cholesterol was added immediately after all the NADPH-oxidase components. The inhibition was less and less important (up to 100% activity) for times longer than 1 min. If cholesterol addition took place 4 min after the mixing, the oxidase activity remained comparable to that of the control. Both results (Figs. 4 and 5) suggest that cholesterol interferes in the interaction between trimera and Cyt b558.
Fig. 5.
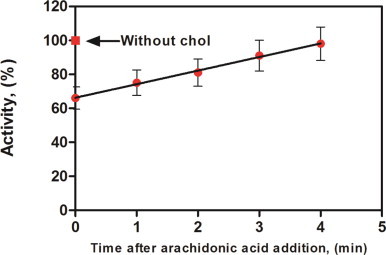
Effect of the addition time of cholesterol on NADPH-oxidase activity. Membrane fractions (MF), trimera, and 40 µM AA were incubated 4 min in reaction buffer. 10 µM cholesterol were added at various times after the addition of AA. Conditions are as described under Materials and methods. Results are expressed as percentages of the control activity (without cholesterol addition). The values represent the means±SEM of three independent experiments.
Structural effects of added cholesterol
Effect of cholesterol on soluble and membrane proteins
To evaluate the sensitivity of each component, the membrane part and the trimera, to cholesterol, superoxide anion production rate was measured after pre-incubation of membrane components or trimera or both, either with AA alone (Fig. 6A) or with a mixture of cholesterol and AA (Fig. 6B). After 10 s of separate pre-incubations of the membrane and of the trimera, both solutions were mixed and left for a second incubation for 4 min. We chose to pre-incubate for 10 s because a preincubation for longer time (30 s) led to a drastic decrease of the activity. This was also observed in the case of separated subunits [71].
Fig. 6.
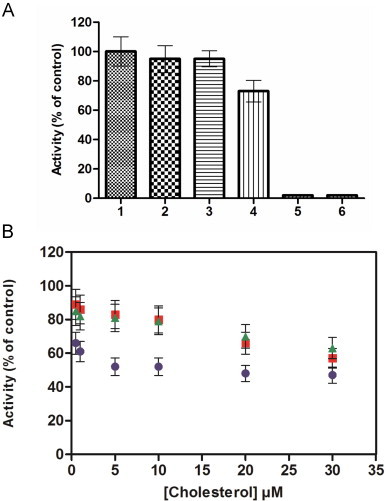
Effect of cholesterol on AA activation of membrane fraction and trimera. (A) AA activation of membrane fraction and trimera (1) In the control experiment, membrane fraction and the trimera were incubated together during 4 min in the presence of 40 µM AA. The rate obtained corresponds to the 100% value (75 mol O2•−/s/mol Cyt b558). (2–4) Membrane fractions (MF) and trimera were separately preincubated during 10 s and mixed together for 4 min as follow: (2) both were preincubated with 40 µM AA, (3) only MF was preincubated with 40 µM AA, (4) only the trimera were preinicubated with 40 µM AA. (5) The rate was measured in absence of trimera. (6) The rate was measured in absence of MF. (B) Membrane fractions (MF) and trimera were separately preincubated during 10 s and mixed together for 4 min as follow: ■ only MF was preincubated with 40 µM AA plus cholesterol as indicated, ▲ only the trimera was preincubated with 40 µM AA plus cholesterol as indicated and ● both were preincubated with 40 µM AA plus cholesterol. Activities in Fig. 6B were expressed as the percent of activities measured of Fig. 6A corresponding to each state to assess the cholesterol effect only.
First, when both membrane fractions and trimera were preincubated separately with AA, the activity was maintained to about 95% of the observed one in standard conditions. The same level of activity (95%) was measured when AA was incorporated only to the membrane fractions. On the other hand, when AA was added only to the trimera, the activity dropped to 73% (Fig. 6A).
The experiment with AA (Fig. 6A) served as control for the ones with cholesterol. In all cases, addition of cholesterol+AA either to the membrane or to the trimera or to both separately led to a decreased rate of superoxide production (Fig. 6B). Incorporating cholesterol+AA only to the membrane fractions or only to the trimera gave comparable effect, indicating that cholesterol could act on both partners. When cholesterol was added to both counterparts, the activity drop was more important, the addition of 5 µM or more of cholesterol to both fractions led to a decrease of the activity down to ~50%. The inhibition always increased by increasing cholesterol concentration, in agreement with the results presented Fig. 2.
Effect of AA and cholesterol on the tryptophan intrinsic fluorescence of the trimera
To probe the effect of AA and cholesterol on the trimera conformational state, we followed the variation of intrinsic tryptophan fluorescence spectra upon addition of AA and/or cholesterol (Fig. 7). Trimera contains a total of 13 tryptophan residues. It was previously shown that addition of AA to the cytosolic subunits p67phox and p47phox induced changes reflected by measurable decrease of the intrinsic tryptophan fluorescence level [68,72].
Fig. 7.
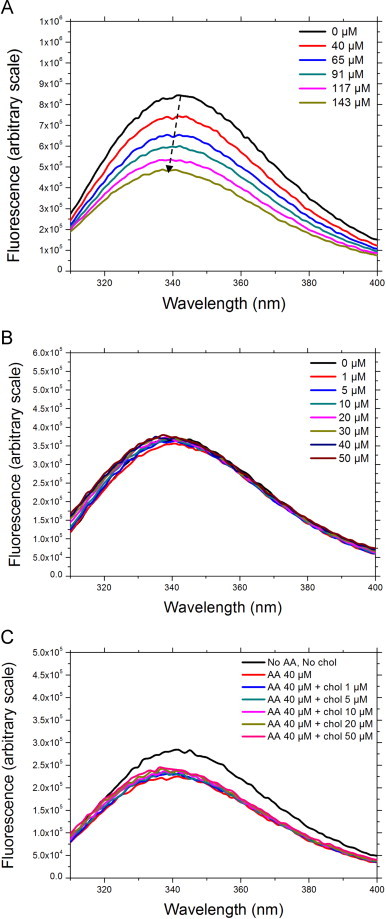
Intrinsic fluorescence of trimera treated with either AA or cholesterol or both. The emission spectra were measured using an excitation wavelength of 290 nm as described in Materials and methods. Results are representative of at least three independent experiments. A: trimera 60 nM, AA as stated in the figure legend. B: trimera 60 nM, cholesterol as stated in the figure legend. C: trimera 60 nM, AA 40 µM, cholesterol as stated in the figure legend.
By increasing AA concentration up to 140 µM, the fluorescence yield of the trimera underwent a concentration dependent decrease (Fig. 7A). A small blue shift up to 5 nm was observed, consistent with a slightly more hydrophobic tryptophan environment.
Surprisingly, the addition of cholesterol had no effect on the fluorescence yield of the trimera but a similar blue shift up to 5 nm was detected by increasing cholesterol concentration to 50 µM (Fig. 7B), indicating that addition of cholesterol does not induce conformational changes in the trimera, as AA does. When cholesterol was added to the trimera treated with 40 µM AA, the initial quenching of 25% due to the presence of AA still was visible, but there was no further lowering due to cholesterol (Fig. 7C). In addition to that the same blue shift up to 5 nm was observed after the addition of both AA and cholesterol.
4. Discussion
In this work we have explored the effect of added cholesterol on the activity of the phagocyte NADPH oxidase. This study is related to events that could happen to Nox2 following hypercholesterolemia. It is also related to the hypothesis of modulation of NADPH oxidase activity by lipid rafts rich in cholesterol. In fact, some studies have reported the distribution and regulation of Nox proteins and oxidase subunits in LRs, (most of the studies were performed in non-human cells [11–14] except those in human neutrophils) [9,73]. Given that, NADPH oxidase activation demands many partners to work together, LRs can provide a useful platform for Nox2 and its subunits to aggregate and then function as an active enzyme complex that produces O 2•−[13,14,74–78]. In particular, it has been demonstrated that NADPH oxidase was assembled and activated in LR of neutrophils, producing O2•−, causing respiratory bursts and killing bacteria [9]. The integrity of LRs would play a crucial role in the regulation of NADPH oxidase activity and cholesterol would be an essential component in LRs for NADPH oxidase in agreement with previous reports [11,79]. Actually, the role of specific protein–lipid interactions in oxidase assembly has been studied in the last decades [32,36,38]. It has been shown that the interaction of the PX domains of p40phox and p47phox with phospholipids constitutes an essential mechanism to orchestrate the assembly of the cytoplasmic components with the phagosomal membrane. The PX domain of p40phox interacts selectively with phosphatidyl-inositol3-phosphate, (PtdIns(3)P) [80,81], while the PX domain of p47phox preferentially recognizes (PtdIns(3,4)P2) [80,82].
On the required cholesterol concentration in neutrophils
The regulatory role of LR was rationalized by the fact that cholesterol exerts a stabilizing role in LRs by filling the void space between sphingolipids and forming hydrogen bonds with them. Thus, cholesterol depletion by MβCD would lead to the breakdown of LRs as it suppresses the glue effect of cholesterol on sphingolipids [83,84]. In addition to that, removal of raft cholesterol leads to dissociation of most proteins from the rafts, rendering them nonfunctional [85]. Shao et al. showed that incubation of the cells with MβCD resulted in a loss of association of gp91phox with the LR fractions [9] while Vilhardt et al. reported that cholesterol depletion by MβCD reduced significantly O2•− production in both intact cells and a cell-free reconstituted system and MβCD effect was joined with a parallel reduction of the translocation of cytosolic components to the membrane [11]. Later, Fuhler et al. further demonstrated that treatment of neutrophils with the MβCD, abrogated fMLP-induced ROS production and activation of protein kinases ERK1/2 and B/Akt in both unprimed and primed neutrophils, further assisting the opinion that LR-associated NADPH oxidase produces ROS and contributes importantly to the onset of phagocytic respiratory bursts [86]. Our results about removal of cholesterol by MβCD are in agreement with the preceding findings since the oxidase activity was decreased to (44±7)%.
On the effect of cholesterol
Our results indicated slight but significant activation of NADPH oxidase complex in cell free system by addition of cholesterol alone at physiological concentrations (ca. 10–30% above the normal level), without AA. Conversely, addition of cholesterol in this range has an inhibitory effect on AA activation of NADPH oxidase activity. Similar amplitude was also observed at 37 °C (data not shown). The effect of cholesterol did not interfere with that of AA (same profile), indicating different binding sites for both compounds. Several facts indicate the presence of two independent inhibitory binding sites. Effectively, in Fig. 2, the curve could be fitted only with a two-site inhibition equation. In addition, cholesterol effect has been observed when it was preincubated either with the membrane or with the trimera alone. When cholesterol (0.2–10 µM) was added to one component (membrane or trimera) (Fig. 7B) a small inhibition was observed (~20%), but when both components were preincubated with the same concentration, a higher inhibition was measured (~50%). It strengthens the idea that cholesterol affects not only the membrane fraction but also the cytosolic ones.
The kinetic parameters in the presence of cholesterol revealed that Vmax for trimera is lower while EC50 is higher, which points out a less stable and imperfect assembly of the complex. Furthermore, cholesterol acts before assembly (Fig. 5), which might reflect that, one of the cholesterol binding sites is in the interaction region between membrane and cytosolic components, in the region hindered after assembly. Indeed, once the complex is formed, cholesterol cannot have access to it and makes no inhibitory effect. This effect has to be related to the observation that the depletion of cholesterol by MβCD also reduced the translocation of cytosolic proteins [10]. The cholesterol concentration found in membrane neutrophil seems to be optimal for NADPH oxidase activity.
4.3. On the conformation of the cytosolic partner
We have shown recently that AA modified the environment of tryptophan residues in the separated cytosolic subunits: both p47phox and p67phox underwent fluorescence decrease, which would be related to structural modifications necessary for their interaction with Cyt b558[58,68]. A similar tryptophan fluorescence decrease was observed for the trimera upon addition of AA. No comparable effect of cholesterol on the trimera was observed, indicating that cholesterol cannot adapt the protein to the membrane subunit.
In conclusion, while the presence of cholesterol at physiological concentration (e.g. in lipid rafts) is important for the NADPH oxidase function, an increase of cholesterol amount might have several consequences: (i) a slight but significant activity in the absence of the usual pro-inflammatory signals such as AA with the possibility of a permanent mild inflammatory state and (ii) an inhibition of the activity of NADPH oxidase in the presence of pro-inflammatory signals. In both cases there would be a modification of the response to the signaling regulation.
Acknowledgments
We are grateful to Prof. E. Pick for the plasmids, to Dr. L. Baciou for helpful discussions and to Dr. F. Lederer for the preparation of neutrophiles. We acknowledge the financial support of the COSTCM1201 Action (Biomimetic models) and of ANR2010-blan-1536-01. We are indebted to EDF for financial support (contract RB 2013-15).
Appendix A. Supplementary material
Supplementary material
References
- 1.Rodiño-Janeiro B.K., Paradela-Dobarro B., Castiñeiras-Landeira M.I., Raposeiras-Roubín S., González-Juanatey J.R., Alvarez E. Current status of NADPH oxidase research in cardiovascular pharmacology. Vascular Health and Risk Management. 2013;9:401–428. doi: 10.2147/VHRM.S33053. 23983473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James C.E., Underwood S.S.C. General and Systematic Pathology. Churchill Livingstone; London: 2009. [Google Scholar]
- 3.Brown M.S., Goldstein J.L. How LDL receptors influence cholesterol and atherosclerosis. Scientific American. 1984;251(5):58–66. doi: 10.1038/scientificamerican1184-58. 6390676 [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar D., Soran H., Durrington P.N. Hypercholesterolaemia and its management. BMJ (Clinical Research Ed.) 2008;337:a993. doi: 10.1136/bmj.a993. 18719012 [DOI] [PubMed] [Google Scholar]
- 5.Crall F.V., Jr., Roberts W.C. The extramural and intramural coronary arteries in juvenile diabetes mellitus: Analysis of nine necropsy patients aged 19–38 years with onset of diabetes before age 15 years. American Journal of Medicine. 1978;64(2):221–230. doi: 10.1016/0002-9343(78)90049-9. 629271 [DOI] [PubMed] [Google Scholar]
- 6.Selby J.V., Friedman G.D., Quesenberry C.P., Jr. Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. American Journal of Epidemiology. 1990;131(6):1017–1027. doi: 10.1093/oxfordjournals.aje.a115593. 2343854 [DOI] [PubMed] [Google Scholar]
- 7.Jin S., Zhou F. Lipid raft redox signaling platforms in vascular dysfunction: features and mechanisms. Current Atherosclerosis Reports. 2009;11(3):220–226. doi: 10.1007/s11883-009-0034-6. 19361354 [DOI] [PubMed] [Google Scholar]
- 8.Simons K., Ehehalt R. Cholesterol, lipid rafts, and disease. Journal of Clinical Investigation. 2002;110(5):597–603. doi: 10.1172/JCI16390. 12208858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao D., Segal A.W., Dekker L.V. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Letters. 2003;550(1–3):101–106. doi: 10.1016/s0014-5793(03)00845-7. 12935894 [DOI] [PubMed] [Google Scholar]
- 10.Han W., Li H., Villar V.A., Pascua A.M., Dajani M.I., Wang X., Natarajan A., Quinn M.T., Felder R.A., Jose P.A., Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51(2):481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. 18195159 [DOI] [PubMed] [Google Scholar]
- 11.Vilhardt F., van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO Journal. 2004;23(4):739–748. doi: 10.1038/sj.emboj.7600066. 14765128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B., Oo T.N., Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2006;20(9):1501–1503. doi: 10.1096/fj.05-5359fje. 16754746 [DOI] [PubMed] [Google Scholar]
- 13.Zhang A.Y., Yi F., Zhang G., Gulbins E., Li P.L. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47(1):74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. 16344372 [DOI] [PubMed] [Google Scholar]
- 14.Zuo L., Ushio-Fukai M., Hilenski L.L., Alexander R.W. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(7):1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. 15142861 [DOI] [PubMed] [Google Scholar]
- 15.Nauseef W.M. How human neutrophils kill and degrade microbes: an integrated view. Immunological Reviews. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. 17850484 [DOI] [PubMed] [Google Scholar]
- 16.Segal A.W. How neutrophils kill microbes. Annual Review of Immunology. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. 15771570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babior B.M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64(5):959–966. 6386073 [PubMed] [Google Scholar]
- 18.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. 17237347 [DOI] [PubMed] [Google Scholar]
- 19.Babior B.M. NADPH oxidase. Current Opinion in Immunology. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. 14734109 [DOI] [PubMed] [Google Scholar]
- 20.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circulation Research. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. 10720409 [DOI] [PubMed] [Google Scholar]
- 21.Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Science. 2009;100(8):1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. 19493276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambeth J.D., Kawahara T., Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radical Biology and Medicine. 2007;43(3):319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. 17602947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassègue B., Griendling K.K. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. 19910640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seger R.A. Chronic granulomatous disease: recent advances in pathophysiology and treatment. Netherlands Journal of Medicine. 2010;68(11):334–340. 21116026 [PubMed] [Google Scholar]
- 25.Sorce S., Krause K.H. NOX enzymes in the central nervous system: from signaling to disease. Antioxidants and Redox Signaling. 2009;11(10):2481–2504. doi: 10.1089/ars.2009.2578. 19309263 [DOI] [PubMed] [Google Scholar]
- 26.Li H., Horke S., Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends in Pharmacological Sciences. 2013;34(6):313–319. doi: 10.1016/j.tips.2013.03.007. 23608227 [DOI] [PubMed] [Google Scholar]
- 27.Varga Z.V., Kupai K., Szűcs G., Gáspár R., Pálóczi J., Faragó N., Zvara A., Puskás L.G., Rázga Z., Tiszlavicz L., Bencsik P., Görbe A., Csonka C., Ferdinandy P., Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. Journal of Molecular and Cellular Cardiology. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. 23722270 [DOI] [PubMed] [Google Scholar]
- 28.Neumann A., Brogden G., Jerjomiceva N., Brodesser S., Naim H.Y., von Köckritz-Blickwede M. Lipid alterations in human blood-derived neutrophils lead to formation of neutrophil extracellular traps. European Journal of Cell Biology. 2014;93:347–354. doi: 10.1016/j.ejcb.2014.07.005. 25172775 [DOI] [PubMed] [Google Scholar]
- 29.Drummond G.R., Sobey C.G. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends in Endocrinology and Metabolism: TEM. 2014;25(9):452–463. doi: 10.1016/j.tem.2014.06.012. 25066192 [DOI] [PubMed] [Google Scholar]
- 30.Carnevale R., Loffredo L., Sanguigni V., Plebani A., Rossi P., Pignata C., Martire B., Finocchi A., Pietrogrande M.C., Azzari C., Soresina A.R., Martino S., Cirillo E., Martino F., Pignatelli P., Violi F. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: lesson from chronic granulomatous disease. Journal of the American Heart Association. 2014;3(3):e000920. doi: 10.1161/JAHA.114.000920. 24973227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Račková L. Cholesterol load of microglia: contribution of membrane architecture changes to neurotoxic power? Archives of Biochemistry and Biophysics. 2013;537(1):91–103. doi: 10.1016/j.abb.2013.06.015. 23831332 [DOI] [PubMed] [Google Scholar]
- 32.Nauseef W.M. Assembly of the phagocyte NADPH oxidase. Histochemistry and Cell Biology. 2004;122(4):277–291. doi: 10.1007/s00418-004-0679-8. 15293055 [DOI] [PubMed] [Google Scholar]
- 33.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A., Marie J.C., Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Experimental and Molecular Medicine. 2009;41(4):217–225. doi: 10.3858/emm.2009.41.4.058. 19372727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard F.R., Kelher M.R., Moore E.E., McLaughlin N.J., Banerjee A., Silliman C.C. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. Journal of Leukocyte Biology. 2005;78(5):1025–1042. doi: 10.1189/jlb.0804442. 16204621 [DOI] [PubMed] [Google Scholar]
- 35.Raad H., Paclet M.H., Boussetta T., Kroviarski Y., Morel F., Quinn M.T., Gougerot-Pocidalo M.A., Dang P.M., El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2009;23(4):1011–1022. doi: 10.1096/fj.08-114553. 19028840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochemical Journal. 2005;386(3):401–416. doi: 10.1042/BJ20041835. 15588255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn M.T., Gauss K.A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. Journal of Leukocyte Biology. 2004;76(4):760–781. doi: 10.1189/jlb.0404216. 15240752 [DOI] [PubMed] [Google Scholar]
- 38.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS Journal. 2008;275(13):3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. 18513324 [DOI] [PubMed] [Google Scholar]
- 39.Grizot S., Grandvaux N., Fieschi F., Fauré J., Massenet C., Andrieu J.P., Fuchs A., Vignais P.V., Timmins P.A., Dagher M.C., Pebay-Peyroula E. Small angle neutron scattering and gel filtration analyses of neutrophil NADPH oxidase cytosolic factors highlight the role of the C-terminal end of p47phox in the association with p40phox. Biochemistry. 2001;40(10):3127–3133. doi: 10.1021/bi0028439. 11258927 [DOI] [PubMed] [Google Scholar]
- 40.Wientjes F.B., Panayotou G., Reeves E., Segal A.W. Interactions between cytosolic components of the NADPH oxidase: p40phox interacts with both p67phox and p47phox. Biochemical Journal. 1996;317(3):919–924. doi: 10.1042/bj3170919. 8760383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs A., Dagher M.C., Fauré J., Vignais P.V. Topological organization of the cytosolic activating complex of the superoxide-generating NADPH-oxidase. Pinpointing the sites of interaction between p47phoz, p67phox and p40phox using the two-hybrid system. Biochimica et Biophysica Acta. 1996;1312(1):39–47. doi: 10.1016/0167-4889(96)00020-1. 8679714 [DOI] [PubMed] [Google Scholar]
- 42.Lapouge K., Smith S.J., Groemping Y., Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. Journal of Biological Chemistry. 2002;277(12):10121–10128. doi: 10.1074/jbc.M112065200. 11796733 [DOI] [PubMed] [Google Scholar]
- 43.Ebisu K., Nagasawa T., Watanabe K., Kakinuma K., Miyano K., Tamura M. Fused p47phox and p67phox truncations efficiently reconstitute NADPH oxidase with higher activity and stability than the individual components. Journal of Biological Chemistry. 2001;276(27):24498–24505. doi: 10.1074/jbc.M101122200. 11333262 [DOI] [PubMed] [Google Scholar]
- 44.Alloul N., Gorzalczany Y., Itan M., Sigal N., Pick E. Activation of the superoxide-generating NADPH oxidase by chimeric proteins consisting of segments of the cytosolic component p67(phox) and the small GTPase Rac1. Biochemistry. 2001;40(48):14557–14566. doi: 10.1021/bi0117347. 11724569 [DOI] [PubMed] [Google Scholar]
- 45.Miyano K., Ogasawara S., Han C.H., Fukuda H., Tamura M. A fusion protein between rac and p67phox (1–210) reconstitutes NADPH oxidase with higher activity and stability than the individual components. Biochemistry. 2001;40(46):14089–14097. doi: 10.1021/bi010882u. 11705402 [DOI] [PubMed] [Google Scholar]
- 46.Mizrahi A., Berdichevsky Y., Ugolev Y., Molshanski-Mor S., Nakash Y., Dahan I., Alloul N., Gorzalczany Y., Sarfstein R., Hirshberg M., Pick E. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. Journal of Leukocyte Biology. 2006;79(5):881–895. doi: 10.1189/jlb.1005553. 16641134 [DOI] [PubMed] [Google Scholar]
- 47.Sarfstein R., Gorzalczany Y., Mizrahi A., Berdichevsky Y., Molshanski-Mor S., Weinbaum C., Hirshberg M., Dagher M.C., Pick E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. Journal of Biological Chemistry. 2004;279(16):16007–16016. doi: 10.1074/jbc.M312394200. 14761978 [DOI] [PubMed] [Google Scholar]
- 48.Gorzalczany Y., Alloul N., Sigal N., Weinbaum C., Pick E. A prenylated p67phox-Rac1 chimera elicits NADPH-dependent superoxide production by phagocyte membranes in the absence of an activator and of p47phox: conversion of a pagan NADPH oxidase to monotheism. Journal of Biological Chemistry. 2002;277(21):18605–18610. doi: 10.1074/jbc.M202114200. 11896062 [DOI] [PubMed] [Google Scholar]
- 49.Berdichevsky Y., Mizrahi A., Ugolev Y., Molshanski-Mor S., Pick E. Tripartite chimeras comprising functional domains derived from the cytosolic NADPH oxidase components p47phox, p67phox, and Rac1 elicit activator-independent superoxide production by phagocyte membranes: an essential role for anionic membrane phospholipids. Journal of Biological Chemistry. 2007;282(30):22122–22139. doi: 10.1074/jbc.M701497200. 17548354 [DOI] [PubMed] [Google Scholar]
- 50.Mizrahi A., Berdichevsky Y., Casey P.J., Pick E. A prenylated p47phox-p67phox-Rac1 chimera is a quintessential NADPH oxidase activator: membrane association and functional capacity. Journal of Biological Chemistry. 2010;285(33):25485–25499. doi: 10.1074/jbc.M110.113779. 20529851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyano K., Fukuda H., Ebisu K., Tamura M. Remarkable stabilization of neutrophil NADPH oxidase using RacQ61L and a p67phox-p47phox fusion protein. Biochemistry. 2003;42(1):184–190. doi: 10.1021/bi0269052. 12515553 [DOI] [PubMed] [Google Scholar]
- 52.Bromberg Y., Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cellular Immunology. 1984;88(1):213–221. doi: 10.1016/0008-8749(84)90066-2. 6090027 [DOI] [PubMed] [Google Scholar]
- 53.Bromberg Y., Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. Journal of Biological Chemistry. 1985;260(25):13539–13545. 2997168 [PubMed] [Google Scholar]
- 54.Curnutte J.T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. Journal of Clinical Investigation. 1985;75(5):1740–1743. doi: 10.1172/JCI111885. 2987311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heyneman R.A., Vercauteren R.E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. Journal of Leukocyte Biology. 1984;36(6):751–759. doi: 10.1002/jlb.36.6.751. 6594417 [DOI] [PubMed] [Google Scholar]
- 56.McPhail L.C., Shirley P.S., Clayton C.C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. Journal of Clinical Investigation. 1985;75(5):1735–1739. doi: 10.1172/JCI111884. 2987310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qualliotine-Mann D., Agwu D.E., Ellenburg M.D., McCall C.E., McPhail L.C. Phosphatidic acid and diacylglycerol synergize in a cell-free system for activation of NADPH oxidase from human neutrophils. Journal of Biological Chemistry. 1993;268(32):23843–23849. 8226922 [PubMed] [Google Scholar]
- 58.Souabni H., Thoma V., Bizouarn T., Chatgilialoglu C., Siafaka-Kapadai A., Baciou L., Ferreri C., Houée-Levin C., Ostuni M.A. Arachidonic acid isomers inhibit NADPH-oxidase activity by direct interaction with enzyme components. Biochimica et Biophysica Acta. 2012;1818:2314–2324. doi: 10.1016/j.bbamem.2012.04.018. 22580228 [DOI] [PubMed] [Google Scholar]
- 59.Ostuni M.A., Gelinotte M., Bizouarn T., Baciou L., Houée-Levin C. Targeting NADPH-oxidase by reactive oxygen species reveals an initial sensitive step in the assembly process. Free Radical Biology and Medicine. 2010;49(5):900–907. doi: 10.1016/j.freeradbiomed.2010.06.021. 20600833 [DOI] [PubMed] [Google Scholar]
- 60.Akasaki T., Koga H., Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. Journal of Biological Chemistry. 1999;274(25):18055–18059. doi: 10.1074/jbc.274.25.18055. 10364257 [DOI] [PubMed] [Google Scholar]
- 61.Mohanty J.G., Jaffe J.S., Schulman E.S., Raible D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. Journal of Immunological Methods. 1997;202(2):133–141. doi: 10.1016/s0022-1759(96)00244-x. 9107302 [DOI] [PubMed] [Google Scholar]
- 62.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Analytical Biochemistry. 1997;253(2):162–168. doi: 10.1006/abio.1997.2391. 9367498 [DOI] [PubMed] [Google Scholar]
- 63.Atger V.M., de la Llera Moya M., Stoudt G.W., Rodrigueza W.V., Phillips M.C., Rothblat G.H. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. Journal of Clinical Investigation. 1997;99(4):773–780. doi: 10.1172/JCI119223. 9045882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kilsdonk E.P., Yancey P.G., Stoudt G.W., Bangerter F.W., Johnson W.J., Phillips M.C., Rothblat G.H. Cellular cholesterol efflux mediated by cyclodextrins. Journal of Biological Chemistry. 1995;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. 7615524 [DOI] [PubMed] [Google Scholar]
- 65.Christian A.E., Haynes M.P., Phillips M.C., Rothblat G.H. Use of cyclodextrins for manipulating cellular cholesterol content. Journal of Lipid Research. 1997;38(11):2264–2272. 9392424 [PubMed] [Google Scholar]
- 66.Rodal S.K., Skretting G., Garred O., Vilhardt F., van Deurs B., Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Molecular Biology of the Cell. 1999;10(4):961–974. doi: 10.1091/mbc.10.4.961. 10198050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pick E., Bromberg Y., Shpungin S., Gadba R. Activation of the superoxide forming NADPH oxidase in a cell-free system by sodium dodecyl sulfate. Characterization of the membrane-associated component. Journal of Biological Chemistry. 1987;262(34):16476–16483. 2824496 [PubMed] [Google Scholar]
- 68.Swain S.D., Helgerson S.L., Davis A.R., Nelson L.K., Quinn M.T. Analysis of activation-induced conformational changes in p47phox using tryptophan fluorescence spectroscopy. Journal of Biological Chemistry. 1997;272(47):29502–29510. doi: 10.1074/jbc.272.47.29502. 9368011 [DOI] [PubMed] [Google Scholar]
- 69.Allen J.A., Halverson-Tamboli R.A., Rasenick M.M. Lipid raft microdomains and neurotransmitter signalling. Nature Reviews. Neuroscience. 2007;8(2):128–140. doi: 10.1038/nrn2059. 17195035 [DOI] [PubMed] [Google Scholar]
- 70.Smart E.J., Anderson R.G. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods in Enzymology. 2002;353:131–139. doi: 10.1016/s0076-6879(02)53043-3. 12078489 [DOI] [PubMed] [Google Scholar]
- 71.Karimi G., Houée Levin C., Dagher M.C., Baciou L., Bizouarn T. Assembly of phagocyte NADPH oxidase: a concerted binding process? Biochimica et Biophysica Acta. 2014;1840(11):3277–3283. doi: 10.1016/j.bbagen.2014.07.022. 25108064 [DOI] [PubMed] [Google Scholar]
- 72.Park H.S., Park J.W. Conformational changes of the leukocyte NADPH oxidase subunit p47(phox) during activation studied through its intrinsic fluorescence. Biochimica et Biophysica Acta. 1998;1387(1–2):406–414. doi: 10.1016/s0167-4838(98)00152-6. 9748657 [DOI] [PubMed] [Google Scholar]
- 73.Guichard C., Pedruzzi E., Dewas C., Fay M., Pouzet C., Bens M., Vandewalle A., Ogier-Denis E., Gougerot-Pocidalo M.A., Elbim C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. Journal of Biological Chemistry. 2005;280(44):37021–37032. doi: 10.1074/jbc.M506594200. 16115878 [DOI] [PubMed] [Google Scholar]
- 74.Bao J.X., Xia M., Poklis J.L., Han W.Q., Brimson C., Li P.L. Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries. American Journal of Physiology. Heart and Circulatory Physiology. 2010;298(3):H992–H1002. doi: 10.1152/ajpheart.00958.2009. 20061541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li P.L., Zhang Y., Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxidants and Redox Signaling. 2007;9(9):1457–1470. doi: 10.1089/ars.2007.1667. 17661535 [DOI] [PubMed] [Google Scholar]
- 76.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's STKE: Signal Transduction Knowledge Environment. 2006;2006(349):re8. doi: 10.1126/stke.3492006re8. 16926363 [DOI] [PubMed] [Google Scholar]
- 77.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxidants and Redox Signaling. 2009;11(6):1289–1299. doi: 10.1089/ars.2008.2333. 18999986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C., Li P.L. Membrane raft redox signalosomes in endothelial cells. Free Radical Research. 2010;44(8):831–842. doi: 10.3109/10715762.2010.485994. 20528560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao Malla R., Raghu H., Rao J.S. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. International Journal of Oncology. 2010;37(6):1483–1493. doi: 10.3892/ijo_00000801. 21042717 [DOI] [PubMed] [Google Scholar]
- 80.Kanai F., Liu H., Field S.J., Akbary H., Matsuo T., Brown G.E., Cantley L.C., Yaffe M.B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nature Cell Biology. 2001;3(7):675–678. doi: 10.1038/35083070. 11433300 [DOI] [PubMed] [Google Scholar]
- 81.Bravo J., Karathanassis D., Pacold C.M., Pacold M.E., Ellson C.D., Anderson K.E., Butler P.J., Lavenir I., Perisic O., Hawkins P.T., Stephens L., Williams R.L. The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Molecular Cell. 2001;8(4):829–839. doi: 10.1016/s1097-2765(01)00372-0. 11684018 [DOI] [PubMed] [Google Scholar]
- 82.Ago T., Takeya R., Hiroaki H., Kuribayashi F., Ito T., Kohda D., Sumimoto H. The PX domain as a novel phosphoinositide-binding module. Biochemical and Biophysical Research Communications. 2001;287(3):733–738. doi: 10.1006/bbrc.2001.5629. 11563857 [DOI] [PubMed] [Google Scholar]
- 83.Bollinger C.R., Teichgräber V., Gulbins E. Ceramide-enriched membrane domains. Biochimica et Biophysica Acta. 2005;1746(3):284–294. doi: 10.1016/j.bbamcr.2005.09.001. 16226325 [DOI] [PubMed] [Google Scholar]
- 84.Gulbins E., Li P.L. Physiological and pathophysiological aspects of ceramide. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;290(1):R11–R26. doi: 10.1152/ajpregu.00416.2005. 16352856 [DOI] [PubMed] [Google Scholar]
- 85.Jin S., Zhou F., Katirai F., Li P.L. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxidants and Redox Signaling. 2011;15(4):1043–1083. doi: 10.1089/ars.2010.3619. 21294649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuhler G.M., Blom N.R., Coffer P.J., Drayer A.L., Vellenga E. The reduced GM-CSF priming of ROS production in granulocytes from patients with myelodysplasia is associated with an impaired lipid raft formation. Journal of Leukocyte Biology. 2007;81(2):449–457. doi: 10.1189/jlb.0506311. 17079651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


