Summary
Background
National estimates for the numbers of babies born small for gestational age and the comorbidity with preterm birth are unavailable. We aimed to estimate the prevalence of term and preterm babies born small for gestational age (term-SGA and preterm-SGA), and the relation to low birthweight (<2500 g), in 138 countries of low and middle income in 2010.
Methods
Small for gestational age was defined as lower than the 10th centile for fetal growth from the 1991 US national reference population. Data from 22 birth cohort studies (14 low-income and middle-income countries) and from the WHO Global Survey on Maternal and Perinatal Health (23 countries) were used to model the prevalence of term-SGA births. Prevalence of preterm-SGA infants was calculated from meta-analyses.
Findings
In 2010, an estimated 32·4 million infants were born small for gestational age in low-income and middle-income countries (27% of livebirths), of whom 10·6 million infants were born at term and low birthweight. The prevalence of term-SGA babies ranged from 5·3% of livebirths in east Asia to 41·5% in south Asia, and the prevalence of preterm-SGA infants ranged from 1·2% in north Africa to 3·0% in southeast Asia. Of 18 million low-birthweight babies, 59% were term-SGA and 41% were preterm. Two-thirds of small-for-gestational-age infants were born in Asia (17·4 million in south Asia). Preterm-SGA babies totalled 2·8 million births in low-income and middle-income countries. Most small-for-gestational-age infants were born in India, Pakistan, Nigeria, and Bangladesh.
Interpretation
The burden of small-for-gestational-age births is very high in countries of low and middle income and is concentrated in south Asia. Implementation of effective interventions for babies born too small or too soon is an urgent priority to increase survival and reduce disability, stunting, and non-communicable diseases.
Funding
Bill & Melinda Gates Foundation by a grant to the US Fund for UNICEF to support the activities of the Child Health Epidemiology Reference Group (CHERG).
Introduction
An estimated 20 million infants are born globally with low birthweight (<2500 g) every year.1 Low birthweight is an important population indicator for tracking neonatal health and includes babies born preterm (<37 completed weeks of gestation) and infants with intrauterine growth restriction. These components of low birthweight have different causes and risks of mortality, morbidity, impaired growth, and non-communicable diseases. Hence, for us to guide interventions to address both prevention and care, we must delineate low birthweight according to preterm birth, intrauterine growth restriction, and their overlap.
National estimates of preterm birth for 184 countries have been published for the year 2010,2 showing a total of 14·9 million preterm births. In the Global Burden of Disease Study,3 77 million (3·1%) disability-adjusted life-years were attributed to preterm birth, similar to the burden of HIV or malaria. In 1998, de Onis and colleagues4 reported estimates of intrauterine growth restriction, using babies born full term and with low birthweight as a proxy measure. They estimated that 13·7 million babies were born at term and with low birthweight every year, but they did not provide national estimates.4 Furthermore, no estimates are available for the co-occurrence of intrauterine growth restriction and preterm birth, or the relation between intrauterine growth restriction and the widely used metric of low birthweight.
The classification of small for gestational age was defined by a 1995 WHO expert committee as infants below the 10th centile of a birthweight-for-gestational-age, gender-specific reference population.5, 6 A major challenge is selection of an appropriate global reference. Small for gestational age is a commonly accepted proxy measure of intrauterine growth restriction.5 However, small for gestational age includes babies who are constitutionally small and in the lower tail of the growth curve, in addition to infants who were growth-restricted in utero because of maternal and environmental factors, such as chronic undernutrition, multiple pregnancy, placental insufficiency, pregnancy complications (eg, pre-eclampsia), infections, and other toxic exposures.7 In settings with high rates of small-for-gestational-age births, growth restriction accounts for a high proportion of these,4 justifying its use as a proxy for intrauterine growth restriction.
Our aim is to estimate the national prevalence and numbers of neonates born small for gestational age at full term (≥37 weeks; term-SGA), and the co-occurrence of small for gestational age with preterm birth (preterm-SGA), in 138 countries of low and middle income. We focus on this group of countries in view of their high burden of disease and the urgent need for data to direct, monitor, and assess public health planning in these regions.
Methods
Definitions
We defined small for gestational age as a birthweight lower than the 10th centile for a specific completed gestational age by gender, using the Alexander reference population8 (US National Center for Health Statistics, 1991; n=3 134 879 livebirths). We defined term-SGA as a baby born small for gestational age at 37 or more completed weeks of gestation, and we classified preterm-SGA as infants born small for gestational age at fewer than 37 weeks of gestation. We defined low birthweight as a baby born weighing less than 2500 g. Finally, we defined appropriate for gestational age as a birthweight on or higher than the 10th centile for gestational age, using the Alexander reference.
Data sources
We obtained data from three sources: (1) systematic literature reviews to identify birth cohorts with information on birthweight and gestational age; (2) research networks of birth cohorts; and (3) the WHO Global Survey on Maternal and Perinatal Health.9 We considered datasets for inclusion if they contained information on: birthweight; gestational age measured by last menstrual period, ultrasound, or clinical assessment; and vital status (required for analyses described elsewhere).10 Our exclusion criteria were: datasets missing more than 25% of data for birthweight or gestational age; inaccurate gestational age ascertained by study investigators (ie, poorly linked gestational age–birthweight data, or gestational age reported in months); and gestational age established by symphysis fundal height. We only included weight in analyses if the measurement was made within 72 h of birth.
We initially searched Medline and WHO regional databases (LILACS, AIM, and EMRO) in September, 2009, to identify potential birth cohorts with data required for a larger scope of secondary analyses related to preterm birth and small-for-gestational-age-related mortality (appendix p 1).10 We identified additional datasets within maternal-neonatal research networks (ongoing maternal-newborn health studies, demographic surveillance sites, and WHO UNIMAPP11 studies). We contacted investigators to ascertain whether their studies met our inclusion criteria and, if so, we asked them to join the Child Health Epidemiology Reference Group (CHERG) SGA-Preterm Birth working group and contribute data for secondary analyses (appendix pp 2–4).10 We did another literature review in February, 2012, to identify published studies reporting the prevalence of both small-for-gestational-age births, using the Alexander reference,8 and low birthweight to use for statistical modelling, since low birthweight was the primary independent modelling predictor. We implemented two strategies: (1) a Medline search using terms (“small-for-gestational-age” OR “intrauterine growth restriction”) AND “low birthweight” AND (“incidence” OR “prevalence”) AND “developing country”; and (2) a Scopus search identifying all published articles that have cited small for gestational age using the Alexander reference.8 Further details on our search strategy are in the appendix (p 5).
We also analysed data from the WHO Global Survey on Maternal and Perinatal Health (appendix p 6),9 which gathered data between 2004 and 2008 from 373 facilities in 24 countries and included 290 610 births. We excluded data from Japan (n=3318) because it is a high-income country. Therefore, a total of 23 countries contributed to the analysis. Details of survey methods are reported elsewhere.9 The WHO Global Survey selected countries randomly from every WHO subregion and then picked facilities at random from the capital city and two other randomly selected provinces. For this facility-based survey, trained data collectors abstracted relevant data from medical records into standardised forms from all births in the facility over a specific period. Several facilities had data with improbable values or unrepresentative data. To exclude these poor data-quality facilities, we omitted those with fewer than 500 births (small sample size), preterm birth rates greater than 40% or less than 3% (outside biological plausibility range), or rates of low birthweight less than 1% (implausible). We aggregated data at the country level.
Datasets analysed by the original study investigators were approved by existing site institutional review boards. For datasets shared with the CHERG working group, personal identifiers were not included and, therefore, were deemed exempt by the Johns Hopkins Bloomberg School of Public Health institutional review board.
Procedures
In the first step of the estimation process, we developed a model to estimate the national prevalence of term-SGA, based on the included input data. We then estimated the prevalence of preterm-SGA, using meta-analytical methods, and we applied these proportions to recent national preterm birth estimates developed by members of our working group.2
We used Stata 11.0 for all analyses. We did random-effects regression with logit(term-SGA prevalence) as the dependent variable and study region as the clustered unit of analysis (appendix p 7). Variables tested included: biological factors (prevalence of low birthweight, neonatal mortality rate); health-care access (proportion of deliveries in a facility, proportion of births by caesarean section, proportion of mothers with more than four antenatal care visits); and demographic factors (proportion of cohort in highest risk categories of maternal age, parity, and maternal education). We created categorical dummy variables for: degree of selection bias (population-based or community-level recruitment, facility-based or antenatal care recruitment with some or minimum selection bias, tertiary care or referral facility); study decade; and method of assessment of gestational age (last menstrual period, ultrasound, clinical). To examine candidate models, we included the natural log of low-birthweight prevalence as the main predictor, added individual predictors, and assessed for significance (p<0·05), improvement in adjusted R2, and Akaike information criterion. To select the final model, we did cross-validation12 to compare prediction accuracy between potential models (appendix p 8).
We undertook sensitivity analyses using two datasets. In the first (modelling dataset A, n=45), we included all birth cohort data. In the second restricted dataset (modelling dataset B, n=20), we included only population-representative studies, excluding facility-based studies that might have selection bias (WHO studies,9 Pakistan Aga Khan University [ZAB], Uganda 200513). Both datasets A and B resulted in similar estimates of variables and model fit; thus, we present results of the larger dataset A, which enabled cross-validation. We also did multiple imputation14 to incorporate infants with missing birthweight back into the individual cohort studies (appendix p 9).
For every cohort in dataset A, we calculated the prevalence of small-for-gestational-age babies within two categories of preterm births: moderate-to-late preterm (between 32 weeks and <37 weeks of gestation) and early preterm (<32 weeks of gestation). We used random-effects meta-analyses to estimate the pooled regional and overall prevalence of infants born small for gestational age between 32 weeks and less than 37 weeks of gestation and those born at less than 32 weeks of gestation. We did sensitivity analyses to look at the effect of region, facility-based versus community-based recruitment, and study quality. We judged studies of a high quality to be those with population-based recruitment, adequate data capture (defined as missing <15% of birthweight data and <30% of birthweight data among neonatal deaths, and the proportion of infants born at <32 weeks comprising at least 5% of preterm births), and biological plausibility (prevalence of small for gestational age >1%).
We used the prediction model to estimate term-SGA prevalence in countries of low and middle income (UN Millennium Development Goal [MDG] classification) for the year 2010. We took national neonatal mortality rates from the UN Interagency Group for Child Mortality Estimation15 and low-birthweight rates from several sources (appendix p 10). To obtain the number of small-for-gestational-age liveborn infants, we multiplied the prevalence of term and preterm small for gestational age by the estimated number of livebirths for 2010.16 We used a bootstrap approach to estimate ranges of uncertainty (appendix p 11).
In every dataset, we calculated the proportion of term-SGA infants who were low birthweight and did meta-analyses, using random effects to pool the estimate at the major regional level. We multiplied this value by term-SGA estimates for every country and summarised them regionally.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
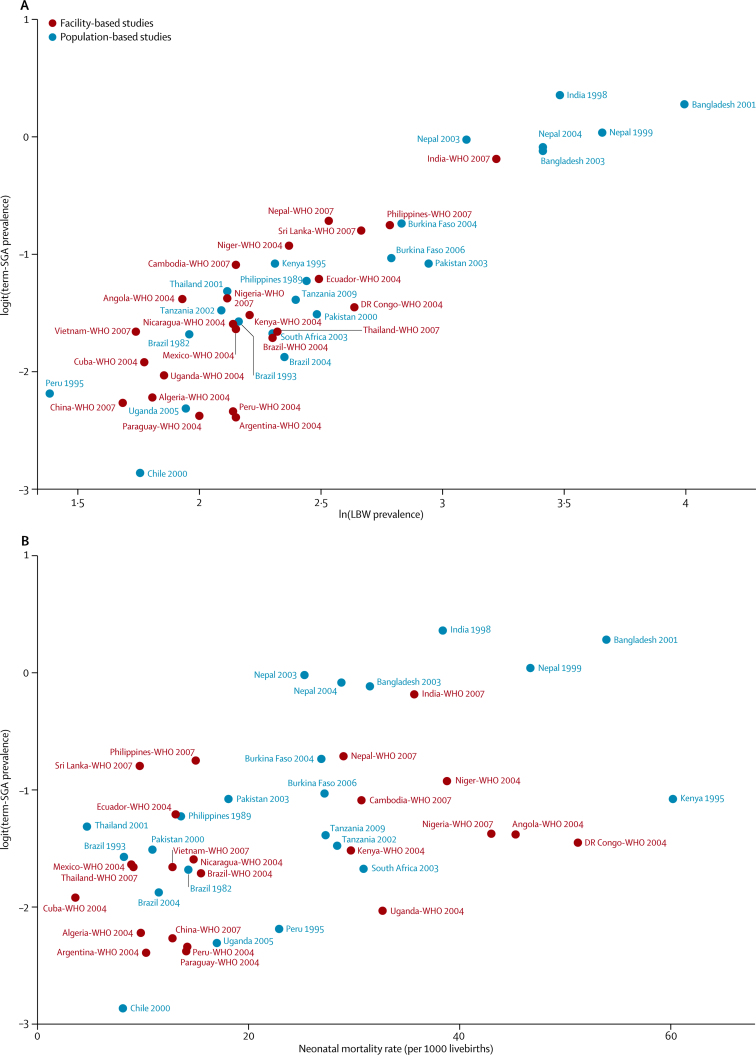
The appendix shows data inputs for the estimation process (p 12), study characteristics of the 22 CHERG cohorts included in our study13, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 (pp 2–4), and survey characteristics for the WHO datasets9 (p 6). From the literature review, we identified six studies reporting prevalence of babies born small for gestational age and the proportion of low-birthweight infants (appendix p 5); however, none of these studies could be used because term-SGA or preterm-SGA rates were not reported. Table 1 shows the final model for term-SGA. Logit(term-SGA prevalence) increased with rising rates of low birthweight and neonatal mortality (figure 1). Regional random effects were applied to account for regional variations of the relations. With low birthweight and neonatal mortality included in the model, socioeconomic covariates were not significant and, thus, not retained in the final model. Data source covariates were retained to control for selection bias and data quality. Regression diagnostic plots are shown in the appendix (p 13); the overall model fit was good (adjusted R2 0·8237).
Table 1.
Final statistical model for logit(term-SGA prevalence)
| Description | Coefficient (95% CI) | p | |
|---|---|---|---|
| ln(LBW prevalence) | Natural log of LBW prevalence | 0·997 (0·732 to 1·262) | <0·0001 |
| Neonatal mortality rate | Neonatal mortality rate | 0·012 (0·003 to 0·022) | 0·010 |
| Population selection dummy variable | Dummy variable to indicate facility recruitment in setting with high institutional delivery | 0·246 (–0·114 to 0·606) | 0·181 |
| Population selection dummy variable | Dummy variable to indicate facility-based recruitment and selection bias | 0·108 (–0·203 to 0·419) | 0·496 |
| _cons | .. | –4·160 (–4·968 to −3·352) | <0·0001 |
Adjusted R2=0·8237. LBW=low birthweight. SGA=small for gestational age.
Figure 1.
Scatterplots showing the relation of term-SGA to LBW and neonatal mortality rate
(A) logit(term-SGA prevalence) versus ln(LBW prevalence). (B) logit(term-SGA prevalence) versus neonatal mortality rate. SGA=small for gestational age. LBW=low birthweight.
Meta-analyses for the prevalence of babies born small for gestational age are presented in the appendix for moderate-to-late preterm infants (32 weeks to <37 weeks of gestation; pp 14–16) and for early preterm infants (<32 weeks of gestation; pp 17–18). Overall, in moderate-to-late preterm infants, 22·0% were small for gestational age (Asia 24·4%; Africa 17·0%; Latin America and the Caribbean 22·7%; appendix p 14). Prevalence of babies born small for gestational age in the moderate-to-late preterm group was similar in community-based and facility-based studies in Asia (appendix p 15), and when restricted to high-quality studies (p 16). For early preterm infants (born at <32 weeks of gestation), the potential for selection bias was greater, in view of early mortality before weighing in community-based cohorts and incomplete data capture. In Asia, prevalence of preterm-SGA was highest in facility-based studies (9·0% in nine facility studies vs 2·1% in six community studies; appendix p 17). With high-quality datasets, the overall prevalence of babies born small for gestational age before 32 weeks of gestation was 11·0% (appendix p 18). In sensitivity analyses, we noted no effect of imputation of missing birthweight data on the prevalence of preterm-SGA in four Asian and two African datasets (appendix p 9).
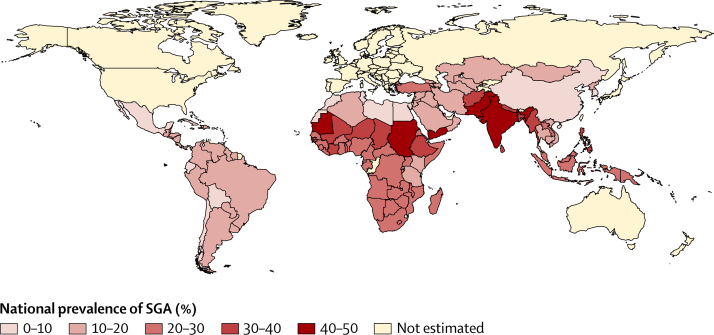
Figure 2 shows the estimated national prevalences of small-for-gestational-age births in low-income and middle-income countries for the year 2010 (a complete list of national estimates is available in the appendix pp 19–23). Table 2 presents the numbers and prevalence of term-SGA, preterm-SGA, and all small-for-gestational-age births, by UN-MDG region. Prevalence of term-SGA ranged from 5·3% in east Asia to as high as 41·5% in south Asia, and preterm-SGA ranged from 1·2% in north Africa to 3·0% in southeast Asia. For all small-for-gestational-age births, the highest prevalence was recorded in south Asia (44·5%), followed by sub-Saharan Africa (25·5%) and southeast Asia (24·3%). The greatest numbers of term-SGA infants were born in south Asia (16·2 million) and sub-Saharan Africa (7·5 million). Although their absolute numbers are lower, preterm-SGA infants carry a higher risk of mortality in the newborn and infant periods than do term-SGA infants;10 numbers of preterm-SGA babies totalled 1·2 million in south Asia and 0·6 million in sub-Saharan Africa. The vast majority of small-for-gestational-age infants (87%, 28·2 million) were born in south Asia, southeast Asia, and sub-Saharan Africa.
Figure 2.
Estimated prevalence of SGA births in 138 low-income and middle-income countries
SGA=small for gestational age.
Table 2.
Estimated prevalence and numbers of preterm and SGA infants, by UN-MDG region in 2010
| Births in 2010 |
Prevalence (uncertainty range) |
Number of births (uncertainty range)* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm birth2 | SGA | Term-SGA | Preterm-SGA | Preterm | SGA | Term-SGA | Term-SGA and LBW | Preterm-SGA | ||
| Caucasus and central Asia | 1 643 000 | 9·2% (6·1–13·0) | 15·0% (10·6–21·4) | 12·9% (8·5–19·2) | 2·1% (1·3–2·9) | 151 300 | 240 700(169 800–342 400) | 207 000 | 82 800 | 33 700 |
| East Asia | 17 490 000 | 7·2% (5·4–9·0) | 7·0% (4·2–11·6) | 5·3% (2·7–10·1) | 1·7% (1·1–2·1) | 1 262 200 | 1 182 300(720 700–1 975 000) | 901 000 | 360 400 | 281 400 |
| Southeast Asia | 10 983 400 | 13·6% (9·3–18·6) | 24·3% (19·5–30·2) | 21·2% (16·7–27·1) | 3·0% (2·0–4·3) | 1 497 500 | 2 670 200(2 143 400–3 318 900) | 2 336 400 | 934 600 | 333 900 |
| South Asia | 38 753 000 | 13·3% (10·1–16·8) | 44·5% (40·0–49·7) | 41·5% (37·4–46·9) | 2·9% (2·1–3·8) | 5 159 300 | 17 350 300(15 600 000–19 400 000) | 16 200 000 | 6 475 100 | 1 150 300 |
| West Asia | 4 855 300 | 10·1% (6·9–14·3) | 21·8% (17·6–27·2) | 19·6% (15·4–25·3) | 2·2% (1·5–3·2) | 488 200 | 1 066 900(863 100–1 334 300) | 958 100 | 383 200 | 108 900 |
| Oceania | 263 100 | 7·4% (4·5–15·6) | 21% (16·2–27·4) | 19·4% (14·6–25·3) | 1·6% (1·0–3·5) | 19 500 | 55 300(42 700–72 000) | 51 000 | 20 400 | 4300 |
| North Africa | 3 543 000 | 7·3% (4·8–10·9) | 9·6% (6·8–13·2) | 8·5% (5·7–11·9) | 1·2% (0·7–1·9) | 259 200 | 337 600(239 400–461 400) | 296 000 | 77 000 | 41 600 |
| Sub-Saharan Africa | 32 085 500 | 12·3% (9·5–15·8) | 25·5% (21·7–28·8) | 23·5% (19·9–26·7) | 2·0% (1·4–2·8) | 3 936 800 | 8 157 300(6 943 600–9 215 500) | 7 525 200 | 1 956 500 | 632 200 |
| Latin America and the Caribbean | 10 844 500 | 8·6% (7·0–12·0) | 12·5% (9·4–16·3) | 10·7% (7·7–14·4) | 1·8% (1·4–2·5) | 929 300 | 1 374 000(1 029 700–1 788 900) | 1 180 100 | 342 200 | 193 900 |
| Total† | 120 461 300 | 11·3% (8·6–14·7) | 27·0% (24·1–30·5) | 24·7% (21·7–28·1) | 2·3% (1·7–2·9) | 13 702 800 | 32 434 800(29 001 600–36 742 300) | 29 654 600 | 10 632 200 | 2 780 100 |
SGA=small for gestational age. LBW=low birthweight.
Uncertainty ranges for all estimates are included in the appendix (pp 19–23).
Total for 138 countries of low and middle income.
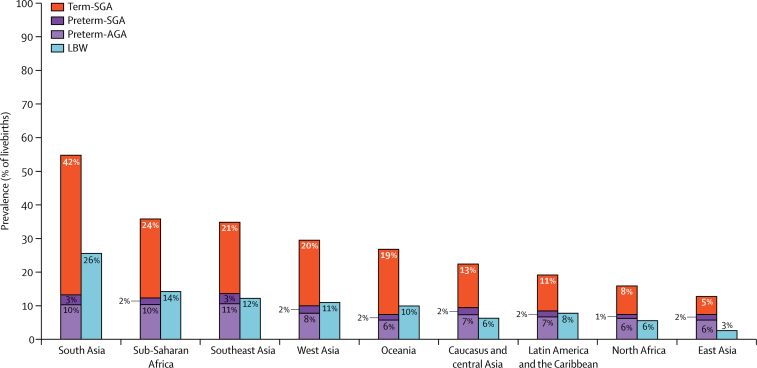
Figure 3 presents prevalence data for term-SGA, preterm-SGA, and preterm appropriate-for-gestational-age births, compared with the prevalence of babies born with low birthweight. Table 2 also shows the estimated numbers of term-SGA infants who weighed less than 2500 g at birth (term-SGA and with low birthweight), by UN-MDG region for 2010. In all regions, the majority (>50%) of term-SGA infants weighed 2500 g or heavier, with high proportions of babies not low birthweight but small for gestational age in Africa (74%) and Latin America and the Caribbean (71%). The highest regional proportion of low-birthweight babies was recorded in south Asia (26%), and the prevalence of term-SGA infants was also very high in this region (42%). Term-SGA accounted for 65% of low-birthweight babies in south Asia and preterm birth accounted for 35%. In sub-Saharan Africa, although preterm birth rates were similar to those in south Asia, the rate of low-birthweight babies was lower (14%) and preterm birth made a relatively larger contribution to the low-birthweight metric (57% preterm birth vs 43% term-SGA). Similarly in Latin America and the Caribbean, preterm birth comprised a larger proportion of the low-birthweight metric (60% preterm birth vs 40% term-SGA). In east Asia, the proportion of low-birthweight infants was very low (2·6%) and consisted mainly of preterm-SGA infants. In regions with lower rates of low-birthweight babies, such as north Africa or east Asia, preterm birth seems the more influential contributor to the low-birthweight metric.
Figure 3.
Prevalence of SGA, preterm births, and LBW by UN-MDG region in 2010
AGA=appropriate for gestational age. SGA=small for gestational age. LBW=low birthweight.
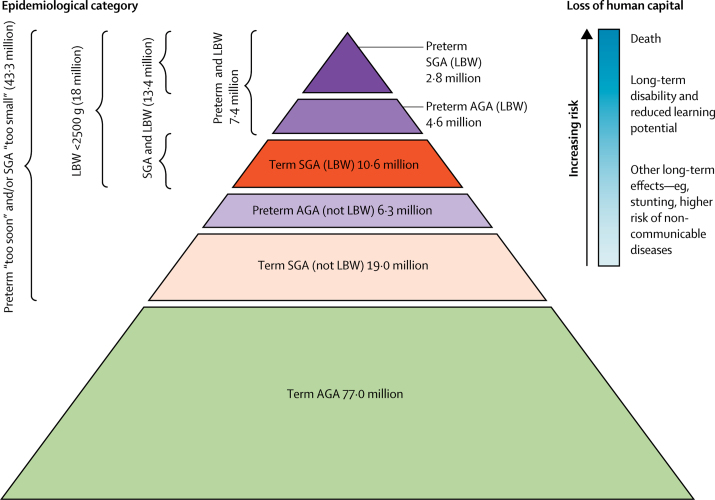
Overall, in countries of low and middle income in 2010, an estimated 43·3 million infants (36% of livebirths) were born either preterm or small for gestational age, or both (figure 4). Of 18 million low-birthweight infants, 59% were term-SGA whereas 41% were preterm (16% preterm-SGA, 25% preterm and appropriate size for gestational age).
Figure 4.
Public health implications of the burden of preterm and SGA births for 120 million births in countries of low and middle income
AGA=appropriate for gestational age. SGA=small for gestational age. LBW=low birthweight. Adapted from reference 37, with permission of WHO.
Table 3 shows the ten countries with the largest numbers of small-for-gestational-age infants born in 2010. An estimated 12·8 million babies were born small for gestational age in India alone (95% CI 11·5–14·3 million), with a prevalence of 47%. Pakistan, Nigeria, Bangladesh, China, and Indonesia had more than 1 million small-for-gestational-age babies.
Table 3.
Top ten countries with the highest numbers of SGA infants born in 2010
| Livebirths in 201016 | NMR 201015 | LBW births | Preterm births2 | Term-SGA births | Preterm-SGA births | Number of SGA births (uncertainty range) | SGA prevalence | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | India | 27 000 000 | 33·1 | 7 507 200 | 3 519 100 | 12 000 000 | 784 600 | 12 800 000 (11 500 000–14 300 000) | 46·9% |
| 2 | Pakistan | 4 700 000 | 36·1 | 1 232 800 | 748 100 | 2 061 300 | 166 800 | 2 228 100 (2 012 200–2 529 800) | 47·0% |
| 3 | Nigeria | 6 300 000 | 40·2 | 740 900 | 773 600 | 1 379 500 | 124 200 | 1 503 800 (1 275 300–1 709 100) | 23·7% |
| 4 | Bangladesh | 3 000 000 | 27·5 | 656 100 | 424 100 | 1 108 500 | 94 600 | 1 203 000 (1 071 800–1 369 200) | 39·6% |
| 5 | China | 17 000 000 | 9·4 | 398 400 | 1 172 300 | 810 700 | 261 400 | 1 072 100 (648 300–1 817 600) | 6·5% |
| 6 | Indonesia | 4 400 000 | 15·9 | 485 300 | 675 700 | 891 600 | 150 700 | 1 042 300 (814 800–1 309 300) | 23·8% |
| 7 | Ethiopia | 2 600 000 | 32·4 | 530 400 | 263 400 | 795 700 | 42 300 | 838 000 (698 900–957 600) | 32·1% |
| 8 | Philippines | 2 300 000 | 12·6 | 459 500 | 348 900 | 708 900 | 77 800 | 786 700 (641 600–937 900) | 33·6% |
| 9 | Democratic Republic of Congo | 2 900 000 | 47·4 | 275 800 | 341 400 | 574 600 | 54 800 | 629 500 (523 000–754 900) | 21·9% |
| 10 | Sudan | 1 400 000 | 31·5 | 438 600 | 188 300 | 565 000 | 30 200 | 595 200 (485 900–696 600) | 41·7% |
NMR=neonatal mortality rate. LBW=low birthweight. SGA=small for gestational age.
Discussion
Our data provide national and regional estimates for the prevalence and number of babies born small for gestational age and the co-occurrence of small for gestational age with preterm birth. 43·3 million infants (36% of livebirths) in countries of low or middle income were born either too small (small for gestational age) or too soon (preterm), or both, in 2010. The estimated burden of babies born small for gestational age is very high; 32·4 million neonates (27% of livebirths) are affected, of whom 29·7 million infants were born at full term (≥37 weeks) and 10·6 million were born at term and with low birthweight (<2500 g). Almost 3 million infants (2%) were born preterm and small for gestational age.
The highest rates and numbers of babies born small for gestational age were in south Asia, where more than half of babies small for gestational age are born and nearly one in two infants are born too small. The prevalence of babies born small for gestational age reached almost 50% in Pakistan and India, predicted largely on national rates for low birthweight, which were very high. The cutoff for small for gestational age at the 10th centile of the reference population was recommended by a WHO expert committee;5 however, a lower cutoff could be considered at the 3rd centile, which would indicate especially severe cases of small for gestational age, particularly in high-burden settings. With a 3rd centile38 cutoff, the prevalence of severe small-for-gestational-age births was 23% in south Asia, affecting 3·9 million infants (analysis not shown). The lowest rates of babies born small for gestational age were noted in east Asia, largely affected by data for China, where the reported low-birthweight rate was 2·4% (WHO Regional Offices, 2008).
A major challenge in estimating the global burden of babies born small for gestational age is selection of a common reference population. The Williams39 reference of Californian livebirths from 1970–76 (n=2 288 806) was recommended in 1995 by WHO6 in view of the multiracial population, representation at lower gestational ages, and association with survival. We chose the 1991 US birth reference population,8 which was published after the original WHO recommendation, because it is more recent than the Williams reference, has a large sample size (n=3 134 879) that better represents low gestational ages, covers a national and diverse multiethnic population, has well characterised methods to smooth centile curves and exclude outlying values, and is the most frequently cited reference in scientific literature. Choosing a common reference for burden estimates is important, since the estimated prevalence of babies born small for gestational age varies substantially depending on the reference population chosen. For example, within a south Indian cohort, the estimated prevalence of babies born small for gestational age ranged from 10·5%40 to 72·5%41 using the 10th centile cutoff of different reference populations, with the Alexander reference providing a prevalence of 56%8 (Joanne Katz, Johns Hopkins Bloomberg School of Public Health; personal communication). Another consideration is use of a birthweight-for-gestation curve versus an ultrasound-based fetal-weight curve. For preterm infants, a birthweight-for-gestation reference might underestimate the true prevalence of intrauterine growth restriction because preterm infants could be small at birth because of pathological effects, which led to the preterm birth, compared with babies who remain in utero.42 However, ultrasound-based fetal-weight estimation methods also have limitations. A standard proposed by WHO43 shifts the Hadlock distribution of fetal weights for every gestational age by a particular country's mean birthweight at 40 weeks, thus setting by default any population-based small-for-gestational-age prevalence close to 10%. This strategy only identifies the most growth-restricted infants in that particular population, rather than establishing the burden of suboptimum growth. Most limitations of available fetal growth references are being addressed in the WHO Intergrowth study, which is currently taking place in eight geographically diverse settings and aims to develop international growth standards to describe optimum fetal growth and newborn nutritional status (completion in 2014).44
Our analyses show important regional differences in babies born small for gestational age and the composition of low birthweight. In south Asia, rates of low birthweight are high and many (65%) low-birthweight births are attributable to term-SGA infants. However, in sub-Saharan Africa and Latin America and the Caribbean, just over 50% of low-birthweight babies are preterm. Furthermore, low birthweight might not fully capture the increased risk of babies born too soon or too small. The median birthweight of an infant born at 33 weeks of gestation is around 2500 g for the Alexander distribution;8 thus, many late preterm infants could weigh 2500 g or heavier. Two-thirds of term-SGA infants weigh 2500 g or more, although these babies are at lower risk of morbidity and mortality than their low-birthweight counterparts, particularly from non-communicable diseases in adulthood.
Estimates of intrauterine growth restriction were reported by de Onis and colleagues in 1998.4 These researchers estimated that 13·7 million infants (11% of births) in low-income and middle-income countries were born at term and with a low birthweight, an indicator that was a proxy for intrauterine growth restriction. By comparison, we estimated that a total of 10·6 million babies were born at term with low birthweight in countries of low and middle income in 2010. However, our estimation of small for gestational age also included two groups missing in the term and low-birthweight indicator: preterm-SGA infants who are at substantially higher risk of adverse outcomes;10 and babies born small for gestational age but weighing 2500 g or more. The estimates made by de Onis and colleagues were based on 1996 rates of low birthweight from WHO and on older data from 1960–96, which used inputs from 60 datasets in low-income and middle-income countries at a time when less attention was paid to metrics for gestational age. Recent findings show temporal changes in the distribution of small-for-gestational-age and preterm births in low-birthweight babies.45
Estimates of preterm, low-birthweight, and small-for-gestational-age rates are imperfect because of gaps and biases in data. The methods used to ascertain gestational age varied between studies and might affect estimation of gestational length. We included studies meeting a priori data-quality criteria for gestational age, and nine studies included ultrasound measures of gestational age. In several studies, last menstrual period was recorded prospectively during monthly pregnancy surveillance and, thus, this information was subject to less recall bias. We included information from both facility-based and community-based or population-based studies, and we attempted to assess biases. National data were available from Chile only.21 The WHO Global Survey was a facility-based survey, which could be biased depending on the nature of the facility, the number of facilities in an area, and the proportion of deliveries that took place in the home. We included a covariate to control for facility bias. In community-based studies, neonatal weight is measured after birth and, therefore, a high proportion of birthweight data can be missing for early neonatal deaths. We excluded datasets that had a substantial amount of missing birthweight data (>25%), and we did sensitivity analyses with imputation of missing birthweight data.10 The prevalence of term-SGA and preterm-SGA did not change substantially. However, data for birthweight might have been missing more frequently among preterm-SGA babies, because these infants are at a higher risk of mortality and they might have died before weighing. Thus, our projections could underestimate the prevalence of preterm-SGA. Furthermore, in view of the use of birthweight rather than an ultrasound growth reference, the prevalence of preterm-SGA could be underestimated, because growth restriction has a relatively higher frequency in babies who are born preterm versus those who remain in utero for the full gestation period. Data for maternal HIV status were limited; HIV infection can be a risk factor for babies born small for gestational age, although risk is not so clearly defined for preterm birth.46 Finally, most of our datasets did not include data on stillbirths, which are more likely to be associated with fetal growth restriction and preterm birth, and our estimates do not capture this burden.
The dearth and quality of data on both birthweight and gestational age in countries of low and middle income have been key barriers to quantification of the burden of small-for-gestational-age babies or intrauterine growth restriction (panel). More than half of infants in low-income and middle-income countries are never weighed at birth, particularly those born outside of facilities,1 and facility-based data are subject to selection biases. Inclusion of birthweight in household surveys (eg, demographic and health survey, multiple indicator cluster survey) since the 1990s has improved data availability, and methods to adjust data quality have been developed.1 Serial fetal ultrasonography is the gold standard for diagnosis of intrauterine growth restriction in high-resource settings, but small for gestational age at birth is the most commonly used indicator in countries of low and middle income. Data for gestational age are also troublesome. In low-income and middle-income countries, ultrasound is generally not available and last menstrual period is used to date most pregnancies, which can be affected by poor maternal recall, lactational amenorrhoea, variation in length of menstrual cycle, or injectable contraception. Last menstrual period has an estimated error of SD 3 weeks, clinical assessment SD 2 weeks, and ultrasound done before 20 weeks of gestation has an error of SD 7 days. Under-registration of very preterm births attributable to early death is also a problem.2
Panel. Research in context.
Systematic review
No systematic national estimates have been published of the burden of babies born small for gestational age and its co-occurrence with preterm birth. To identify birth cohorts with birthweight and gestational age data required for secondary analysis, we did a systematic literature review of Medline and WHO regional databases with the terms: “preterm birth”, “intrauterine/fetal growth restriction”, OR “small for gestational age”, AND “developing countries”. We identified 45 birth cohorts from low-income and middle-income countries with adequate data and investigators willing to join the CHERG SGA-Preterm Birth Working Group. After fitting the statistical model with these data, we observed a high correlation between low birthweight and prevalence of small-for-gestational-age births. To include more data in the model, we did an additional literature review to identify studies that reported low birthweight and prevalence of small-for-gestational-age births using the 1991 US national birthweight reference (Alexander, 1991).8 We searched Medline and Scopus to identify studies reporting either the prevalence of small-for-gestational-age and low-birthweight births or the prevalence of small-for-gestational-age babies using the Alexander reference, using prespecified inclusion criteria. Search terms included [“fetal growth restriction”, “intrauterine growth restriction”, OR “small for gestational age”] AND “low birthweight”, using MESH subject heading terms. Six reports were identified that reported prevalence of small for gestational age and low birthweight; however, none reported the prevalence of babies born at term and small for gestational age (term-SGA) or preterm and small for gestational age (preterm-SGA) and were therefore excluded. Secondary analyses and statistical modelling were done to estimate the prevalence of term-SGA for 138 countries of low and middle income for the year 2010. We also estimated the proportion of preterm-SGA using meta-analyses.
Interpretation
In the year 2010, 32·4 million (27%) small-for-gestational-age livebirths were estimated, of which 2·8 million babies (2% of births) were preterm-SGA. The prevalence of term-SGA ranged from 5·3% in east Asia to 41·5% in south Asia, and preterm-SGA ranged from 1·2% in north Africa to 3·0% in southeast Asia. Of the 18 million low-birthweight babies born every year, about 59% are because of growth restriction in term infants and 41% are attributable to prematurity. Previously, babies born at term and low birthweight were a proxy for intrauterine growth restriction; last estimates date from 1998, when about 13·7 million infants (11% of births) in countries of low and middle income were born at term and low birthweight, compared with our estimate of 10·6 million babies (9% of births) for the year 2010. However, the number of babies born at term and low birthweight does not fully capture the burden of growth restriction and misses infants born small for gestational age above the 2500 g cutoff in addition to those who are both preterm and small for gestational age. These babies might have increased risk of morbidity or mortality. Globally, a huge burden of fetal growth restriction exists, particularly concentrated in south Asia. Implementation of simple and cost-effective interventions that increase survival and reduce morbidity of these babies born too small is an urgent priority.
Our findings have important programmatic and research implications for newborn health and survival, particularly because 43% of under-5 deaths happen during the neonatal period. Evidence for the primary prevention of preterm birth and fetal growth restriction is limited. In an analysis modelling high coverage of five evidence-based interventions in countries with a high development index, little reduction was seen in preterm birth rates.47 However, the findings underline the importance of further research in settings of low-income and middle-income countries about birth spacing and treatment of maternal infections. Nutritional supplementation programmes (balanced protein–energy supplementation) for women during pregnancy can reduce the risk of small-for-gestational-age births by a third,48 although evidence of effectiveness at scale is scarce. Multiple micronutrient supplementation reduces the risk of babies born small for gestational age by 17%;49 however, the effect of supplementation varies across populations, with differing baseline rates of malnutrition and access to obstetric care.
By contrast, interventions that improve the care and survival of preterm and small-for-gestational-age infants have major potential for immediate effects and should be prioritised—eg, early feeding support (initiation of breastfeeding, alternative oral feeding methods), kangaroo mother care,50 early detection and treatment of neonatal infections,51 and neonatal resuscitation.52 These common interventions improve the management of small babies—whether due to preterm birth or intrauterine growth restriction—and have been proven to have great effect, or are even judged standard care, in high-income settings. Yet, these simple low-cost interventions do not reach those small babies in the settings of greatest need.
Moving beyond birthweight metrics alone and delineating preterm birth and intrauterine growth restriction are important for advancing a healthy start in life. In 2010 in countries of low and middle income, 32·4 million neonates, or one in every four babies, were classified as small for gestational age, closely linked to 13·7 million babies born too soon. Half of infants born small for gestational age were in south Asia, where one of two babies was born too small. To improve the epidemiology and adequately monitor the effect of interventions, systems are needed urgently to better capture and track pregnancy outcomes and to increase the quantity and quality of both birthweight and gestational age data. Effective low-technology interventions are available now to deliver care to these most vulnerable babies born too small or too soon.
This online publication has been corrected. The corrected version first appeared at thelancet.com/lancetgh on July 3, 2013
Acknowledgments
Acknowledgments
We thank additional members of the CHERG SGA-Preterm Birth working group: Subarna Khatry and Christentze Schmiegelow; Joanna Schellenberg for support; and Ramesh Adhikari, Fernando Barros, Christian Coles, Anthony Costello, Gary Darmstadt, Sheela Devi, Hermann Lanou, Steve LeClerq, Dharma Manandhar, Daniel Minja, R D Thulasiraj, Laeticia Celine Toe, Willy Urassa, Cesar G Victora, Keith West, and Nelly Zavaleta, who worked on the individual studies. Funding was provided by the Bill & Melinda Gates Foundation (810-2054), by a grant to the US fund for UNICEF to support the work of CHERG. Financial support for analysis was offered to investigators through a subcontract mechanism administered by the US fund for UNICEF. Funding sources of the individual studies are as follows: Bangladesh (2005)—United States Agency for International Development (USAID), Saving Newborn Lives program by Save the Children (US), Bill & Melinda Gates Foundation (BMGF); Brazil (1982)—International Development Research Center for Canada, WHO, UK Overseas Development Administration; Brazil (1993)—UN Development Fund for Women; Brazil (2004)—Wellcome Trust; Burkina Faso (2004)—Nutrition Third World, Belgian Ministry of Development; Burkina Faso (2006)—Flemish University Council, Nutrition Third World, Belgian Ministry of Development, Nutriset; India (2000)—Center for Human Nutrition (JHSPH), Office of Health and Nutrition (USAID), BMGF, Task Force Sight and Life; Kenya (1995)—USAID, Royal Netherlands Embassy, Netherlands Foundation for the Advancement of Tropical Research; Nepal (1999)—USAID, UNICEF Country Office (Kathmandu, Nepal), BMGF; Nepal (2003)—Wellcome Trust; Nepal (2004)—National Institutes of Health (NIH), BMGF, USAID, Proctor and Gamble; Pakistan (2003)—UNICEF, UN System Standing Committee on Nutrition; Peru (1995)—Office of Health and Nutrition (USAID); Philippines (1983)—NIH, Nestle's Coordinating Center for Nutritional Research, Wyeth International, Ford Foundation, US National Academy of Science, WHO, Carolina Population Center, USAID; South Africa (2001)—Wellcome Trust; South Africa (2004)—USAID, National Vaccine Program Office and CDC's Antimicrobial Resistance Working Group, BMGF; Tanzania (1998)—Wellcome Trust; Tanzania (2001)—National Institute of Child Health and Human Development; Tanzania (2008)—European Union Framework 7; Thailand (2001)—Thailand Research Fund, Health System Research Office, Ministry of Public Health, Thailand; and Uganda (2005)—Gates Malaria Partnership (BMGF).
Contributors
ACL was responsible for study design, data collection, the literature reviews, statistical modelling, data analysis, and wrote the report. JK was responsible for study design, data collection, interpretation of results, and helped write the report. HB did data analysis, and HB, SC, and JEL provided technical input on statistical modelling and helped write the report. NK did literature reviews, data collection, and helped write the report. JPV, AS, BAW, JN, JKN, HER, MFS, and AV helped analyse primary datasets and reviewed the report. LA, AHB, ZAB, LEC, PC, SEC, WF, RG, LH, SK, PK, JL, TM, MM, AM, LCM, M-LN, DO, DR, and JT contributed data to the analysis and reviewed the report. ME and REB provided important assistance with study design and reviewed the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.WHO. UNICEF . Low birthweight: country, regional and global estimates. World Health Organization; Geneva: 2004. [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52(suppl 1):S5–15. [PubMed] [Google Scholar]
- 5.WHO Expert Committee on Physical Status . Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. World Health Organization; Geneva: 1995. [PubMed] [Google Scholar]
- 6.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64:650–658. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 7.Smith G, Lees C. Disorders of fetal growth and assessment of fetal well-being. In: Edmonds DK, editor. Dewhurst textbook of obstetrics and gynaecology. 8th edn. John Wiley and Sons; Chichester: 2012. [Google Scholar]
- 8.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 9.Shah A, Faundes A, Machoki M. Methodological considerations in implementing the WHO Global Survey for Monitoring Maternal and Perinatal Health. Bull World Health Organ. 2008;86:126–131. doi: 10.2471/BLT.06.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz J, Lee AC, Kozuki N, the CHERG Small-for-Gestational-Age-Preterm Birth Working Group Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013 doi: 10.1016/S0140-6736(13)60993-9. published online June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fall CH, Fisher DJ, Osmond C, Margetts BM, for the Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(4 suppl):S533–S546. doi: 10.1177/15648265090304S408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Johnson HL, Cousens S, for the Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 13.Ndyomugyenyi R, Clarke SE, Hutchison CL, Hansen KS, Magnussen P. Efficacy of malaria prevention during pregnancy in an area of low and unstable transmission: an individually-randomised placebo-controlled trial using intermittent preventive treatment and insecticide-treated nets in the Kabale Highlands, southwestern Uganda. Trans R Soc Trop Med Hyg. 2011;105:607–616. doi: 10.1016/j.trstmh.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 15.ChildInfo Inter-agency Group for Child Mortality Estimation (IGME) July, 2012. http://www.childinfo.org/mortality_igme.html (accessed Oct 18, 2012).
- 16.UNICEF . State of the world's children 2012. UNICEF; New York: 2012. [Google Scholar]
- 17.Schmiegelow C, Minja D, Oesterholt M. Factors associated with and causes of perinatal mortality in northeastern Tanzania. Acta Obstet Gynecol Scand. 2012;91:1061–1068. doi: 10.1111/j.1600-0412.2012.01478.x. [DOI] [PubMed] [Google Scholar]
- 18.Santos IS, Barros AJ, Matijasevich A, Domingues MR, Barros FC, Victora CG. Cohort profile: the 2004 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2011;40:1461–1468. doi: 10.1093/ije/dyq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victora CG, Barros FC. Cohort profile: the 1982 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2006;35:237–242. doi: 10.1093/ije/dyi290. [DOI] [PubMed] [Google Scholar]
- 20.Victora CG, Hallal PC, Araujo CL, Menezes AM, Wells JC, Barros FC. Cohort profile: the 1993 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2008;37:704–709. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Merialdi M, Lincetto O. Reduction in neonatal mortality in Chile between 1990 and 2000. Pediatrics. 2006;117:e949–e954. doi: 10.1542/peds.2005-2354. [DOI] [PubMed] [Google Scholar]
- 22.Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. J Nutr. 1999;129:1563–1568. doi: 10.1093/jn/129.8.1563. [DOI] [PubMed] [Google Scholar]
- 23.Fawzi WW, Msamanga GI, Urassa W. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 24.Bland R, Coovadia H, Coutsoudis A, Rollins N, Newell M. Cohort profile: management of the Africa center vertical transmission study. Int J Epidemiol. 2010;39:351–360. doi: 10.1093/ije/dyp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huybregts L, Roberfroid D, Lanou H. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2009;90:1593–1600. doi: 10.3945/ajcn.2009.28253. [DOI] [PubMed] [Google Scholar]
- 26.Roberfroid D, Huybregts L, Lanou H. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–1340. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 27.Isaranurug S, Mo-suwan L, Choprapawon C. A population-based cohort study of effect of maternal risk factors on low birthweight in Thailand. J Med Assoc Thai. 2007;90:2559–2564. [PubMed] [Google Scholar]
- 28.Adair LS. Low birth weight and intrauterine growth retardation in Filipino infants. Pediatrics. 1989;84:613–622. [PubMed] [Google Scholar]
- 29.Bhutta ZA, Rizvi A, Raza F. A comparative evaluation of multiple micronutrient and iron-folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food Nutr Bull. 2009;30(4 suppl):S496–S505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 30.Christian P, Klemm R, Shamim AA. Effects of vitamin A and beta-carotene supplementation on birth size and length of gestation in rural Bangladesh: a cluster-randomized trial. Am J Clin Nutr. 2013;97:188–194. doi: 10.3945/ajcn.112.042275. [DOI] [PubMed] [Google Scholar]
- 31.Baqui AH, El-Arifeen S, Darmstadt GL, for the Projahnmo Study Group Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–1944. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 32.Rahmathullah L, Tielsch JM, Thulasiraj RD. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christian P, West KP, Khatry SK. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–1202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 34.Osrin D, Vaidya A, Shrestha Y. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 35.Tielsch JM, Darmstadt GL, Mullany LC. Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–e340. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ter Kuile FO, Terlouw DJ, Kariuki SK. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4 suppl):68–77. [PubMed] [Google Scholar]
- 37.March of Dimes. Partnership of Maternal. Newborn and Child Health. Save the Children. WHO Born too soon: the global action report on preterm birth—care for the preterm baby. May 2, 2012. http://www.who.int/pmnch/media/news/2012/borntoosoon_chapter5.pdf (accessed May 14, 2013).
- 38.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–632. [PubMed] [Google Scholar]
- 40.Boersma ER, Mbise RL. Intrauterine growth of live-born Tanzanian infants. Trop Geogr Med. 1979;31:7–19. [PubMed] [Google Scholar]
- 41.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–449. [PubMed] [Google Scholar]
- 42.Ehrenkranz RA. Estimated fetal weights versus birth weights: should the reference intrauterine growth curves based on birth weights be retired? Arch Dis Child Fetal Neonatal Ed. 2007;92:F161–F162. doi: 10.1136/adc.2006.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikolajczyk RT, Zhang J, Betran AP. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377:1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 44.The International Fetal and Newborn Growth Consortium About study. http://www.intergrowth21.org.uk/about.aspx?lang=1 (accessed Oct 18, 2012).
- 45.Barros FC, Barros AJ, Villar J, Matijasevich A, Domingues MR, Victora CG. How many low birthweight babies in low- and middle-income countries are preterm? Rev Saude Publica. 2011;45:607–616. doi: 10.1590/s0034-89102011005000019. [DOI] [PubMed] [Google Scholar]
- 46.Ndirangu JNM, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Hum Reprod. 2012;27:1846–1856. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang HH, Larson J, Blencowe H, on behalf of the Born Too Soon preterm prevention analysis group Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imdad A, Bhutta ZA. Maternal nutrition and birth outcomes: effect of balanced protein-energy supplementation. Paediatr Perinat Epidemiol. 2012;26(suppl 1):178–190. doi: 10.1111/j.1365-3016.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 49.Ramakrishnan U, Grant FK, Goldenberg T, Bui V, Imdad A, Bhutta ZA. Effect of multiple micronutrient supplementation on pregnancy and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(suppl 1):153–167. doi: 10.1111/j.1365-3016.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- 50.Lawn JE, Mwansa-Kambafwile J, Barros FC, Horta BL, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to pre-term birth complications. Int J Epidemiol. 2011;40:525–528. doi: 10.1093/ije/dyq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bang AT, Baitule SB, Reddy HM, Deshmukh MD, Bang RA. Low birth weight and preterm neonates: can they be managed at home by mother and a trained village health worker? J Perinatol. 2005;25(suppl 1):S72–S81. doi: 10.1038/sj.jp.7211276. [DOI] [PubMed] [Google Scholar]
- 52.Lee ACC, Cousens S, Wall SN. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11(suppl 3):S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.