Abstract
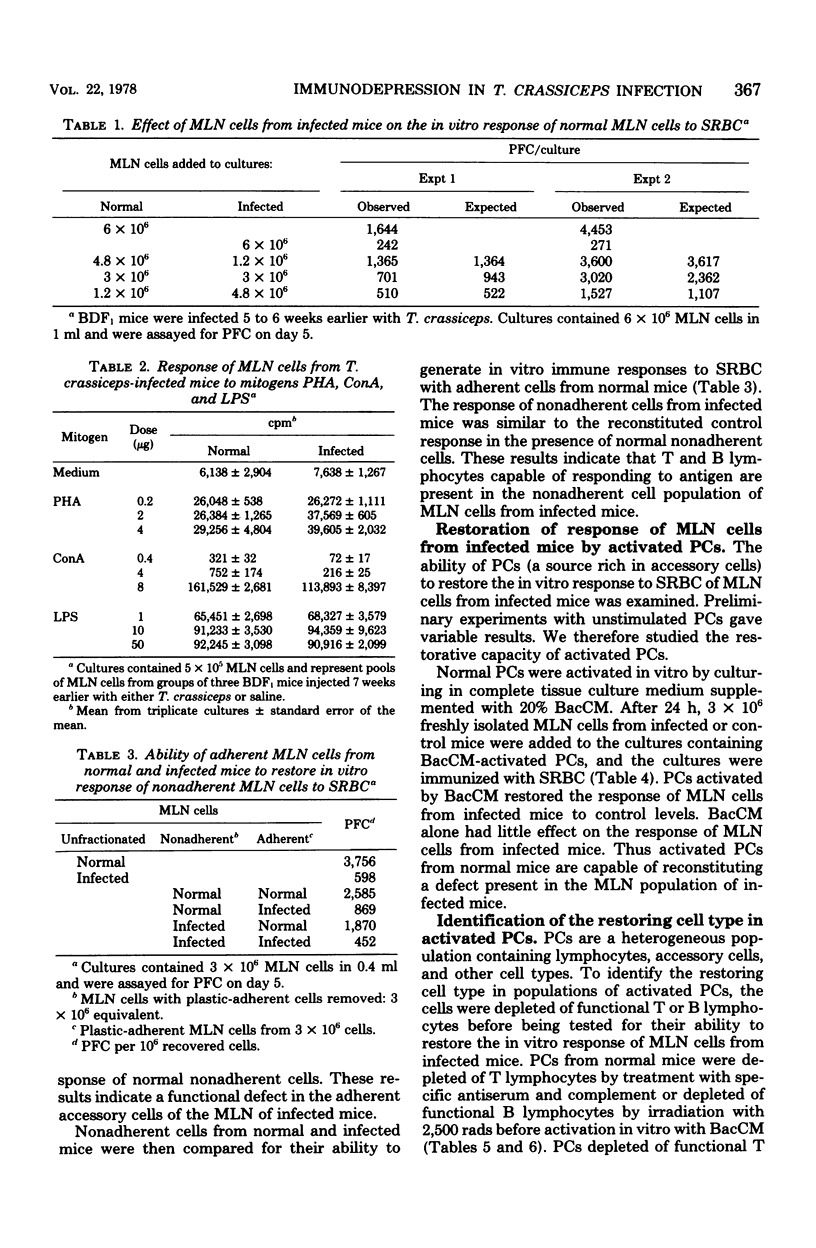
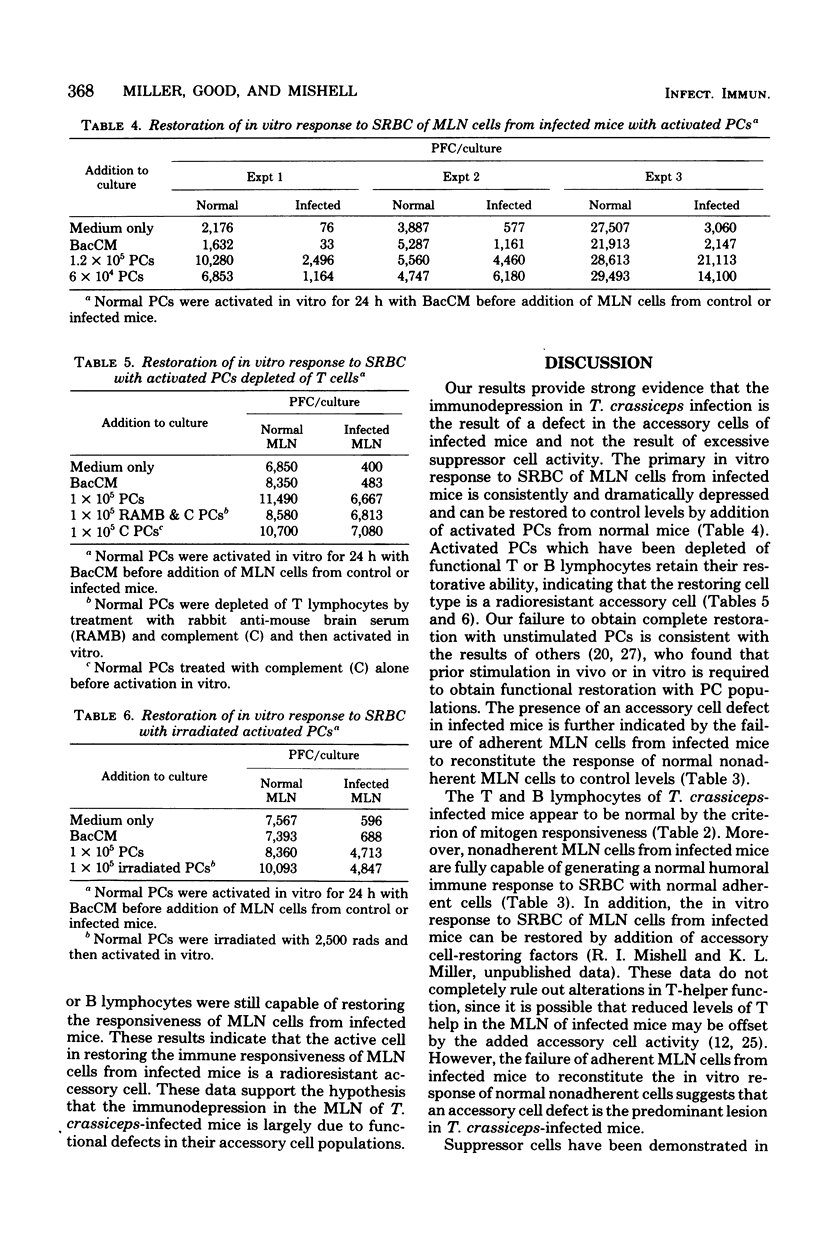
The in vitro response to sheep erythrocytes of mesenteric lymph node cells from mice infected with the larval cestode Taenia crassiceps is significantly depressed and can be restored to control levels by addition of activated peritoneal cells depleted of functional T or B lymphocytes. Adherent mesenteric lymph node cells from infected mice are unable to reconstitute the in vitro response to sheep erythrocytes of normal nonadherent cells. The responses of mesenteric lymph node cells from infected mice to the T-lymphocyte mitogens concanavalin A and phytohemagglutinin and the B-lymphocyte mitogen lipopolysaccharide are normal. Mesenteric lymph node cells from infected mice do not suppress the in vitro response to sheep erythrocytes of normal mesenteric lymph node cells. These results suggest that the immunodepression in T. crassiceps-infected mice is primarily the result of alterations in functional accessory cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown I. N., Watson S. R., Sljivić V. S. Antibody response in vitro of spleen cells from Plasmodium yoelii-infected mice. Infect Immun. 1977 May;16(2):456–460. doi: 10.1128/iai.16.2.456-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Goodgame R. W. Immune responses during human schistosomiasis mansoni. IV. Induction of suppressor cell activity by schistosome antigen preparations and concanavalin A. J Immunol. 1978 Apr;120(4):1225–1232. [PubMed] [Google Scholar]
- Coulis P. A., Lewert R. M., Fitch F. W. [Splenic suppressor cells and cell-mediated cytotoxicity in murine schistosomiasis]. J Immunol. 1978 Jan;120(1):58–60. [PubMed] [Google Scholar]
- Crandall C. A., Crandall R. B. Ascaris suum: immunosuppression in mice during acute infection. Exp Parasitol. 1976 Dec;40(3):363–372. doi: 10.1016/0014-4894(76)90102-8. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Faubert G., Tanner C. E. Trichinella spiralis: inhibition of sheep hemagglutinins in mice. Exp Parasitol. 1971 Aug;30(1):120–123. doi: 10.1016/0014-4894(71)90077-4. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Good A. H., Miller K. L. Depression of the immune response to sheep erythrocytes in mice infected with Taenia crassiceps larvae. Infect Immun. 1976 Aug;14(2):449–456. doi: 10.1128/iai.14.2.449-456.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R., Kontiainen S., Mitchison N. A., Tigelaar R. E. Antigen--antibody complexes as blocking factors on the T lymphocyte surface. Soc Gen Physiol Ser. 1974;29:143–154. [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K., Galanos C., Koenig S., Oettgen H. F. B-cell activation by lipopolysaccharide. Distinct pathways for induction of mitosis and antibody production. J Exp Med. 1977 Dec 1;146(6):1640–1647. doi: 10.1084/jem.146.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Crandall C. A., Crandall R. B. T-dependent suppression of the primary antibody response to sheep erythrocytes in mice infected with Trichinella spiralis. Cell Immunol. 1976 Nov;27(1):102–110. doi: 10.1016/0008-8749(76)90158-1. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Lucas A., Mishell B. B. The role of activated accessory cells in preventing immunosuppression by hydrocortisone. J Immunol. 1977 Jul;119(1):118–122. [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Studies on immune responses to larval cestodes in mice: a simple mechanism of non-specific immunosuppression in Mesocestoides corti-infected mice. Aust J Exp Biol Med Sci. 1977 Oct;55(5):615–622. doi: 10.1038/icb.1977.59. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Rowland E. C., Kuhn R. E. Suppression of cellular responses in mice during Trypanosoma cruzi infections. Infect Immun. 1978 May;20(2):393–397. doi: 10.1128/iai.20.2.393-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W. Mechanism of activation of the bone marrow-derived lymphocyte. 3. A distinction between a macrophage-produced triggering signal and the amplifying effect on triggered B lymphocytes of allogeneic interactions. J Exp Med. 1973 Dec 1;138(6):1466–1480. doi: 10.1084/jem.138.6.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigi S. M., Capwell R. R., Grabstein K. H., Mishell R. I. Sera and the in vitro induction of immune responses. III. Adjuvant obtained from gliding bacteria with properties distinct from enteric bacterial lipopolysaccharide. J Immunol. 1977 Aug;119(2):679–684. [PubMed] [Google Scholar]
- Specter S. C., Bendinelli M., Ceglowski W. S., Friedman H. Macrophage-induced reversal of immunosuppression by leukemia viruses. Fed Proc. 1978 Jan;37(1):97–101. [PubMed] [Google Scholar]
- Strickland G. T., Petitt L. E., Voller A. Immunodepression in mice infected with Toxoplasma gondii. Am J Trop Med Hyg. 1973 Jul;22(4):452–455. doi: 10.4269/ajtmh.1973.22.452. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Waki S., Takada S., Suzuki M. Plasmodium berghei: suppressed response of antibody-forming cells in infected mice. Exp Parasitol. 1977 Oct;43(1):143–152. doi: 10.1016/0014-4894(77)90017-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- White R. G. The adjuvant effect of microbial products on the immune response. Annu Rev Microbiol. 1976;30:579–600. doi: 10.1146/annurev.mi.30.100176.003051. [DOI] [PubMed] [Google Scholar]



