Structured Abstract
Purpose
Little is known about pregnancy attempts among female young cancer survivors (YCS). We sought to determine fertility preservation (FP), demographic, cancer and reproductive characteristics associated with pregnancy attempts after cancer.
Methods
We recruited 251 female YCS (ages 18-44) to complete a survey on reproductive health outcomes. We used log-binomial regression models to estimate relative risks (RR) for characteristics associated with pregnancy attempts.
Results
For the entire cohort, median time since cancer diagnosis was 2.4 years (interquartile range 4.0). Fifty-two YCS (21%) attempted pregnancy after cancer diagnosis. In unadjusted analyses, lack of FP therapy prior to cancer treatment, older age, partnered relationship, higher income, history of stem cell or bone marrow transplant, and longer duration of survivorship were significantly associated with pregnancy attempts. In multivariable analyses, YCS who did not undergo FP therapy were more than twice as likely to attempt pregnancy as those who did undergo FP therapy (RR 2.4, 95%CI 1.3, 4.3). Partnered status (RR 7.1, 95%CI 2.5, 20.2) and >2 years since cancer diagnosis (RR 2.3, 95%CI 1.3, 4.1) were also significantly associated with attempts.
Conclusions
In YCS, milestones including partnered relationships and longer duration of cancer survivorship are important to attempting pregnancy. A novel, inverse association between FP therapy and pregnancy attempts warrants further study.
Implications for Cancer Survivors
Pregnancy attempts after cancer were more likely after attaining both social and cancer-related milestones. As these milestones require time, YCS should be made aware of their potential for concomitant, premature loss of fertility in order to preserve their range of fertility options.
Keywords: fertility preservation therapy, cancer, pregnancy attempts, young adults, cancer survivorship
Introduction
Over 380,000 female adolescent and young adult cancer survivors live in the United States [1]. Because of improved long-term survival, young adult-aged cancer survivors (YCS) expect to raise their own families and prefer biologic children over adoption and third party reproduction [2,3]. The decision to attempt pregnancy for any young adult is complex. Young adults are often at a pivotal stage of their life development, in terms of pursuing educational and career goals, financial stability, and committed romantic relationships [4–7]. With prior cancer and cancer treatment, YCS face not only these psychosocial factors, but also uncertainty on cancer status, higher risks of chronic medical conditions, potential for impaired fecundity and premature ovarian aging, all of which may affect the decision to attempt pregnancy [8–15].
Little is known about what influences the decision to attempt pregnancy after cancer. Oncology, reproductive medicine and pediatric professional societies recommend that health care providers discuss with reproductive-aged patients the potential threats that cancer and cancer treatments pose to future fertility, and refer for fertility preservation (FP) counseling and treatments as indicated [16–18]. Whether FP counseling and therapy impacts pregnancy attempts after cancer is not known. Because many female YCS face a narrowed reproductive window, it is necessary to identify factors that drive the decision to attempt pregnancy to understand how to best support their reproductive goals. Therefore, the objective of this study was to examine the association between pregnancy attempts in YCS and prior fertility preservation, demographic, cancer history, and reproductive characteristics.
Materials and Methods
Study Population
Participants were female YCS who were recruited to the Fertility Information Research Study (FIRST), an ongoing prospective cohort study of reproductive health outcomes after cancer. Participants were recruited through social media outreach by cancer advocacy groups and six university-based FP programs [19]. Eligible individuals were consented over the telephone and completed the study questionnaire via either telephone interview or the Internet. To be eligible for FIRST, participants must be female, aged 18 to 44 at study enrollment, and have a personal history of cancer or cancer treatment. Participants represent variable durations since cancer diagnosis. Participants who underwent a hysterectomy and/or bilateral oophorectomy were excluded from the current analysis. This study was approved by the institutional review board at the University of California, San Diego.
Questionnaire data
Demographics, cancer and treatment characteristics, medical conditions, and reproductive health questions were included in the study questionnaire completed by participants at the time of recruitment. Demographic data included: age at study enrollment, race/ethnicity, relationship status, education, and annual household income. Additionally, questions were asked to assess self-reported health habits (e.g., smoking status), body mass index (BMI) and current medical conditions (e.g., asthma, diabetes, depression). Participants reported information about cancer type and stage, cancer treatments (e.g., radiation, surgery, chemotherapy), cancer recurrence, and year of cancer diagnosis. Regarding reproductive health, participants reported on history of hysterectomy and/or oophorectomy, menstrual cycle pattern, pregnancy, and infertility using questions derived from the National Survey of Family Growth [20], the Penn Ovarian Aging Study [21] and the Olsen time to pregnancy questionnaire [22].
Participants completed questions assessing their experience with FP prior to their cancer treatment. To assess FP referral, women were asked, “Before your cancer treatment began, were you and/or your family ever referred to a fertility specialist to talk about fertility preservation?” To assess use of FP therapy, women answered the following questions: 1) “Have you ever used any therapies or interventions to preserve your fertility?” and 2) “What therapies or medical interventions have you used to preserve your fertility?” The answer choices for the latter question were embryo banking, egg banking, ovarian tissue banking, ovarian suppression with medication, ovarian shielding during radiation, ovarian transposition, conservative gynecologic surgery, or other.
Pregnancy Attempt after Cancer
Women were classified as having attempted pregnancy after cancer based on a series of questions ascertaining pregnancy, attempted pregnancy, and history of infertility that occurred after the date of cancer diagnosis. First, participants were asked, “Are you trying to become pregnant now?” Those who answered yes were classified as attempting pregnancy. Next, participants were asked, “Have you ever tried to become pregnant for at least a year without becoming pregnant?” Participants who answered yes were asked to provide the date when they first started trying to get pregnant. Those participants who provided a date after their cancer diagnosis date were classified as attempting pregnancy after cancer. Lastly, women were asked, “Have you ever been pregnant?” Of those who responded in the affirmative, the date of the end of their pregnancy, length of gestation and time to pregnancy were ascertained, from which the start of their pregnancy attempts after cancer diagnosis could be calculated. Women with a pregnancy attempt start date after their cancer diagnosis date were classified as having attempted pregnancy after cancer.
Statistical Methods
Descriptive statistics were calculated as frequencies and percentages for categorical data and median and interquartile ranges (IQR) for continuous data. The primary exposure of interest was FP therapy prior to cancer treatment. Participants who answered yes to using any FP therapy were classified as having undergone fertility preservation. The primary outcome was attempting pregnancy after cancer diagnosis.
In order to identify factors associated with pregnancy attempts after cancer diagnosis, bivariable analyses were conducted. Factors considered in these analyses included participant demographics (e.g., age at study enrollment, race, education, income, relationship status), cancer and treatment characteristics (e.g., cancer type, stage, time since cancer diagnosis, treatment type), and fertility preservation characteristics (e.g., FP referral, FP therapy). Analysis was performed using Fisher's Exact, Chi-square or Student's t-test, as appropriate. Time since cancer diagnosis was dichotomized into ≤2 years and >2 years based on the common recommendation to defer pregnancy for at least 2 years post-cancer diagnosis. Comorbid medical conditions were categorized as 0 conditions versus 1 or more conditions, and cancer treatments received were dichotomized as yes versus no. For example, receipt of any kind of chemotherapy was classified as yes or no.
Log-binomial regression models were used to estimate relative risks (RR) for characteristics associated with pregnancy attempts after cancer diagnosis. FP therapy (the primary exposure of interest) along with variables associated with pregnancy attempts at p<0.05 in the bivariable analyses were included in the final adjusted regression model. Significance for all analyses was set at p<0.05. All analyses were conducted using SAS statistical software v9.3 (Cary, NC).
Results
A total of 295 participants completed the study questionnaire between May 2011 and February 2013. For the current analysis, 251 participants were included, while 26 participants were excluded due to prior hysterectomy and/or bilateral oophorectomy, and 18 participants were excluded due to missing data on the main study outcome (pregnancy attempt after cancer diagnosis).
Table 1 depicts baseline characteristics of participants. The mean age at study enrollment (standard deviation [SD]) of the participants was 31.3 (5.6) years, and the median time since cancer diagnosis (interquartile range) was 2.4 (4.0) years. The majority of participants were white (79%), college graduates (86%), in a partnered relationship (57%), and endorsed the desire to have a baby in the future (88%). The two most common cancer types were breast cancer (32%) and lymphoma (28%). Approximately 81% of participants received chemotherapy, and 51% received radiation therapy. For FP characteristics, 45% of participants received a referral to a fertility specialist for a consultation, and 35% underwent FP therapy prior to their cancer treatment. Specifically, 24% underwent embryo or oocyte cryopreservation.
Table 1. Participant characteristics by attempting pregnancy after cancer in a cohort of female adolescent and young adult survivors (N=251).
| Participant Characteristics | Overall | Pregnancy Attempt N=52 | No Pregnancy Attempt N=199 | P valuea |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Demographics | ||||
| Age at Study Enrollment, years | <0.01 | |||
| Mean [SD] | 31.3 [5.6] | 33.8 [4.7] | 30.6 [5.6] | |
| 19 to 25 | 47 (18.8) | 3 (5.8) | 44 (22.2) | |
| 26 to 31 | 86 (34.4) | 14 (26.9) | 72 (36.4) | |
| 32 to 37 | 85 (34.0) | 25 (48.1) | 60 (30.3) | |
| 38 to 44 | 32 (12.8) | 10 (19.2) | 22 (11.1) | |
| Race | 0.63 | |||
| White | 198 (79.2) | 39 (75.0) | 159 (80.3) | |
| Black | 8 (3.2) | 1 (1.9) | 7 (3.5) | |
| Asian | 13 (5.2) | 3 (5.8) | 10 (5.1) | |
| Other | 31 (12.4) | 9 (17.3) | 22 (11.1) | |
| Body Mass Index (kg/m2) | 0.59 | |||
| < 25 | 146 (58.2) | 27 (51.9) | 119 (59.8) | |
| 25 – 29.9 | 58 (23.1) | 14 (26.9) | 44 (22.1) | |
| ≥ 30 | 47 (18.7) | 11 (21.2) | 36 (18.1) | |
| Education | 0.82 | |||
| College graduate | 214 (86.3) | 46 (88.5) | 168 (85.7) | |
| Did not graduate from college | 34 (13.7) | 6 (11.5) | 28 (14.3) | |
| Income | <0.01 | |||
| ≤ $50,000 | 80 (31.9) | 6 (11.5) | 74 (37.2) | |
| > $50,000 | 120 (47.8) | 37 (71.2) | 83 (41.7) | |
| Declined to answer | 51 (20.3) | 9 (17.3) | 42 (21.1) | |
| Current Smoker | 6 (2.4) | 2 (3.9) | 4 (2.0) | 0.61 |
| Comorbid Medical Conditionsb | 1.00 | |||
| 0 | 89 (35.5) | 18 (34.6) | 71 (35.7) | |
| 1 or more | 162 (64.5) | 34 (65.4) | 128 (64.3) | |
| Reproductive Characteristics | ||||
| Relationship Status | <0.01 | |||
| Partnered | 143 (57.0) | 48 (92.3) | 95 (47.7) | |
| Not partnered | 108 (43.0) | 4 (7.7) | 104 (52.3) | |
| Live Birth Before Cancer Diagnosis | 41 (16.3) | 12 (23.1) | 29 (14.6) | 0.15 |
| Desire to Have a Baby in the Future | 220 (87.6) | 43 (91.5) | 177 (100.0) | <0.01 |
| Cancer & Treatment Characteristics | ||||
| Cancer Diagnosis | 0.21 | |||
| Breast | 81 (32.3) | 17 (32.7) | 64 (32.2) | |
| Lymphoma | 69 (27.5) | 12 (23.1) | 57 (28.7) | |
| Blood/Leukemia | 19 (7.6) | 4 (7.7) | 15 (7.5) | |
| Thyroid | 14 (5.5) | 7 (13.5) | 7 (3.5) | |
| Gynecologic (cervix/uterus/ovary) | 13 (5.2) | 2 (3.8) | 11 (5.5) | |
| Other | 55 (21.9) | 10 (19.2) | 45 (22.6) | |
| Cancer Stage | 0.16 | |||
| I | 53 (21.5) | 11 (21.6) | 42 (21.5) | |
| II | 76 (30.9) | 17 (33.3) | 59 (30.3) | |
| III | 45 (18.3) | 6 (11.7) | 39 (20.0) | |
| IV | 18 (7.3) | 1 (2.0) | 17 (8.7) | |
| Unknown | 54 (22.0) | 16 (31.4) | 38 (19.5) | |
| Time Since Cancer Diagnosis | <0.01 | |||
| Median [IQR], years | 2.4 [4.0] | 5.0 [7.1] | 2.1 [3.3] | |
| ≤ 2 years | 108 (43.0) | 11 (21.2) | 97 (48.7) | |
| > 2 years | 143 (57.0) | 41 (78.8) | 102 (51.3) | |
| Surgery | 151 (60.2) | 35 (67.3) | 116 (58.3) | 0.27 |
| Chemotherapy | 204 (81.3) | 38 (73.1) | 166 (83.4) | 0.11 |
| Radiation Therapy | 127 (50.6) | 28 (53.9) | 99 (49.8) | 0.64 |
| Endocrine Therapy | 48 (19.1) | 11 (21.2) | 37 (18.6) | 0.69 |
| Bone Marrow or Stem Cell Transplant | 15 (6.0) | 0 (0.0) | 15 (7.5) | 0.05 |
Note: Due to missing data, some variables do not add up to 251.
Fisher's exact tests were used for categorical variables, and Student's t-tests were performed for continuous variables.
Comorbid medical conditions included asthma/lung disease, high blood pressure, diabetes/high blood sugar, being overweight (obesity), overactive/underactive thyroid, depression/bipolar disorder, eating disorder, rheumatologic diseases, Crohn's disease/ulcerative colitis, seizures/neurologic disorders, TIA/stroke.
Fifty-two YCS (21%) attempted pregnancy after cancer diagnosis. Among these YCS, 7 women (13%) had their first pregnancy attempt < 1 year, 11 women (21%) at 1-2 years, 11 women (21%) at 2-3 years, 6 women (12%) at 3-4 years, and 17 women (33%) > 4 years after cancer diagnosis. Twenty-seven women conceived after their first pregnancy attempt, of which 17 achieved live births. Of the remaining 10 pregnancies, there were 5 spontaneous abortions, 2 elective terminations, 1 tubal pregnancy, and 2 ongoing pregnancies. Twenty-three of the 52 YCS are currently continuing to attempt pregnancy. Two women were diagnosed with infertility and are no longer attempting pregnancy.
In unadjusted analyses, older age, partnered relationship status, income > $50K, and longer duration of cancer survivorship were significantly associated with pregnancy attempts (Table 1). None of the 15 participants who underwent prior stem cell or bone marrow transplants attempted pregnancy (p=0.05). Cancer diagnosis, stage and treatment, age at cancer diagnosis as well as comorbid medical conditions were not associated with pregnancy attempts.
Among fertility preservation variables, referral to FP counseling prior to cancer treatment was not associated with pregnancy attempts after cancer treatment (p=0.35) (Figure 1). However, participants who did not undergo FP therapy were more likely to attempt pregnancy compared to participants who had undergone FP therapy prior to cancer treatment (26% vs. 11%, p=0.008). Similarly, participants who did not undergo embryo or oocyte cryopreservation were more likely to attempt pregnancy compared to participants who banked embryos or oocytes (25% vs. 8%, p=0.006). Compared to participants who underwent FP therapy, those who did not undergo FP therapy were more likely to self-report as white or Asian than black (p=0.01), were more likely to have had a live birth before cancer diagnosis (p=0.03), and were more likely to undergo radiation therapy than not undergo radiation therapy (p=0.03). While YCS with thyroid cancer were less likely to undergo FP therapy (p=0.005), FP therapy was not associated with other cancer types. Age at diagnosis, additional demographic and reproductive characteristics, cancer stage, and receipt of cancer treatment other than radiation therapy (i.e., surgery, chemotherapy, endocrine therapy and bone marrow or stem cell transplant) were not associated with FP therapy (data not shown).
Fig. 1. Proportions of participants attempting pregnancy by fertility preservation (FP) characteristics (N=251).
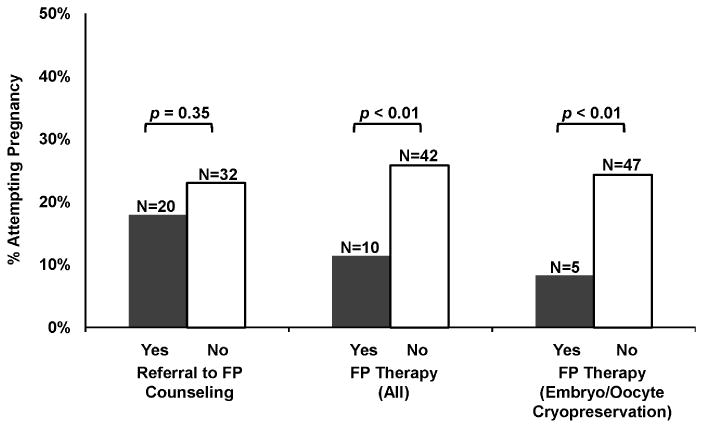
Estimates from multivariable modeling of pregnancy attempts after cancer are shown in Table 2. In a model adjusting for age at study enrollment, relationship status, income, and survivorship duration, YCS who did not undergo FP therapy were more than twice as likely to attempt pregnancy as women who underwent FP therapy [RR 2.4, 95% Confidence Interval (CI) 1.3, 4.3]. Women who were in a partnered relationship status were seven times more likely to attempt pregnancy than those who were not partnered (RR 7.1, 95%CI 2.5, 20.2). Women who were at least 2 years post-cancer diagnosis were twice as likely to attempt pregnancy compared to women within 2 years of their cancer diagnosis (RR 2.3, 95%CI 1.3, 4.1). In this model, age and income were no longer significantly associated with pregnancy attempts. Further, when restricting the type of FP therapy to embryo and/or oocyte cryopreservation, women who did not undergo these FP procedures were 3 times more likely to attempt pregnancy than women who underwent embryo/oocyte cryopreservation (RR 3.0, 95% CI 1.3, 6.8).
Table 2. Participant characteristics associated with attempting pregnancy after cancer in a cohort of female adolescent and young adult survivors.
| Characteristic | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P value | RR | (95% CI) | P value | |
| Non-use of Fertility Preservation (FP) Therapy (vs. FP Use) | 2.27 | (1.20 – 4.30) | 0.01 | 2.38 | (1.31 – 4.32) | <0.01 |
| Partnered (vs. Not Partnered) | 9.06 | (3.37 – 24.36) | <0.01 | 7.08 | (2.48 – 20.22) | <0.01 |
| > 2 Years Since Cancer Diagnosis (vs. ≤ 2) | 2.82 | (1.52 – 5.22) | <0.01 | 2.33 | (1.31 – 4.13) | <0.01 |
| Age ≥ 32 years (vs. <32) | 2.34 | (1.39 – 3.95) | <0.01 | 1.30 | (0.79 – 2.15) | 0.30 |
| Income > $50,000 (vs. ≤ $50,000 or Decline to Answer) | 2.69 | (1.56 – 4.66) | <0.01 | 1.53 | (0.94 – 2.49) | 0.09 |
Note: The final log-binomial regression model was adjusted for all variables shown in the table.
Discussion
In this contemporary cohort of young female cancer survivors, partnered relationships and longer duration of cancer survivorship were significantly associated with pregnancy attempts. Conversely, young women who underwent fertility preservation therapy prior to cancer treatment were less likely to attempt pregnancy. The decision to attempt pregnancy after cancer appears to involve achieving both social and cancer-related milestones. As these milestones require time, YCS should be made aware of their potential for concomitant, premature loss of fertility in order to preserve their range of fertility options.
The finding that YCS who underwent FP therapy were less likely to attempt pregnancy after cancer is novel and may have important clinical implications. While further research is needed to explore why those who underwent FP therapy were less likely attempt pregnancy after cancer, it is possible that YCS who underwent FP therapy feel that they have preserved their fertility potential and hence defer childbearing to pursue other life goals, such as education, career, and financial stability. This would be of concern if YCS have misperceptions related to FP therapy and the success of assisted reproduction via embryo or oocyte freezing. Among cancer survivors, Balthazar and colleagues (2012) reported that FP knowledge after a single FP counseling visit was poor, with the median knowledge score about FP equal to 6 out of 13 possible points [23]. Since the average age-dependent percentages of thawed embryo transfer cycles resulting in live births range from 17% to 39% in the U.S. [24], YCS need to be adequately educated about their future reproductive potential derived from FP therapy.
Young adult cancer survivors and healthy young adults have some similarities and differences regarding timing of childbearing. We found that YCS who were in a partnered relationship were significantly more likely to attempt pregnancy after cancer, which is consistent with data from healthy women [25,26] and a smaller cohort of YCS [27]. In addition to a partnered relationship, studies have shown that in young healthy women, education, financial stability and age have been shown to be highly influential on the timing of childbearing [28–32]. However among YCS in this study, age, education, and income were not significant factors influencing pregnancy attempts in the adjusted model. Since YCS experience considerable developmental and life course interruptions as a result of their cancer experience, it is possible that these milestones are less important to YCS than to healthy women [14,33,34]. Alternatively, it is also possible that the relative homogeneity in age, education and income of this cohort limited our power to detect independent associations with our outcome of interest.
We found that longer duration of cancer survivorship was significantly associated with attempting pregnancy. Women who were at least 2 years post-cancer diagnosis were more likely to have attempted pregnancy than those diagnosed more recently. This finding may be due to the common physician recommendation for cancer patients to wait at least 2 years post-diagnosis before attempting pregnancy. It should be noted that this recommendation is generally considered for breast cancer patients with estrogen-sensitive tumors for several reasons: 1) higher risk of recurrence within 2 years after the initial diagnosis [35,36] and 2) benefit of endocrine therapy on survival outcomes [37,38]. However, Azim and colleagues (2013) found no difference in disease-free survival among breast cancer patients who became pregnant within 2 years of diagnosis and those who became pregnant after 2 years [39]. Additionally, cohort studies generally show that pregnancy after breast cancer is not associated with adverse cancer outcomes [39–42]. In a meta-analysis, investigators found a 41% reduced risk of death in women who became pregnant after breast cancer compared to those who did not [40]. These findings can inform decisions on attempting pregnancy, particularly after breast cancer. Thus, updated knowledge about the risks associated with pregnancy after breast cancer should be communicated to YCS and their health care providers.
Among remaining cancer and treatment characteristics, only history of bone marrow or stem cell transplant was associated with a lower rate of pregnancy attempt. Because conditioning treatments that precede transplant confer severe gonadotoxicity, it is possible that these YCS did not attempt pregnancy because they experienced ovarian failure. Indeed, 5/15 (33.3%) reported no periods over the prior 12 months, while 9/15 (60.0%) reported being on birth control pills or menopausal hormone therapy. Although these numbers were very low, this is an interesting finding that warrants further research. We anticipated a healthy survivor bias, i.e. more pregnancy attempts in women with better prognosis. This was not observed, possibly because we recruited an ambulatory population with few participants with metastatic disease (n=18) and significant co-morbidities. It is likely that the study was underpowered to detect an association between health status and pregnancy attempts.
Modest rates of fertility preservation consultation and treatments were reported by participants of this study. The National Comprehensive Cancer Network (NCCN) adolescent and young adult clinical practice guidelines [14] and the American Society of Clinical Oncology (ASCO) guidelines [16] support FP discussions in reproductive-aged cancer patients. Our study findings showed that 45% received a FP consultation referral and 35% (66% of those who received an FP referral) underwent FP treatments. Other studies have found similar rates of FP therapy among YCS [43,44]. Kim and colleagues (2012) reported data from three fertility preservation centers showing 58% of breast cancer patients who underwent a FP consultation subsequently completed FP therapy. In contrast, some studies have shown much lower rates of FP use [45,46]. For example, among 981 female YCS recruited from the California Cancer Registry, only 4% pursued FP treatments [46]. Also Partridge and colleagues (2008) reported that 56% of the young breast cancer survivors in their study desired a future pregnancy at cancer diagnosis, yet only 10% underwent FP therapy [45]. This discrepancy in rates of FP therapy may be due to an increased awareness of fertility preservation in the past few years. Higher rates in recent studies may also be due to access to reproductive specialists. For example, our study recruited more recently diagnosed participants from university-based FP programs; whereas, Letourneau and colleagues recruited participants diagnosed between 1993 and 2007 from the California Cancer Registry [46]. Overall, these findings suggest increasing exposure to FP counseling and therapy, but many reproductive-aged patients are still lacking access to FP care prior to their cancer treatment.
The strengths of this study are the large cohort size of female reproductive-aged YCS, diversity of cancer types and stages, and comprehensive data collection on cancer and treatment characteristics and reproductive health outcomes (e.g., pregnancy, infertility, FP therapy), which allowed us to consider a large number of important covariates and potential confounding factors. Our cohort of YCS primarily diagnosed within the past 5 years allowed us to examine current FP practices and pregnancy attempts. It should be noted that the use of social media outreach to recruit participants was successful at reaching a geographically diverse sample of YCS. While this study provides needed insight into the complex factors associated with pregnancy attempts after cancer, it also raises additional unanswered questions related to fertility outcomes for YCS, such as prospectively determining factors that influence pregnancy attempts, how to intervene on patients and providers to improve access and use of FP, and the use of banked embryos or oocytes to achieve pregnancy.
Several limitations to the study should be noted. The primary limitation is the reliance on self-report for the study variables, which may result in misclassification. For example, the definition of our main study outcome was based on 3 self-report questions. Some women may have been attempting pregnancy after their cancer diagnosis yet failed to respond affirmatively to any of the 3 questions chosen to define a pregnancy attempt and were therefore not classified as attempting pregnancy. Conversely, some women may have unintentionally become pregnant after their cancer diagnosis, yet were classified as attempting pregnancy based on an affirmative response to having ever been pregnant after their cancer diagnosis. In addition, we did not ascertain information on whether participants sought or received medical clearance for pregnancy, which may impact the timing of pregnancy attempts. However, in an ongoing follow up of this cohort with data on 231 participants to date, nearly half (45%) report that they have received no recommendations on timing of pregnancy from a medical provider. We will explore this in future analyses of our longitudinal data. Our study findings are based on cross-sectional data collected at study enrollment, resulting in our inability to comment on causative relationships. Another limitation is that participants were recruited from FP programs and social media and elected to participate in a study about fertility and cancer, possibly indicating a heightened interest in future fertility to be noted when considering the generalizability of the study. Additional generalizability concerns include that most participants were white, well educated, reported a yearly income > $50,000, and were within 5 years of cancer diagnosis.
This is the first study to examine factors associated with pregnancy attempts among young adult female cancer survivors. For this population, factors such as being in a stable relationship and duration of cancer survivorship are critical to decisions regarding pregnancy attempts, but take time to achieve. As accelerated ovarian aging occurs concurrent to known delays in reaching these milestones, awareness of the impact of these competing factors on fertility must be raised in YCS. The new finding of an inverse association between FP therapy and pregnancy attempts warrants further study.
Acknowledgments
Research related to the development of this paper was supported by the National Institutes of Health, grants UL1 RR024926 pilot and HD-058799-01, and by the American Cancer Society, grants MRSG-08-110-01-CCE and 120500-PFT-11-008-01-CPPB. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health. The authors would like to thank the FIRST participants, Clarisa Gracia, Janet McLaren, Kathleen Lin, Samantha Roberts, Susan Faerber, Kristin Smith, Stupid Cancer foundation and Fertile Action for their contributions to this study.
Footnotes
Conflict of Interest
Sally A. Dominick, Brian W. Whitcomb, and Jessica R. Gorman declare that they have no conflict of interest.
Jennifer E. Mersereau, Karine Chung, and H. Irene Su have served on the Advisory Board for Ferring Pharmaceuticals.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Contributor Information
Sally A. Dominick, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Drive, #0901, La Jolla, CA 92093-0901, USA.
Brian W. Whitcomb, Division of Biostatistics & Epidemiology, School of Public Health & Health Sciences , 408 Arnold House, 715 North Pleasant Street , University of Massachusetts , Amherst, MA 01003-9304, USA.
Jessica R. Gorman, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Drive #0901, La Jolla, CA 92093-0901, USA.
Jennifer E. Mersereau, Department of Obstetrics & Gynecology, University of North Carolina, UNC Reproductive Endocrinology, Old Clinic Building, Campus Box 7570, Chapel Hill, NC 27599-7570, USA.
Karine Chung, Division of Reproductive Endocrinology & Infertility, University of Southern California, Keck School of Medicine, 1127 Wilshire Boulevard, 14th Floor, Los Angeles, CA 90017, USA.
H. Irene Su, Department of Reproductive Medicine, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Drive #0901, La Jolla, CA 92093-0901, USA.
References
- 1.Society AC. Cancer Treatment and Survivorship Facts and Figures 2012-2013. Atlanta. 2012 [Google Scholar]
- 2.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–41. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 3.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–9. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 4.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117:2289–94. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 5.Haase JE, Phillips CR. The adolescent/young adult experience. J Pediatr Oncol Nurs. 2004;21:145–9. doi: 10.1177/1043454204264385. [DOI] [PubMed] [Google Scholar]
- 6.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55:469–80. [PubMed] [Google Scholar]
- 7.Bellizzi KM, Smith A, Schmidt S, Keegan THM, Zebrack B, Lynch CF, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118:5155–62. doi: 10.1002/cncr.27512. [DOI] [PubMed] [Google Scholar]
- 8.Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14:873–81. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DM, Sklar CA, Boice JD, Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClellan W, Klemp JR, Krebill H, Ryan R, Nelson EL, Panicker J, et al. Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncol. Nurs. Forum. 2013;40:254–62. doi: 10.1188/13.ONF.254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118:4884–91. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penrose R, Beatty L, Mattiske J, Koczwara B. The psychosocial impact of cancer-related infertility on women: a review and comparison. Clin J Oncol Nurs. 2013;17:188–93. doi: 10.1188/13.CJON.188-193. [DOI] [PubMed] [Google Scholar]
- 13.Ruddy KJ, Partridge AH. Fertility (male and female) and menopause. J Clin Oncol. 2012;30:3705–11. doi: 10.1200/JCO.2012.42.1966. [DOI] [PubMed] [Google Scholar]
- 14.Coccia PF, Altman J, Bhatia S, Borinstein SC, Flynn J, George S, et al. Adolescent and Young Adult Oncology. J Natl Compr Canc Netw. 2012;10:1112–50. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]
- 15.Biasoli I, Falorio S, Luminari S, Spector N, Federico M. Fertility in female survivors of Hodgkin's lymphoma. Rev Bras Hematol Hemoter. 2012;34:48–53. doi: 10.5581/1516-8484.20120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallat ME, Hutter J. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121:e1461–9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]
- 18.The Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100:1214–23. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Mersereau JE, Goodman LR, Deal AM, Gorman JR, Whitcomb BW, Su HI. To preserve or not to preserve: How difficult is the decision about fertility preservation? Cancer. 2013;119:4044–50. doi: 10.1002/cncr.28317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groves RM, Mosher WD, Lepkowski JM, Kirgis NG. Planning and development of the continuous National Survey of Family Growth. Vital Health Stat 1. 2009:1–64. [PubMed] [Google Scholar]
- 21.Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83:383–92. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 22.Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–24. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- 23.Balthazar U, Deal AM, Fritz MA, Kondapalli LA, Kim JY, Mersereau JE. The current fertility preservation consultation model: are we adequately informing cancer patients of their options? Hum. Reprod. 2012;27:2413–9. doi: 10.1093/humrep/des188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Society for Assisted Reproductive Technology (SART) IVF Success Rates: National Data Summary. 2013 Internet. cited 2013 Nov 1. Available from https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0.
- 25.Proudfoot S, Wellings K, Glasier A. Analysis why nulliparous women over age 33 wish to use contraception. Contraception. 2009;79:98–104. doi: 10.1016/j.contraception.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Cooke A, Mills TA, Lavender T. Advanced maternal age: Delayed childbearing is rarely a conscious choice. Int J Nurs Stud. 2012;49:30–9. doi: 10.1016/j.ijnurstu.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: results from a prospective cohort study of cancer survivors. Pediatr. Blood Cancer. 2013;60:2001–6. doi: 10.1002/pbc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benzies K, Tough S, Tofflemire K, Frick C, Faber A, Newburn-Cook C. Factors influencing women's decisions about timing of motherhood. J. Obstet. Gynecol. Neonatal Nurs. 2006;35:625–33. doi: 10.1111/j.1552-6909.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 29.Peterson BD, Pirritano M, Tucker L, Lampic C. Fertility awareness and parenting attitudes among American male and female undergraduate university students. Hum. Reprod. 2012;27:1375–82. doi: 10.1093/humrep/des011. [DOI] [PubMed] [Google Scholar]
- 30.Heck KE, Schoendorf KC, Ventura SJ, Kiely JL. Delayed childbearing by education level in the United States, 1969–1994. Matern. Child Health J. 1997;1:81–8. doi: 10.1023/a:1026218322723. [DOI] [PubMed] [Google Scholar]
- 31.Bretherick KL, Fairbrother N, Avila L, Harbord SHA, Robinson WP. Fertility and aging: do reproductive-aged Canadian women know what they need to know? Fertil Steril. 2010;93:2162–8. doi: 10.1016/j.fertnstert.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 32.Tough S, Benzies K, Fraser-Lee N, Newburn-Cook C. Factors influencing childbearing decisions and knowledge of perinatal risks among Canadian men and women. Matern. Child Health J. 2007;11:189–98. doi: 10.1007/s10995-006-0156-1. [DOI] [PubMed] [Google Scholar]
- 33.Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology. 2012;21:134–43. doi: 10.1002/pon.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 35.Pagani O, Price KN, Gelber RD, Castiglione-Gertsch M, Holmberg SB, Lindtner J, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117:319–24. doi: 10.1007/s10549-008-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jatoi I, Anderson WF, Jeong JH, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29:2301–4. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis. 2013;5:S36–46. doi: 10.3978/j.issn.2072-1439.2013.05.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–41. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 39.Azim HA, Kroman N, Paesmans M, Gelber S, Rotmensz N, Ameye L, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31:73–9. doi: 10.1200/JCO.2012.44.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azim HA, Santoro L, Pavlidis N, Gelber S, Kroman N, Azim H, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47:74–83. doi: 10.1016/j.ejca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Pagani O, Partridge A, Korde L, Badve S, Bartlett J, Albain K, et al. Pregnancy after breast cancer: if you wish, ma'am. Breast Cancer Res Treat. 2011;129:309–17. doi: 10.1007/s10549-011-1643-7. [DOI] [PubMed] [Google Scholar]
- 42.Valachis A, Tsali L, Pesce LL, Polyzos NP, Dimitriadis C, Tsalis K, et al. Safety of pregnancy after primary breast carcinoma in young women: a meta-analysis to overcome bias of healthy mother effect studies. Obstet Gynecol Surv. 2010;65:786–93. doi: 10.1097/OGX.0b013e31821285bf. [DOI] [PubMed] [Google Scholar]
- 43.Lawrenz B, Jauckus J, Kupka MS, Strowitzki T, von Wolff M. Fertility preservation in >1,000 patients: patient's characteristics, spectrum, efficacy and risks of applied preservation techniques. Arch Gynecol Obstet. 2011;283:651–6. doi: 10.1007/s00404-010-1772-y. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Oktay K, Gracia C, Lee S, Morse C, Mersereau JE. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97:671–6. doi: 10.1016/j.fertnstert.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Partridge AH, Gelber S, Peppercorn J, Ginsburg E, Sampson E, Rosenberg R, et al. Fertility and menopausal outcomes in young breast cancer survivors. Clin Breast Cancer. 2008;8:65–9. doi: 10.3816/CBC.2008.n.004. [DOI] [PubMed] [Google Scholar]
- 46.Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118:4579–88. doi: 10.1002/cncr.26649. [DOI] [PMC free article] [PubMed] [Google Scholar]


