Abstract
Purpose of review
Renal involvement is a major cause of morbidity and mortality in systemic lupus erythematosus (SLE). In this review we provide an update on recent discoveries in the pathogenesis, diagnosis, and treatment of lupus nephritis (LN).
Recent findings
Localized long-lived plasma cells have been identified as playing an important role in LN. In addition, the roles of aberrant expression of microRNAs and pro-inflammatory cytokines have been explored. Early diagnosis is important for effective treatment and multiple biomarkers have been identified; however, none have been yet validated for clinical use. Biomarker panels may turn out to be more accurate than each individual component. Biologic agents for the treatment of LN are being studied, including Belimumab which was recently approved for non-renal SLE. Rituximab has not proven itself in large, placebo-controlled trials, although it is still being used in refractory cases of LN.
Summary
LN is a potentially devastating complication of SLE. Immune cells, cytokines, and epigenetic factors have all been recently implicated in LN pathogenesis. These recent discoveries may enable a paradigm shift in the treatment of this complex disease, allowing the tailoring of treatment to target specific pathogenic mediators at specific points in time in the progression of disease.
Keywords: Lupus nephritis, long-lived plasma cells, microRNA, biomarkers
Introduction
Systemic lupus erythematosus (SLE) is characterized by a loss of self-tolerance and the development of autoantibodies (autoAbs) to ubiquitous nuclear self-antigens. This autoAb production is initiated by exposure of the immune system to self-antigens via various mechanisms, including abnormal clearance of apoptotic material [1]. In addition, the threshold for the autoimmune response is lowered by activation of the type I interferon (IFN) pathway [2].
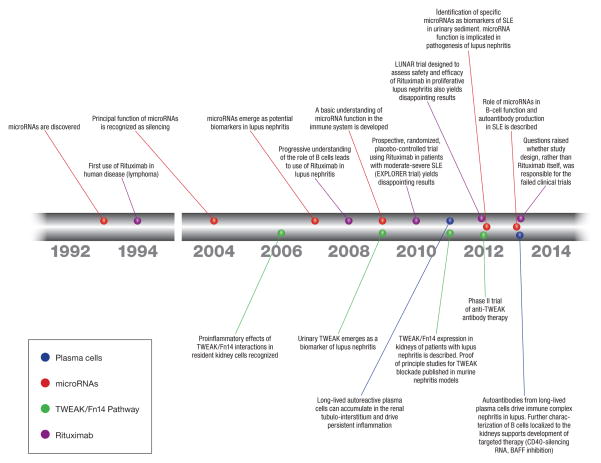
SLE affects mostly women of reproductive age, with up to 20% of the cases beginning in childhood [3]. It is a potentially devastating disease, which can involve practically any organ system. Renal involvement, termed lupus nephritis (LN), significantly increases the morbidity and mortality of SLE patients and requires aggressive immunosuppressive therapy, which unfortunately is associated with a plethora of side effects. The LN histology-based classification system currently in use gives the clinician a tool to predict outcome and to tailor therapy, albeit, with moderate to good success at best [4•]. In this review, we will discuss recent advances in the understanding of LN pathogenesis, diagnosis and monitoring of disease, as well as novel therapies (Figure 1).
Figure 1.
Timeline of recent developments in the pathogenesis and treatment of lupus nephritis
Pathogenesis of Lupus Nephritis
In recent years, we have learned a great deal about activation of B cells and their contribution to the maintenance of autoAb production, as well as the importance of local renal expression of inflammatory cytokines promoting the influx of immune cells [5••]. In this section, we will mainly focus on newly identified pathogenic mechanisms contributing to the local inflammatory environment in the kidney, and to events leading to the influx, persistence, and autoAb production of long-lived plasma cells (PCs).
B Cells and Plasma Cells
Long-lived memory PCs play a central role in the production of autoAbs and their maintenance, and can be detected in the peripheral blood of SLE patients during a disease flare [6]. However, their exact contribution to the development of SLE and LN was unclear. Cheng et al elegantly demonstrated the role of long-lived memory PCs in the pathogenesis of SLE by infusing PCs from lupus mice into Rag1−/− mice lacking B cells and PCs. The infused cells homed to the spleen and bone marrow of the recipient mice, and resulted in generation of autoAbs to dsDNA and the development of immune complex nephritis within 21 weeks of the adoptive transfer [7••]. Furthermore, Espeli et al described localization of autoreactive PCs in the kidney, in addition to the spleen and bone marrow, of NZB/W F1 mice [8], a lupus prone mouse strain that develops nephritis [9]. In fact, most IgG anti-dsDNA-specific PCs were found in the kidneys, followed by the bone marrow and spleen. These cells were more prevalent in mice with LN, and were located in the tubulointerstitium. In lupus patients, PCs could be found in the medulla of those with the most severe kidney disease, particularly patients with combined class III/IV and V LN. The PCs in the kidneys also distinguished themselves from those in lymphoid organs in that more than 90% of them were not actively undergoing cell cycle changes. The fact that most of the PCs in the kidneys are not dividing and are localized to the deeper areas of the kidneys may explain some of the difficulty in treating LN with standard immunosuppression, as well as emphasizing the importance of local chemotactic factors. Further support for the centrality of B cell activation in LN can be found in a study by Ripoll et al, in which CD40, a co-stimulatory molecule for B cell activation, was silenced by small interfering RNA (siRNA) technology in the kidneys of NZB/W F1 mice. The authors compared the clinical and histological changes in CD40-siRNA treated mice to those receiving established treatments for LN, such as cyclophosphamide. CD40-siRNA resulted in a decrease in influx of immune cells as well as prevention of progression comparable to the “standard of care” [10•].
Cytokines and chemokines are important for B cell survival and the orchestration of inflammatory cell migration. MRL/lpr mice are a mouse strain that is particularly susceptible to develop LN [9]. In this mouse model, Moreth et al demonstrated a role for the proteoglycan biglycan in triggering CXCL13 overexpression, leading to an increased influx of B cells, worsening proteinuria, and more severe kidney damage [11].
Anti-B cell activating factor (BAFF) monoclonal Ab was approved for SLE in 2011, although a specific benefit for LN has not been demonstrated to date. In a prospective study, Sun et al assessed the correlation between local expression of BAFF, localization of infiltrating CD20+ B cells in LN biopsies, and nephritis severity. Infiltrating B cells and intrarenal BAFF were predominantly localized in the interstitium, and both correlated with proteinuria as well as serum levels of BUN and creatinine. Interestingly, there was no correlation between intrarenal BAFF expression and plasma BAFF levels [12•].
MicroRNAs (miRNAs) are small noncoding RNAs that modulate gene expression at the posttranscriptional level by binding to the 3′ untranslated region of their target, thereby affecting the translation or stability of the transcripts [13]. Emerging evidence has demonstrated that miRNAs play a vital role in autoimmunity [14,15], and in LN in particular [16]. Recently, Liu et al. demonstrated that miR-30a was significantly increased in B cells from SLE patients, and overexpressed miR-30a in vitro could lower the level of Lyn, a member of the Src family protein tyrosine kinases, in B cells [17]. Interestingly, Lyn-deficient mice develop an autoimmune-type disease, characterized by the development of autoAbs in the serum, and deposition of immune complexes in the kidney – pathologic features reminiscent of SLE [18].
The role of miR-15a was assessed in the IFN-accelerated NZB/W F1 model of SLE. IFN treatment elevated miR-15a levels, which in turn correlated with lower levels of regulatory B cell subpopulations, particularly B-10. The authors concluded that IFN-induced miR-15a overexpression may have a specific negative regulatory effect on this B cell subpopulation [19].
Macrophages
Glomerular immune-complex deposition leads to the influx of multiple immune cells, including macrophages. Sialoadhesin (Sn), a sialic acid-binding immunoglobulin-like lectin, is a macrophage-restricted adhesion molecule that can be induced under the influence of IFN-α, and which serves as a biomarker for a type I IFN-signature [20]. In SLE, Sn-expressing monocytes have been shown to correlate with disease severity [21]. Sn was also thought to have a functional component, as its deficiency leads to amelioration of murine autoimmune neuropathy [22]. However, Kidder et al demonstrated that in NZB/NZW F1 mice, Sn-deficiency did not reduce LN severity or delay disease progression [23•]. Therefore, while Sn may have a role as a biomarker for disease severity in LN, it will not necessarily serve as a future therapeutic target.
Inflammatory Cytokines
In parallel with the recognition that intrarenal inflammation has its own spectrum of immune cells, the past years have also shed more light on the roles of particular cytokines and their receptors, which have moved on to becoming therapeutic targets. One example is TNF-like weak inducer of apoptosis (TWEAK), a member of the TNF superfamily. TWEAK is an inflammatory cytokine that is believed to play an important role in LN. Its receptor, Fn14, is present on mesangial cells, podocytes, endothelial cells, and tubular cells, and is upregulated in LN [24]. TWEAK/Fn14 interactions in the kidney induce expression of multiple inflammatory mediators, including RANTES, monocyte chemoattractant protein (MCP)-1, IP-10, and VCAM-1, all highly relevant to the pathogenesis of LN [25]. Importantly, blocking TWEAK/Fn14 interactions in several murine models of inflammatory kidney disease was shown to be beneficial [26•,27•]. These findings supported the development of a human anti-TWEAK Ab as an add-on treatment to the standard-of-care, a concept currently being tested in a multicenter phase II clinical trial (Anti-TWEAK in Lupus Nephritis (ATLAS), ClinicalTrials.gov Identifier: NCT01499355).
Diagnosis and Disease Monitoring
The incidence of end-stage renal disease from LN did not change from 1996 to 2004, despite the introduction of several new efficacious therapies. This may be explained by the limitations of the current treatment options, poor access to healthcare or medication non-compliance, as well as delayed diagnosis or treatment [28]. Timely diagnosis of LN is still a challenge. The gold standard for diagnosis is renal biopsy which can be associated with significant morbidity, as well as inadequacies due to the “blind”-nature of the procedure. Furthermore, a one-time diagnosis is often not sufficient, as the histopathology can change over time and therapy needs to be tailored appropriately. As serial biopsies are not usually done and current markers such as proteinuria have proved to be lacking [29,30], a more accurate, yet not invasive, diagnostic method is sought after.
The pathogenesis of LN is now known to involve multiple mediators, and the efforts to identify a reliable biomarker have recently spread in multiple directions: inflammatory cells [31–33•], cytokines/chemokines such as MCP-1, neutrophil gelatinase-associated lipocalin (NGAL), IL-6, VCAM-1, CXCL16, TWEAK, and IP-10 [34,35••], as well as miRNA [36–40]. Furthermore, in an effort to cast an even wider net, more comprehensive methods are now being used to identify such biomarkers, including microarray chips [38–40], proteomics [41], and combinations of markers that may together reflect disease activity more accurately than each component in isolation [42•,43,44,45•].
Several of the studied biomarkers are quite promising and may even prove to have therapeutic potential, as in many of the cases it is presumed that the biomarker is directly related to the pathogenesis of the disease (rather than just merely a side-product of the inflammatory process). As mentioned previously, TWEAK/Fn14 interactions play a role in LN, and their neutralization has been shown to ameliorate disease progression. Several recent studies have confirmed prior reports that urinary TWEAK levels reflect renal disease activity, and correlate well with other potential biomarkers, such as MCP-1 [46–48]. While most publications highlight biomarkers that correlate with some aspect of disease status, Treamtrakanpon et al provided evidence that the serum levels of the cytokine APRIL could potentially predict treatment resistance – a finding which, if validated, could be critical in therapy decisions for individual LN patients [49].
While prior microarray studies on peripheral blood mononuclear cells (PBMCs) of SLE patients showed differential miRNA expression between patients and healthy controls [50], more specific studies also indicated differential expression of miRNAs between LN and non-LN SLE patients [38–39]. Via a similar microarray method, miR-126 was found to be upregulated in CD4+ T cells of patients with SLE. At the same time, its levels were inversely associated with the CD4+ T cell expression of Dnmt1 (DNA methyltransferase 1), a gene expression modulator that suppresses certain genes by the methylation of their promoter regions [51]. Dnmt1 in lupus patients has reduced enzymatic activity, likely leading to T cell DNA hypomethylation which is generally associated with a permissive transcriptional environment. Indeed, T cell DNA hypomethylation levels correlate with SLE disease activity, suggesting an epigenetic factor in the development of SLE [52,53•].
Two separate studies have recently demonstrated the biomarker potential of urinary T cells levels. In a primarily cross-sectional study comparing 147 SLE patients (both LN and non-LN), 31 patients with nephropathies from other causes, and 20 healthy individuals, Enghard et al demonstrated a close correlation between urinary CD4+ T cells and LN activity, with excellent discrimination between active and non-active LN patients (AUC 0.9969). Furthermore, urinary CD4+ T levels were higher in patients with proliferative disease (class IV and IV/V) compared with class I or pure class V [33•]. Similarly, in a smaller-scale study of urinary CD8+ cells levels comparing 22 active LN patients with 24 non-LN SLE patients, Dolff et al showed good correlation with disease activity. The main clinical use for urinary CD8+ cells may be to distinguish between patients with active LN to those with a recent history of LN but no current activity, as proteinuria may not be sensitive enough in this patient population to diagnose an early flare [31].
The fact that in each individual LN patient there are likely multiple, distinct molecular pathways that are activated, makes the likelihood of finding one unifying biomarker quite low. This has led many researchers to look for a combination of biomarkers, with the thought that the whole will be bigger (and more accurate) than the sum of its parts. Thus, Sui et al, in a large retrospective study including 589 SLE patients, found that simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone Abs was associated with a higher proportion of proliferative renal lesions (class III+IV), and a higher rate of recurrence and poor renal outcome [44]. Brunner et al analyzed multiple candidate urinary biomarkers in LN patients that underwent renal biopsies, and compared them with specific histologic features. They found that different biomarker combinations reflect different specific tissue changes: the combination of MCP-1, α1-acid glycoprotein (AAG), ceruloplasmin, and protein:creatinine ratio was useful in reflecting LN activity (AUC 0.85); MCP-1, NGAL and creatinine clearance were best at predicting LN chronicity (AUC 0.83); and the combination of MCP-1, AAG, transferrin, creatinine clearance, and C4 proved to be a good diagnostic tool for membranous LN (AUC 0.75) [42•].
Therapies
Recent years have been quite exciting in terms of therapeutic advancements in SLE in general, and LN in particular. While traditional treatments such as corticosteroids and immunosuppressants are non-specific and associated with numerous side effects, we are now seeing a rise in target-oriented therapies with promising prospects [54••,55•,56]. As the pathogenesis of the disease continues to be elucidated, many specific targets are being singled out for therapy, and conventional treatment protocols are being enhanced [57•].
Depletion of immune cells
The most talked-about B cell depletion drug in recent years has been Rituximab. It is a monoclonal chimeric anti-CD20 mAb, targeting mature B cells, but not plasma cells [58]. As SLE is characterized by loss of B cell tolerance and production of antibodies to self-antigens, the rationale behind depleting self-antigen presenting cells, as well as the precursors to the autoantibody-secreting cells, is apparent. In addition, as B cells also play a role in T cell activation and cytokine production, their depletion can affect different aspects of SLE pathogenesis [59•]. Indeed, there were multiple small-scale open-label studies that have demonstrated the efficacy of Rituximab in refractory SLE [60••]. This explains the disappointment following the publication of results from two large randomized control trials, EXPLORER (Exploratory Phase II/III SLE Evaluation of Rituximab) [61] and LUNAR (Lupus Nephritis Assessment with Rituximab) [62], which failed to demonstrate benefit from Rituximab in renal and non-renal lupus when added to the standard-of-care regimen. However, debate continues regarding the implication of these studies, which may have been problematic in terms of their endpoint definitions, lack of power to demonstrate the pre-specified endpoints, and the background regimens being too aggressive to prove added benefits from additional drugs [63•,64•]. Importantly, guidelines from both the American College of Rheumatology (ACR) [65] and European League against Rheumatism (EULAR) [66] support the use of Rituximab in the treatment of refractory LN. Of note, recently Condon et al demonstrated a steroid-sparing effect of Rituximab in an observational study in which 50 LN patients were treated with Rituximab and low-dose mycophenolate mofetil (MMF). Good disease control was achieved, while at the same time only 2 patients required oral steroids after 2 years of follow up [67•].
One explanation for the negative results in the Rituximab trials may be that the antibody-secreting cells driving the kidney inflammation in LN are long-lived PCs, which do not display CD20 and are, therefore, not targeted by the drug [7••,68]. Interestingly, it has recently been shown by Wang et al that a prolonged course of Rituximab in NZB/NZW F1 mice significantly reduced kidney PCs levels, as well as decreased antibody levels and disease activity significantly better than a short course of the drug [69]. It is yet unknown how this PC depletion was brought about by Rituximab, but one reasonable explanation may be that the prolonged course led to an extended period of B cell depletion, which in turn prevented ongoing maturation and differentiation of B cells into PCs [69]. While this effect of Rituximab on PCs needs to be further studied, there are reports regarding the efficacy of a plasma-cell depletor, Bortezomib, in LN [70–72]. Bortezomib inhibits the proteasome within PCs, resulting in inability to degrade misfolded proteins, which eventually leads to apoptosis. While Bortezomib has shown promise in reducing disease activity and decreasing autoAbs concentrations while increasing complement levels [71], this drug has been associated with serious neurotoxicity [73].
Cytokine modulation
As mentioned previously, several cytokines and chemokines are thought to play major roles in the triggering and perpetuation of inflammation in SLE, and may prove to be effective therapeutic targets. Of those, one of the most clinically relevant is BAFF, which promotes the formation and survival of memory B cells and Ab-secreting plasmablasts [74••]. Belimumab, a BAFF inhibitor, has been the first new treatment to be approved by the US Food and Drug Administration for non-renal SLE in 50 years. In two phase III RCTs, BLISS 52 [75] and BLISS 76 [76], Belimumab demonstrated added benefit as compared with placebo when used together with standard therapy. A post-hoc analysis of the trials to determine the drug’s efficacy in LN also showed a trend toward increased response in the Belimumab group compared with placebo [77]. Currently, a phase III randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of Belimumab in LN is underway (ClinicalTrials.gov Identifier: NCT01639339).
Interestingly, Rovin et al discuss the possibility of achieving the best disease control by stepwise use of the different agents, according to the chronology of the pathogenesis of LN [78••]. As the major, most immediate culprits in the renal inflammation are most likely the autoAb-producing PCs, it may be useful to first administer a course of a proteasome inhibitor in order to deplete the PCs, followed by B cell depletion to prevent further differentiation into PCs. Finally, B cell depletion is associated with an increase in BAFF, likely as a reaction to the reduced B cell counts. Increased BAFF likely induces more auto-reactive B cells once the B cells are reconstituted post-treatment. Therefore, the use of BAFF-inhibitors such as Belimumab at the time of B cell depletion may be beneficial in preventing the return of autoreactive B cells and sustaining remission [78••]. This idea of tailoring therapy to defined targets at specific points in time is intriguing, and may herald future target-specific and pathogenesis-directed management of complicated diseases.
Conclusion
If LN were a soap opera and we had to summarize the events of the past few seasons, we could say that we watched some interesting developments: good guys (maybe) going bad (Rituximab), the introduction of a whole new set of characters (long-lived PCs in the renal interstitium, microRNAs as biomarkers and potential therapeutic targets), and the development of a passionate, yet intriguing, long-term relationship (treatment with biologics, with its ups and downs). We have much to look forward to in the “episodes” ahead.
Supplementary Material
Key points.
There is increasing appreciation for the contribution of interstitial autoantibody-producing long-lived plasma cells to the renal inflammation in LN.
Epigenetic changes in LN patients most likely contribute to disease pathogenesis, and these changes themselves and their facilitators, e.g. hypomethylation of T cell DNA and microRNA’s, may serve as biomarkers of disease activity.
Understanding the multiple pathways contributing to LN may eventually allow physicians to better tailor individualized therapeutic regimens, through the application of target-specific and disease-stage-specific treatment protocols.
Footnotes
Conflict of Interest:
Drs. Schwartz and Goilav have no conflicts to report. Dr. Putterman has received research funds from Biogen Idec, is co-holder on a patent for the diagnostic use of TWEAK in LN, and is an investigator in the anti-TWEAK mAb in LN clinical trial.
References and recommended reading
- 1.Herrmann M, Voll RE, Zoller OM, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Elkon KB, Stone VV. Type I interferon and systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:803–812. doi: 10.1089/jir.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995;34:866–872. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 4•.Giannico G, Fogo AB. Lupus nephritis: is the kidney biopsy currently necessary in the management of lupus nephritis? Clin J Am Soc Nephrol. 2013;8:138–145. doi: 10.2215/CJN.03400412. An overview of the limitations of the current renal biopsy classification system in lupus nephritis. [DOI] [PubMed] [Google Scholar]
- 5••.Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357–1366. doi: 10.1681/ASN.2013010026. Review of the current understanding of pathogenic mechanisms in LN, as well as reasoning for the latest treatment strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiepe F, Dörner T, Hauser AE, et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 7••.Cheng Q, Mumtaz IM, Khodadadi L, et al. Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann Rheum Dis. 2013;72:2011–2017. doi: 10.1136/annrheumdis-2013-203455. LN was induced by infusion of long-lived plasma cells from lupus mice into Rag1(−/−) immunodeficient mice, implicating these cells as major contributors to LN pathogenesis. [DOI] [PubMed] [Google Scholar]
- 8.Espeli M, Bökers S, Giannico G, et al. Local Renal Autoantibody Production in Lupus Nephritis. J Am Soc Nephrol. 2011;22:296–305. doi: 10.1681/ASN.2010050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews BS, Eisenberg RS, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Ripoll E, Merino A, Goma M, et al. CD40 gene silencing reduces the progression of experimental lupus nephritis modulating local milieu and systemic mechanisms. PLoS ONE. 2013;8:e65068. doi: 10.1371/journal.pone.0065068. An interesting study of the use of CD40-silencing RNA in NZB/W F1 mice, and its effectiveness in ameliorating renal disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreth K, Brodbeck R, Babelova A, et al. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120:4251–4272. doi: 10.1172/JCI42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Sun C, Shen Y, Chen X, et al. The characteristics and significance of locally infiltrating B cells in lupus nephritis and their association with local BAFF expression. Int J Rheumatol. 2013:954292. doi: 10.1155/2013/954292. Local BAFF expression is associated with increased renal localization of B cells in lupus nephritis, and with increased disease severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 14.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chafin CB, Reilly CM. MicroRNA implicated in the immunopathogenesis of lupus nephritis. Clinc Dev Immunol. 2013;2013:430239. doi: 10.1155/2013/430239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Dong J, Mu R, et al. MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn. Arthritis Rheum. 2013;65:1603–1611. doi: 10.1002/art.37912. [DOI] [PubMed] [Google Scholar]
- 18.Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases LYN and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Kasar S, Underbayev C, et al. Role of microRNA-15a in autoantibody production in interferon-augmented murine model of lupus. Mol Immunol. 2012;52:61– 70. doi: 10.1016/j.molimm.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaas M, Oetke C, Lewis LE, et al. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J Immunol. 2012;189:2414–2422. doi: 10.4049/jimmunol.1200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biesen R, Demir C, Barkhudarova F, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1136–1145. doi: 10.1002/art.23404. [DOI] [PubMed] [Google Scholar]
- 22.Ip CW, Kroner A, Crocker PR, et al. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol Dis. 2007;25:105–111. doi: 10.1016/j.nbd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 23•.Kidder D, Richards HE, Lyons PA, et al. Sialoadhesin deficiency does not influence the severity of lupus nephritis in New Zealand Black x New Zealand White F1 mice. Arthritis Res Ther. 2013;15:R175. doi: 10.1186/ar4364. Sn-KO mice had similar disease progression and severity to wild-type lupus-prone mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Kwan BC, Lai FM, et al. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patient with lupus nephritis. Nephrology. 2011;16:426–432. doi: 10.1111/j.1440-1797.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell S, Michaelson J, Burkly LC, et al. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- 26•.Michaelson JS, Wisniacki N, Burkly LC, Putterman C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J Autoimmun. 2012;39:130–142. doi: 10.1016/j.jaut.2012.05.003. A review summarizing the development of the interest in TWEAK, from the elucidation of the cytokine’s importance in disease pathogenesis, to the current phase 2 trial for the anti-TWEAK antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Cheng E, Armstrong CL, Galisteo R, Winkles JA. TWEAK/Fn14 axis-targeted therapeutics: moving basic science discoveries to the clinic. Front Immunol. 2013;4:473. doi: 10.3389/fimmu.2013.00473. A thorough review of the TWEAK/Fn14 pathway and its role in inflammatory disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996–2004. J Rheumatol. 2009;36:63–67. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunnarsson I, Sundelin B, Jonsdottir T, et al. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 2007;56:1263–1272. doi: 10.1002/art.22505. [DOI] [PubMed] [Google Scholar]
- 30.Lightstone L. Lupus nephritis: where are we now? Curr Opin Rheumatol. 2010;22:252–256. doi: 10.1097/BOR.0b013e3283386512. [DOI] [PubMed] [Google Scholar]
- 31.Dolff S, Abdulahad WH, Arends S, et al. Urinary CD8+ T-cell counts discriminate between active and inactive lupus nephritis. Arthritis Res Ther. 2013;15:R36. doi: 10.1186/ar4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XY, Wang HY, Zhao XY, et al. Th22, but not Th17 might be a good index to predict the tissue involvement of systemic lupus erythematosus. J Clinc Immunol. 2013;33:767–774. doi: 10.1007/s10875-013-9878-1. [DOI] [PubMed] [Google Scholar]
- 33•.Enghard P, Rieder C, Kopetschke K, et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann Rheum Dis. 2014;73:277–283. doi: 10.1136/annrheumdis-2012-202784. Urinary CD4 T cells are sensitive for detection of proliferative lupus nephritis, and their levels reflect disease activity. [DOI] [PubMed] [Google Scholar]
- 34.Reyes-Thomas J, Blanco I, Putterman C. Urinary biomarkers in Lupus Nephritis. Clinc Rev Allergy Immunol. 2011;40:138–150. doi: 10.1007/s12016-010-8197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Watson L, Beresford MW. Urine biomarkers in juvenile-onset SLE nephritis. Pediatr Nephrol. 2013;28:363–374. doi: 10.1007/s00467-012-2184-y. A review of traditional biomarkers with their limitations, as well as an overview of potential novel biomarkers for juvenile LN. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Tam LS, Li EKM, et al. Serum and urinary free microRNA level in patients with systemic lupus erythematosus. Lupus. 2011;20:493–500. doi: 10.1177/0961203310389841. [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Tam LS, Kwan BCH, et al. Expression of miRNA-146a and miRNA-155 in the urinary sediment of systemic lupus erythematosus. Clin Rheumatol. 2012;31:435–440. doi: 10.1007/s10067-011-1857-4. [DOI] [PubMed] [Google Scholar]
- 38.Dai Y, Sui W, Lan H, et al. Comprehensive analysis of micro RNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29:749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 39.Te JL, Dozmorov IM, Guthridge JM, et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS ONE. 2010;5:e10344. doi: 10.1371/journal.pone.0010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai R, Zhang Y, Khan D, et al. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS ONE. 2010;5:e14302. doi: 10.1371/journal.pone.0014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu T, Du Y, Han J, et al. Urinary angiostatin – a novel putative marker of renal pathology chronicity in lupus nephritis. Mol Cell Proteomics. 2013;12:1170–1179. doi: 10.1074/mcp.M112.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Brunner HI, Bennett MR, Mina R, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012;64:2687–2697. doi: 10.1002/art.34426. Different combinations of non-invasive biomarkers can reflect distinct aspects of LN, including LN activity, severity, and membranous histology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Nagaraja HN, Nadasdy T, et al. A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int. 2012;81:401–406. doi: 10.1038/ki.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sui M, Lin Q, Xu Z, et al. Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis. J Clin Immunol. 2013;33:378–387. doi: 10.1007/s10875-012-9825-6. [DOI] [PubMed] [Google Scholar]
- 45•.Petri M, Van Vollenhoven RF, Buyon J, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III Belimumab trials. Arthritis Rhem. 2013;65:2143–2153. doi: 10.1002/art.37995. A post-hoc analysis of the large-scale phase 3 Belimumab trials that identified a combination of variables that can predict a LN flare. [DOI] [PubMed] [Google Scholar]
- 46.Xuejing Z, Jiazhen T, Jun L, et al. Urinary TWEAK level as a marker of lupus nephritis activity in 46 cases. J Biomed Biotechnol. 2012:359647. doi: 10.1155/2012/359647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-shehaby A, Darweesh H, El-Khatib M, et al. Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clinc Immunol. 2001;31:848–856. doi: 10.1007/s10875-011-9555-1. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz N, Rubinstein T, Burkly LC, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treamtrakanpon W, Tantivitayakul P, Benjachat T, et al. APRIL, a proliferation-inducing ligand, as a potential marker of lupus nephritis. Arthritis Res Ther. 2012;14:R252. doi: 10.1186/ar4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai Y, Huang YS, Tang M, et al. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007;16:939–946. doi: 10.1177/0961203307084158. [DOI] [PubMed] [Google Scholar]
- 51.Zhao S, Wang Y, Liang Y, et al. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 52.Richardson B, Scheinbart L, Strahler J, et al. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33:1665–1673. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- 53•.Zhang Y, Zhao M, Sawalha AH, et al. Impaired DNA methylation and its mechanisms in CD4+T cells of systemic lupus erythematosus. J Autoimmun. 2013;41:92–99. doi: 10.1016/j.jaut.2013.01.005. The authors provide a detailed review of DNA methylation processes and their possible effect on the development of SLE. [DOI] [PubMed] [Google Scholar]
- 54••.Liu Z, Davidson A. Taming lupus – a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012:871–882. doi: 10.1038/nm.2752. An excellent review of the current mechanisms thought to be involved in the pathogenesis of SLE, and their implications for the development of novel treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Paz Z, Tsokos GC. New therapeutics in systemic lupus erythematosus. Curr Opin Rheumatol. 2013;25:297–303. doi: 10.1097/BOR.0b013e32835fd682. A comprehensive overview of prospective new treatments in SLE. [DOI] [PubMed] [Google Scholar]
- 56.Koutsokeras T, Healy T. Systemic lupus erythematosus and lupus nephritis. Nat Rev Drug Discov. 2014;13:173–174. doi: 10.1038/nrd4227. [DOI] [PubMed] [Google Scholar]
- 57•.Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97–107. doi: 10.1038/nrrheum.2013.157. A useful outline of current management approaches for SLE, with emphasis on specific disease manifestations, including lupus nephritis. [DOI] [PubMed] [Google Scholar]
- 58.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 59•.Anolik JH. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus. 2013;22:342–349. doi: 10.1177/0961203312471576. A summary of B cell development, function, and regulation, the role of B cell dysfunction in the pathogenesis of SLE, as well as the potential of B cell-directed therapy. [DOI] [PubMed] [Google Scholar]
- 60••.Weidenbusch M, Rommele C, Schrottle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28:106–111. doi: 10.1093/ndt/gfs285. A systematic review evaluating the use of Rituximab as treatment for refractory LN. [DOI] [PubMed] [Google Scholar]
- 61.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rovin BH, Furie R, Latinis K, et al. LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 63•.Wofsy D, Hillson JL, Diamond B. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum. 2013;65:1586–1591. doi: 10.1002/art.37940. An important paper demonstrating that the use of alternate endpoint definitions in the EXPLORER and LUNAR trials would have indicated a significant benefit to the use of Rituximab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Isenberg DA, Rahman A. Taking a closer look at biologic therapy for SLE. Nat Rev Rheumatol. 2014;10:71–72. doi: 10.1038/nrrheum.2013.203. A succinct review detailing the issues around the Rituximab trials and their problematic endpoints. [DOI] [PubMed] [Google Scholar]
- 65.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–82. doi: 10.1136/annrheumdis-2012-201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72:1280–1286. doi: 10.1136/annrheumdis-2012-202844. A study that demonstrates effective induction of LN remission and maintenance with Rituximab and MMF (and no steroids) [DOI] [PubMed] [Google Scholar]
- 68.Chang A, Henderson SG, Brandt D, et al. In situ B cell mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W, Rangel-Moreno J, Owen T, et al. Long-term B cell depletion in murine lupus eliminates autoantibody-secreting cells and is associated with alterations in the kidney plasma cell niche. J Immunol. 2014;192:3011–3020. doi: 10.4049/jimmunol.1302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 71.Hiepe F, Alexander T, Peukert R, et al. Refractory SLE patients respond to the proteazome inhibitor bortezomib. Ann Rheum Dis. 2012;71:A15–16. [Google Scholar]
- 72.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 73.Corthals SL, Kuiper R, Johnson DC, et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica. 2011;96:1728–1732. doi: 10.3324/haematol.2011.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Stohl W. Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin Ther Targets. 2014;18:473–489. doi: 10.1517/14728222.2014.888415. A detailed overview of the role of the BAFF/APRIL axis in SLE, with discussion of the benefits of anti-BAFF/APRIL therapies. [DOI] [PubMed] [Google Scholar]
- 75.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 76.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–6930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dooley MA, Houssiau F, Aranow C, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. 2013;22:63–72. doi: 10.1177/0961203312465781. [DOI] [PubMed] [Google Scholar]
- 78••.Rovin BH, Parikh SV. Lupus nephritis: the evolving role of novel therapeutics. Am J Kidney Dis. 2014;63:677–690. doi: 10.1053/j.ajkd.2013.11.023. The authors summarize new therapies for LN, and discuss their potential role in treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.