Abstract
Objective
To document the prevalence of multidrug resistance among people newly diagnosed with – and those retreated for – tuberculosis in Malawi.
Methods
We conducted a nationally representative survey of people with sputum-smear-positive tuberculosis between 2010 and 2011. For all consenting participants, we collected demographic and clinical data, two sputum samples and tested for human immunodeficiency virus (HIV).The samples underwent resistance testing at the Central Reference Laboratory in Lilongwe, Malawi. All Mycobacterium tuberculosis isolates found to be multidrug-resistant were retested for resistance to first-line drugs – and tested for resistance to second-line drugs – at a Supranational Tuberculosis Reference Laboratory in South Africa.
Findings
Overall, M. tuberculosis was isolated from 1777 (83.8%) of the 2120 smear-positive tuberculosis patients. Multidrug resistance was identified in five (0.4%) of 1196 isolates from new cases and 28 (4.8%) of 581 isolates from people undergoing retreatment. Of the 31 isolates from retreatment cases who had previously failed treatment, nine (29.0%) showed multidrug resistance. Although resistance to second-line drugs was found, no cases of extensive drug-resistant tuberculosis were detected. HIV testing of people from whom M. tuberculosis isolates were obtained showed that 577 (48.2%) of people newly diagnosed and 386 (66.4%) of people undergoing retreatment were positive.
Conclusion
The prevalence of multidrug resistance among people with smear-positive tuberculosis was low for sub-Saharan Africa – probably reflecting the strength of Malawi’s tuberculosis control programme. The relatively high prevalence of such resistance observed among those with previous treatment failure may highlight a need for a change in the national policy for retreating this subgroup of people with tuberculosis.
Résumé
Objectif
Documenter la prévalence de la résistance polymédicamenteuse de la tuberculose parmi les personnes nouvellement diagnostiquées et les personnes traitées à nouveau au Malawi.
Méthodes
Nous avons mené une enquête nationale représentative des personnes atteintes de tuberculose à frottis d'expectoration positif entre 2010 et 2011. Pour tous les participants consentants, nous avons recueilli les données démographiques et cliniques, deux échantillons d'expectoration et effectué le dépistage du virus de l'immunodéficience humaine (VIH). Les échantillons ont subi des tests de résistance au Laboratoire central de référence de Lilongwe, au Malawi. Tous les isolats de Mycobacterium tuberculosis qui ont présenté une résistance polymédicamenteuse ont été retestés pour la résistance aux médicaments de première intention - et testés pour la résistance aux médicaments de deuxième intention – dans un laboratoire de référence supranational pour la tuberculose en Afrique du Sud.
Résultats
Dans l'ensemble, M. tuberculosisM. tuberculosis a été isolé chez 1777 (83,8%) des 2120 patients atteints de tuberculose à frottis positif. La résistance polymédicamenteuse a été identifiée dans 5 (0,4%) des 1196 isolats obtenus à partir des nouveaux cas et dans 28 (4,8%) des 581 isolats obtenus à partir des personnes qui recevaient à nouveau un traitement. Parmi les 31 isolats issus des cas retraités qui ont connu un échec de traitement, 9 (29%) isolats ont présenté une résistance polymédicamenteuse . Bien que la résistance aux médicaments donnés en deuxième intention ait été identifiée, aucun cas de tuberculose ultrarésistante aux médicaments n'a été détecté. Les dépistages du VIH des personnes à partir desquelles les isolats de M. tuberculosisM. tuberculosis ont été obtenus ont montré que 577 (48,2%) des personnes nouvellement diagnostiquées et 386 (66,4%) des personnes recevant à nouveau le traitement étaient séropositives.
Conclusion
La prévalence de la résistance polymédicamenteuse chez les personnes atteintes de tuberculose à frottis positif était faible en Afrique subsaharienne – reflétant probablement la force du programme de contrôle de la tuberculose du Malawi. La prévalence relativement élevée de cette résistance observée chez les personnes pour lesquelles le traitement précédent a échoué peut mettre en évidence un besoin de changement dans la politique nationale en matière de retraitement de ce sous-groupe de personnes atteintes de tuberculose.
Resumen
Objetivo
Documentar la prevalencia de la resistencia a medicamentos múltiples entre pacientes a quienes se ha diagnosticado recientemente o han vuelto a recibir tratamiento para la tuberculosis en Malawi.
Métodos
Llevamos a cabo una encuesta representativa a nivel nacional de pacientes con tuberculosis que dieron positivo en el análisis de esputo entre 2010 y 2011. Para todos los participantes adultos, se recogieron datos demográficos y clínicos, dos muestras de esputo y realizamos pruebas del virus de inmunodeficiencia humana (VIH). Las muestras se sometieron a pruebas de resistencia en el Laboratorio Central de Referencia en Lilongwe (Malawi). Se volvieron a examinar todas las cepas de Mycobacterium tuberculosis multirresistentes para probar la resistencia a los medicamentos de primera línea y se probó su resistencia a los medicamentos de segunda línea en un laboratorio de referencia supranacional para la tuberculosis en Sudáfrica.
Resultados
En general, la M. tuberculosisM. tuberculosis se aisló en 1777 (83,8%) de los 2120 pacientes de tuberculosis con baciloscopia positiva. Se detectó multirresistencia a medicamentos en cinco (0,4%) de las 1196 cepas de casos nuevos y en 28 (4,8%) de las 581 cepas de pacientes que se volvieron a someter al tratamiento. De las 31 cepas de casos de repetición del tratamiento que no habían respondido previamente al tratamiento, nueve (29,0%) mostraron multirresistencia a medicamentos. Pese a que se halló resistencia a los medicamentos de segunda línea, no se detectaron casos de tuberculosis con resistencia extendida a medicamentos. Las pruebas del VIH de quienes se obtuvieron cepas de M. tuberculosisM. tuberculosis mostraron que 577 (48,2%) de los pacientes con diagnóstico reciente y 386 (66,4%) de los pacientes que se volvieron a someter al tratamiento dieron positivo.
Conclusión
La prevalencia de la multirresistencia a medicamentos entre los pacientes con tuberculosis que dieron positivo en la baciloscopia positiva fue baja en el África subsahariana, lo cual probablemente refleja la eficacia del programa de control de la tuberculosis de Malawi. La prevalencia relativamente alta de dicha resistencia observada entre los pacientes que no respondieron al tratamiento anterior puede poner de manifiesto la necesidad de un cambio en la política nacional para volver a tratar a este subgrupo de pacientes con tuberculosis.
ملخص
الغرض
توثيق انتشار مقاومة الأدوية المتعددة بين الأشخاص الذين جرى تشخيص إصابتهم حديثاً بالسل – والذين تكرر علاجهم من السل - في ملاوي.
الطريقة
أجرينا دراسة استقصائية تمثيلية على الصعيد الوطني للأشخاص المصابين بالسل وسجلوا نتائج إيجابية لاختبار لطاخة البلغم بين 2010 و2011. وقمنا بجمع البيانات الديمغرافية والسريرية وعينتين من البلغم واختبارهما لتحديد الإصابة بفيروس العوز المناعي البشري من جميع المشاركين الذين أبدوا موافقتهم. وتم إجراء اختبار المقاومة للعينات في المختبر المرجعي المركزي في ليلونغوي، بملاوي. وتم تكرار اختبار جميع مستفردات البكتريا المتفطرة السليّة التي تبين مقاومتها للأدوية المتعددة من أجل تحديد مقاومتها لأدوية الخط الأول – واختبارها من أجل تحديد مقاومتها لأدوية الخط الثاني – في مختبر مرجعي للسل على الصعيد فوق الوطني في جنوب أفريقيا.
النتائج
بشكل عام، تم استفراد البكتريا المتفطرة السليّة من 1777 (83.8 %) مريضاً من أصل 2120 مريضاً بالسل سجلوا نتائج إيجابية لاختبار اللطاخة. وتم تحديد مقاومة الأدوية المتعددة في خمس (0.4 %) من أصل 1196 مستفردة من حالات جديدة و28 (4.8 %) من أصل 581 مستفردة من الأشخاص الذين تكرر علاجهم. وأظهرت تسع (29.0 %) من أصل 31 مستفردة من الحالات التي تكرر علاجها بعد فشل علاجها في السابق مقاومة للأدوية المتعددة. وعلى الرغم مما تبين من مقاومة لأدوية الخط الثاني، لم يتم اكتشاف حالات للسل الشديد المقاوم للأدوية. وتبين من اختبار فيروس العوز المناعي البشري للأشخاص الذين تم الحصول على مستفردات البكتريا المتفطرة السليّة منهم أن 577 (48.2 %) من الأشخاص الذين جرى تشخيصهم حديثاً و386 (66.4 %) من الأشخاص الذين تكرر علاجهم سجلوا نتائج إيجابية.
الاستنتاج
كان انتشار مقاومة الأدوية المتعددة بين الأشخاص المصابين بالسل الذين سجلوا نتائج إيجابية لاختبار اللطاخة منخفضاً في أفريقيا جنوب الصحراء الكبرى – بما يوضح على نحو محتمل قوة برنامج مكافحة السل في ملاوي. من المحتمل أن يؤكد الارتفاع النسبي لانتشار هذه المقاومة التي لوحظت بين الأشخاص الذين فشل علاجهم في السابق على الحاجة للتغيير في السياسة الوطنية من أجل تكرار علاج هذه الفئة الفرعية من الأشخاص المصابين بالسل.
摘要
目的
记录马拉维肺结核新诊以及复治人群多耐药性流行率。
方法
我们针对2010年和2011年之间痰涂片阳性肺结核患者进行了具有全国代表性的调查。对于所有的参与者,我们都收集了人口和临床数据、两份唾液样本并进行艾滋病毒(HIV)检测。这些样本在马拉维隆圭中央参考实验室接受耐药性检测。在南非超国家结核病参考实验室对发现具有多耐药性的所有分枝杆菌肺结核分离菌再次进行一线药物的耐药性检测,然后进行二线药物耐药性检测。
结果
总的来说,在2120名痰涂片阳性肺结核患者中,从1777名(83.8%)患者中分离出肺结核分枝杆菌。从新患者的1196个分离菌中确定了5个(0.4%)有多耐药性,从复治患者的581个分离菌中确定了28个(4.8%)有多耐药性。在曾经治疗失败的复治病例中获得的31个分离菌中,9个(29.0%)显示出多耐药性。尽管发现二线药物耐药性,但未发现广泛耐药性的肺结核病例。对获得肺结核分枝杆菌分离菌人群的艾滋病毒检测显示,新患者中有577例(48.2%)为阳性,复治患者中有386例(66.4%)为阳性。
结论
撒哈拉以南非洲痰涂片阳性肺结核患者多耐药性的流行率较低——可能反映了马拉维的肺结核病控制规划的效力。在先前治疗失败的人群中观察这种耐药性的流行率相对较高,这可能凸显了对这个肺结核患者子群的全国性复治政策作出改变的需求。
Резюме
Цель
Задокументировать распространенность множественной лекарственной устойчивости при первичном и повторном лечении больных туберкулезом в Малави.
Методы
В 2010-2011 гг. было проведено национальное репрезентативное исследование больных туберкулезом легких с бактериовыделением. У всех согласившихся принять участие в исследовании были собраны демографические и клинические данные и взяты два образца мокроты; кроме того, они прошли тестирование на вирус иммунодефицита человека (ВИЧ). Лекарственная устойчивость полученных образцов была проверена в Центральной референс-лаборатории г. Лилонгве, Малави. Все изоляты микобактерий туберкулеза с выявленной множественной лекарственной устойчивостью были подвергнуты дополнительному тестированию на устойчивость к лекарственным препаратам первой и второй линии в наднациональной туберкулезной референс-лаборатории в Южной Африке.
Результаты
В итоге, наличие микобактерий туберкулеза было выявлено у 1777 (83,8%) из 2120 больных туберкулезом легких с бактериовыделением. Множественная лекарственная устойчивость была обнаружена у пяти (0,4%) из 1196 изолятов, взятых у лиц, получавших первичное лечение туберкулеза, и у 28 (4,8%) из 581 изолятов, взятых у лиц, получавших повторное лечение. Из изолятов, взятых у лиц, получавших повторное лечение после неудачного первичного, множественная лекарственная устойчивость была выявлена в девяти (29%) случаях из 31. Притом, что устойчивость к лекарствам второй линии была обнаружена, случаев туберкулеза с широкой лекарственной устойчивостью выявлено не было. Тестирование на ВИЧ лиц, у которых были выделены изоляты микобактерий туберкулеза, показало наличие вируса у 577 (48,2%) больных, у которых туберкулез был выявлен впервые, и у 386 (66,4%) больных, получавших повторное лечение.
Вывод
Распространенность лекарственной устойчивости среди больных туберкулезом легких с бактериовыделением в странах Африки южнее Сахары невелика, что, по-видимому, свидетельствует об эффективности противотуберкулезной программы Малави. В то же время, довольно высокая распространенность таких случаев среди лиц, лечение которых в прошлом не принесло результата, может указывать на необходимость изменения национального подхода к повторному лечению этой подкатегории больных туберкулезом.
Introduction
Although the World Health Organization (WHO) has monitored the emergence of drug resistance of Mycobacterium tuberculosis since 1994,1 there have been few national surveys of such resistance in sub-Saharan Africa.2
In 2012, it was estimated that about 1.9% of people newly diagnosed and 9.4% of those undergoing retreatment in Africa had multidrug-resistant (MDR) tuberculosis.3 The prevalence of MDR tuberculosis in Africa varies between countries4 and might be generally increasing.3,5
Over several years, attempts have been made – at the Central Reference Laboratory in Lilongwe – to isolate M. tuberculosis from all smear-positive patients undergoing retreatment in Malawi to investigate drug susceptibility. In 2008, about 8% of people investigated in this manner were found to have MDR tuberculosis (James Mpunga, Malawi National Tuberculosis Control Programme, personal communication, 2008) – although most of the samples came from urban centres and the laboratory’s attempts to isolate M. tuberculosisM. tuberculosis often failed.6 The only published data on MDR tuberculosis in Malawi indicated that just 0.5% of people newly diagnosed with tuberculosis and 0.9% of people being retreated in Karonga district had MDR tuberculosis in 1996–1998.7
In 2007, the nationally recommended treatment regimen for people newly diagnosed with tuberculosis in Malawi changed. The initial supervised treatment remained the same – i.e. daily isoniazid, rifampicin, pyrazinamide and ethambutol for 2 months – but the unsupervised continuation phase changed from 6 months of isoniazid and ethambutol to 4 months of isoniazid and rifampicin.8,9 There are four problems since this change that need monitoring. The first is that poor adherence during this currently-recommended continuation phase could lead to the emergence of MDR tuberculosis. Another problem is that nothing is known about the resistance of Malawian isolates of M. tuberculosis to the second-line drugs that began to be used routinely in Malawi in 2007. A third problem is the high prevalence of human immunodeficiency virus (HIV) infection among people with tuberculosis.10 In 2010, 63% of Malawian tuberculosis patients tested for HIV were found positive.4 Finally, the national prevalence of drug-resistant tuberculosis may be affected by migration of people from neighbouring countries, where such outbreaks have occurred.11 Given these issues, we conducted a national survey of resistance to anti-tuberculosis drugs in Malawi.
Methods
Study setting and design
We engaged all of Malawi’s 48 tuberculosis registration centres to conduct a prospective, cross-sectional survey. The centres were grouped into three zones – northern, central and southern – for phased sample collection.
Data collection and management
Health workers in each registration centre formed a recruitment team and attended a three-day training course about the survey protocol. They subsequently collected data on each consenting smear-positive tuberculosis patient, including the patient’s age, sex, level of education, occupation, marital status and HIV status – if known – and details of any previous tuberculosis treatment. After each patient was asked if they had received tuberculosis treatment, the patient’s medical records at the health facility of recruitment were checked for evidence of such treatment.
Following national policy in Malawi,8 each participant in the survey was offered HIV testing and counselling. At the time of the survey, two rapid blood tests – Uni-Gold Recombigen HIV-1/2 (Trinity Biotech, Bray, Ireland) and Determine HIV-1/2 (Alere, Waltham, United States of America) were used in the registration centres. Any samples giving inconclusive results were sent to the Central Reference Laboratory for retesting.
Data were collected on piloted forms and double-entered into an Epi Info (Centers for Disease Control and Prevention, Atlanta, United States of America) spreadsheet.
Participants and case definitions
Using the definitions recommended by WHO,12 new cases were defined as people who had never been treated for tuberculosis – or had previously received anti-tuberculosis medications for less than one month – and retreatment cases were defined as those who had previously received tuberculosis treatment for at least one month. Retreatment cases were grouped according to the outcome of previous treatment: cured, completed, defaulted or failed. A patient was defined as cured when the person was smear-negative at, or one month before, treatment completion and on at least one previous occasion. A completed treatment was defined as a patient who completed treatment but without smear microscopy proof of cure. Persons who had treatment interruption for two consecutive months or more were grouped as defaulted. Those who remained smear-positive when tested five or six months after initiation of their previous treatment were defined as treatment failures.
For our survey, sputum samples were collected from each newly-diagnosed person with sputum-smear-positive tuberculosis seen at a registration centre in the northern, central and southern zones in May–July 2010, August–October 2010 and November 2010–January 2011, respectively. Sputum samples were also collected from each person with smear-positive tuberculosis undergoing retreatment at any registration centre between February 2010 and March 2011.
Drug resistance definition
Isolates of M. tuberculosis were defined as MDR if they were at least resistant to isoniazid and rifampicin, and extremely drug resistant (XDR) if they were also resistant to an injectable drug and a quinolone of the second-line medications.
Sample size projections
Assuming that 1.8% and 20% of the people newly diagnosed would have MDR tuberculosis and be lost to follow-up, respectively, we estimated that we needed to enrol 1260 new cases to estimate the prevalence of MDR tuberculosis among such cases with a precision of ± 1%. Similarly, assuming that 5.0% and 20% of our retreatment sample would have MDR tuberculosis and be lost to follow-up, respectively, we estimated that we would have to enrol 770 people undergoing retreatment to estimate the prevalence of MDR tuberculosis with a precision of ± 2.0%.
Laboratory procedures
Prior to enrolment, each participant had been found positive for tuberculosis by the microscopic examination of three smears of sputum.12,13 Each month, a random selection of sputum smears from the registration centres – five from each health centre and 25 from each district hospital – was re-examined by a visiting laboratory supervisor. Concordance between the registration centres’ results and the supervisor’s remained above 96% during our survey.
For our survey, two additional sputum samples were collected – under supervision and approximately one hour apart – from each enrolled patient and stored at 2–8 °C in the registration centre. Efforts were made to ensure that these samples were collected before anti-tuberculosis treatment was commenced. The samples were transported to the Central Reference Laboratory, in cooler boxes, by bus or in a district health vehicle or study team vehicle.
Once a sample had reached the laboratory, it was decontaminated and further homogenized.14 Part of the pellet produced by centrifuging the sample was smeared, stained with auramine phenol stain and then checked for acid-fast bacilli. Another part was inoculated into two tubes of Lowenstein–Jensen medium – one containing glycerol and the other containing sodium pyruvate – which were examined for growth weekly for up to 8 weeks. Each contaminated culture was discarded and replaced with a new culture that was set up using another part of the relevant pellet – which had been kept in a refrigerator. The Capilia tuberculosis test15 was used to identify isolates belonging to the M. tuberculosis complex. Indirect susceptibility testing to isoniazid, rifampicin, ethambutol and streptomycin was performed, on one isolate per participant, using the proportion method on Lowenstein–Jenson medium.16
All isolates defined as MDR tuberculosis were sent to the South African Medical Research Council’s Supranational Reference Laboratory in Pretoria. There, they were retested for their susceptibility to first-line drugs – using a line probe assay and automated liquid culture17,18 – and tested for their susceptibility to the second-line drugs amikacin, kanamycin, capreomycin, ofloxacin and ethionamide – using automated liquid culture.
Statistical analysis
For our final analysis, we excluded those cases from which M. tuberculosis was not isolated in culture. Categorical and non-parametric continuous variables were compared using χ2 and Wilcoxon rank-sum tests, respectively. Data on new tuberculosis cases were analysed independently from retreatment cases. Associations between MDR tuberculosis and patient age, sex, HIV status, year of previous tuberculosis treatment and outcome of previous tuberculosis treatment were compared using Poisson logistic regression analysis. Unadjusted and adjusted incidence rate ratios (IRRs) were calculated in univariate and multivariate analyses, respectively. Stata 10.0 (StataCorp. LP, College Station, United States of America) was used for the statistical analysis.
Ethical considerations
Ethical approval was granted by the Malawi National Health Sciences Research Committee in April 2009. This study commenced in 2009, before requirements for review of all WHO-supported research by the WHO research ethics review committee were fully implemented. Written informed consent was obtained from adult participants and the caregivers of child participants. As recommended by the relevant national guidelines,8 all cases of MDR tuberculosis were given six months of capreomycin, levofloxacin, ethionamide, cycloserine and pyrazinamide followed by 18 months of levofloxacin, ethionamide and cycloserine.
Results
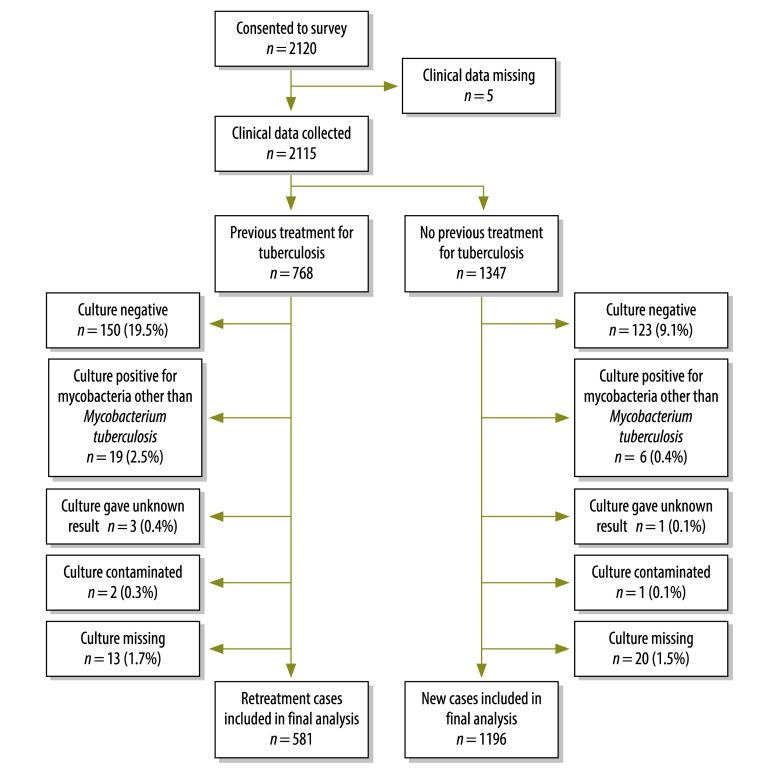
During the study period, 2120 smear-positive individuals consented to participate. Five were excluded as their baseline data were missing, another 1347 were classified as newly diagnosed with tuberculosis and the remaining 768 were classified as retreatment cases (Fig. 1). M. tuberculosis was isolated from 1196 (88.8%) of the new cases. There was no difference in the distribution of age, sex, region or HIV status between these and new cases from which M. tuberculosis was not isolated. M. tuberculosis was isolated from 581 (75.7%) of people undergoing retreatment. Those in whom M. tuberculosis was not isolated were older than the other retreatment cases, with mean ages of 40.7 and 36.4 years, respectively.
Fig. 1.
Flowchart to determine multidrug resistance in people diagnosed with tuberculosis in Malawi, 2010–2011
Compared with the new cases, people undergoing retreatment were more frequently found to be culture-negative or to be culture-positive for mycobacteria other than M. tuberculosis.
Of 86 treatment failures, 31 samples were culture-positive for M. tuberculosis, six were culture-positive for other mycobacteria and 49 were culture-negative.
The median transit time of all samples, from collection to arrival at the Central Reference Laboratory was 4 days (interquartile range, IQR: 2–7 days). Transit time had no apparent effect on the probability that a sample would be found culture-positive for M. tuberculosis (P = 0.71).
Culture-positive tuberculosis
Culture-positive individuals in both new and retreatment groups were similar in terms of their sociodemographic characteristics (Table 1).
Table 1. Characteristics of people newly-diagnosed with, and retreated for, tuberculosis, Malawi, 2010–2011.
| Characteristic | New cases (n = 1196) |
Retreatment cases (n = 581) |
|||
|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | ||
| Mean age (years) | 1196 | 35.6 (34.8–36.4) | 581 | 36.4 (35.5–37.4) | |
| Sex (% male) | 1196 | 53.7 (50.8–56.5) | 581 | 60.6 (56.6–64.6) | |
| Marital status (%) | |||||
| Married | 750 | 63.2 (60.5–66.0) | 346 | 60.3 (56.3–64.3) | |
| Single | 232 | 19.6 (17.3–21.8) | 106 | 18.5 (15.3–21.7) | |
| Divorced | 103 | 8.7 (7.1–10.3) | 70 | 12.2 (9.5–14.9) | |
| Widowed | 101 | 8.5 (6.9–10.1) | 52 | 9.1 (6.7–11.4) | |
| Occupation (%) | |||||
| Business | 226 | 19.5 (17.2–21.8) | 120 | 21.1 (17.8–24.5) | |
| Formal employment | 195 | 16.8 (14.7–19.0) | 126 | 22.2 (18.8–25.6) | |
| Subsistence farmer | 330 | 28.4 (25.8–31.0) | 148 | 26.1 (22.4–29.7) | |
| Unemployed | 409 | 35.3 (32.5–38.0) | 174 | 30.6 (26.8–34.4) | |
| Educational level achieved (%) | |||||
| Tertiary | 23 | 2.0 (1.2–2.8) | 13 | 2.3 (1.0–3.5) | |
| Secondary | 262 | 22.5 (20.1–24.9) | 171 | 29.9 (26.1–33.7) | |
| Primary | 729 | 62.6 (59.8–65.4) | 319 | 55.8 (51.7–59.9) | |
| None | 151 | 13.0 (11.0–14.9) | 69 | 12.1 (9.4–14.7) | |
| HIV status (%) | |||||
| Positive | 577 | 48.2 (45.4–51.1) | 386 | 66.4 (62.6–70.3) | |
| Negative | 474 | 39.6 (36.9–42.4) | 165 | 28.4 (24.7–32.1) | |
| Unknown | 145 | 12.1 (9.4–14.8) | 30 | 5.2 (2.6–7.7) | |
| Region of residence (%) | |||||
| Northern | 115 | 9.6 (7.9–11.3) | 85 | 14.6 (11.8–17.5) | |
| Central west | 283 | 23.7 (21.3–26.1) | 108 | 18.6 (15.4–21.8) | |
| Central east | 135 | 11.3 (9.5–13.1) | 46 | 7.9 (5.7–10.1) | |
| South-west | 359 | 30.0 (27.4–32.6) | 207 | 35.6 (31.7–39.5) | |
| South-east | 304 | 25.4 (22.9–27.9) | 135 | 23.2 (19.8–26.7) | |
| Outcome of previous treatment (%) | |||||
| Cureda | NA | NA | 389 | 67.0 (63.1–70.8) | |
| Completedb | NA | NA | 104 | 17.9 (14.8–21.0) | |
| Defaultedc | NA | NA | 49 | 8.4 (6.2–10.7) | |
| Failedd | NA | NA | 31 | 5.3 (3.5–7.2) | |
| Unknown | NA | NA | 8 | 1.4 (0.4–2.3) | |
| Smear score (%)e | |||||
| Scanty | 101 | 8.6 (7.0–10.2) | 66 | 11.6 (8.9–14.2) | |
| 1+ | 135 | 11.5 (9.6–13.3) | 66 | 11.6 (8.9–14.2) | |
| 2+ | 295 | 25.0 (22.6–27.5) | 115 | 20.1 (16.8–23.4) | |
| 3+ | 647 | 54.9 (52.1–57.8) | 324 | 56.7 (52.7–60.8) | |
CI: confidence interval; HIV: human immunodeficiency virus; NA: not applicable.
a Cured defined as a smear-positive patient who was smear-negative at, or one month before, treatment completion and on at least one previous occasion.12
b Treatment completed defined as a patient who completed treatment but without smear microscopy proof of cure.12
c Defaulted defined as treatment interruption for two consecutive months or more.12
d Failed defined as remaining smear-positive when tested five or six months after initiation of previous treatment.12
e Smear scores indicate the density of acid-fast bacilli seen on a sputum smear.13
Note: Data are missing for some characteristics. The sum of the percentages for some characteristics may not equal 100 due to rounding.
Overall, 66.4% (386) of the retreatment cases and 48.2% (577) of the new cases were known or found to be infected with HIV, demonstrating a significantly higher HIV prevalence among people retreated (P < 0.01). The retreatment cases reported that they had received tuberculosis treatment between 1978 and 2010 with a median of 2.4 years (IQR: 1.1–5.9 years) before their enrolment. Just 31 (5.3%) of the culture-positive retreatment cases had failed their previous treatment (Table 1).
Among the 1196 M. tuberculosis isolates from new cases, ethambutol, isoniazid, rifampicin and streptomycin resistance was present in 0.5%, 3.2%, 0.8% and 4.2%, respectively (Table 2). The corresponding values for the 581 isolates from the retreatment cases were all higher (Table 2).
Table 2. Resistance to first-line anti-tuberculosis drugs among Mycobacterium tuberculosis isolates, Malawi, 2010–2011.
| Resistance | Isolates from new cases (n = 1196) |
Isolates from retreatment cases (n = 581) |
|||
|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | ||
| Fully sensitive | 1116 | 93.3 (91.7–94.7) | 470 | 80.9 (77.5–84.0) | |
| Any resistancea | |||||
| R | 9 | 0.8 (0.4–1.4) | 38 | 6.5 (4.7–8.9) | |
| H | 38 | 3.2 (2.3–4.3) | 66 | 11.4 (8.9–14.2) | |
| E | 6 | 0.5 (0.2–1.1) | 18 | 3.1 (1.9–4.9) | |
| S | 50 | 4.2 (3.1–5.5) | 49 | 8.4 (6.3–11.0) | |
| Multidrug resistance | 5 | 0.4 (0.1–1.0) | 28 | 4.8 (3.2–6.9) | |
| RH | 2 | 0.2 (0.0–0.6) | 13 | 2.2 (1.2–3.8) | |
| RHE | 0 | 0.0 (0.0–0.3) | 1 | 0.2 (0.0–1.0) | |
| RHS | 1 | 0.1 (0.0–0.5) | 6 | 1.0 (0.4–2.2) | |
| RHES | 2 | 0.2 (0.0–0.6) | 8 | 1.4 (0.6–2.7) | |
| Other forms of resistance | 75 | 6.3 (5.0–7.8) | 83 | 14.3 (11.5–17.4) | |
| R only | 3 | 0.3 (0.1–0.7) | 9 | 1.5 (0.7–2.9) | |
| H only | 22 | 1.8 (1.2–2.8) | 32 | 5.5 (3.8–7.7) | |
| E only | 2 | 0.2 (0.0–0.6) | 4 | 0.7 (0.2–1.8) | |
| S only | 35 | 2.9 (2.1–4.1) | 30 | 5.2 (3.5–7.3) | |
| RS | 1 | 0.1 (0.0–0.5) | 0 | 0.0 (0.0–0.6) | |
| RE | 0 | 0.0 (0.0–0.3) | 1 | 0.2 (0.0–1.0) | |
| HE | 1 | 0.1 (0.0–0.5) | 2 | 0.3 (0.0–1.2) | |
| HS | 10 | 0.8 (0.4–1.5) | 3 | 0.5 (0.1–1.5) | |
| ES | 1 | 0.1 (0.0–0.5) | 1 | 0.2 (0.0–1.0) | |
| HES | 0 | 0.0 (0.0–0.3) | 1 | 0.2 (0.0–1.0) | |
CI: confidence interval; E: ethambutol; H: isoniazid; R: rifampicin; S: streptomycin.
a Any resistance indicates resistance to the anti-tuberculosis medication tested, independent of resistance results to the other medications.
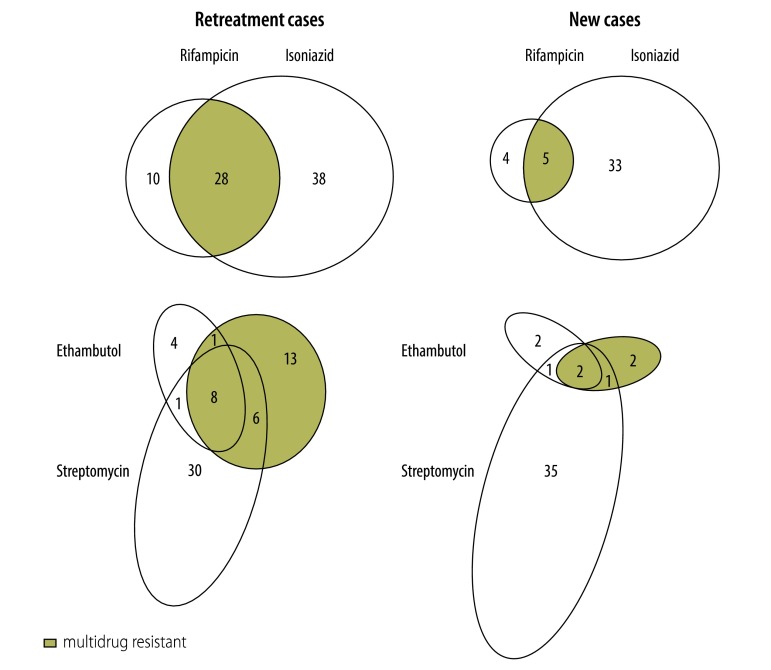
Five (0.4%) of the 1196 new cases had MDR tuberculosis (Table 2 and Fig. 2). Other types of resistance (mono-resistance or any combination of drug resistance excluding MDR tuberculosis) were identified in 75 (6.3%) of the new cases but the remaining 1116 (93.3%) M. tuberculosis isolates from new cases were found to be sensitive to all four first-line drugs.
Fig. 2.
Resistance patterns of Mycobacterium tuberculosis to anti-tuberculosis drugs, Malawi, 2010–2011
Twenty-eight (4.8%) of the 581 M. tuberculosis isolates from retreatment cases showed multidrug resistance (Table 2). Other types of resistance were identified in 83 (14.3%) of the retreatment cases but the remaining 470 (80.9%) M. tuberculosis isolates from retreatment cases were found to be sensitive to all four first-line drugs (Table 2 and Fig. 2).
In the multivariate analysis, sex, age and HIV status were not found to be significantly associated with MDR tuberculosis among new or retreatment cases. There was also no evidence of a significant association between region of residence and MDR tuberculosis. All of the 28 retreatment cases with MDR tuberculosis had received treatment in the previous five years – 23 (82%) within the previous two years. MDR tuberculosis in people undergoing retreatment was found to be significantly and inversely associated with time since previous treatment (adjusted IRR: 0.7, 95% confidence interval, CI: 0.5–0.9). Previous treatment failure – but no other previous treatment outcome – was strongly associated with MDR tuberculosis (adjusted IRR: 3.7, 95% CI: 1.6–8.4). Of the 31 treatment failures, nine (29.0%) cultured multi-drug resistant M. tuberculosis.
Of the 33 isolates of M. tuberculosis found to be multidrug-resistant in Malawi, 30 successfully underwent retesting in South Africa, and 11 of these were sensitive to either isoniazid or rifampicin or both of these drugs. If the results from South Africa are used as the gold standard, this indicates a 36.7% false-positive rate (11/30 in Table 3). When a random sample of 106 isolates of M. tuberculosis found not to be multidrug-resistant in Malawi were retested in South Africa, one was identified as MDR tuberculosis – giving a 0.9% false-negative rate (1/106 in Table 3).
Table 3. Comparison of anti-tuberculosis drug susceptibility testing of Malawian Mycobacterium tuberculosis isolates, 2010–2011.
| Malawian result | South African resulta |
|||
|---|---|---|---|---|
| Sensitive to all drugs | Resistant to rifampicin only | Resistant to isoniazid only | MDR | |
| Sensitive to all drugs | 87 | 1 | 2 | 0 |
| Resistant to rifampicin only | 2 | 0 | 0 | 0 |
| Resistant to isoniazid only | 6 | 0 | 7 | 1 |
| MDR | 4 | 2 | 5 | 19 |
MDR: multidrug-resistant.
a The table shows the numbers of M. tuberculosis isolates, from Malawian cases of smear-positive pulmonary tuberculosis, that were tested for resistance to isoniazid, rifampicin, ethambutol and streptomycin in both the Central Reference Laboratory (Lilongwe, Malawi) and the South African Medical Research Council’s Supranational Reference Laboratory (Pretoria, South Africa).
The 20 isolates found to show multidrug resistance in South Africa were re-cultured in South Africa and tested for resistance to several second-line drugs. Although 18 of these isolates were successfully re-cultured and tested, none showed extensive drug resistance (Table 4).
Table 4. Resistance to second-line anti-tuberculosis drugs among 18 multidrug-resistant Mycobacterium tuberculosis isolates, Malawi, 2010–2011.
| Isolate | Resistancea |
||||
|---|---|---|---|---|---|
| Amikacin | Kanamycin | Capreomycin | Ofloxacin | Ethionamide | |
| 1–14 | susceptible | susceptible | susceptible | susceptible | resistant |
| 15 | susceptible | resistant | susceptible | susceptible | resistant |
| 16 | susceptible | susceptible | resistant | susceptible | resistant |
| 17 | resistant | resistant | resistant | susceptible | resistant |
| 18 | resistant | susceptible | resistant | susceptible | resistant |
a Amikacin, kanamycin, capreomycin, ofloxacin and ethionamide were tested at concentrations up to 1.0, 5.0, 2.5, 2.0 and 5.0 µg/mL, respectively.
Discussion
This is the first national survey of anti-tuberculosis drug resistance done in Malawi. We found the prevalence of MDR tuberculosis among people newly diagnosed to be low, at 0.4%. As about 7200 new cases of smear-positive tuberculosis have occurred annually in Malawi over recent years,4 we can expect there to be 29 cases of primary MDR tuberculosis in Malawi annually. Although we found the prevalence of MDR tuberculosis among retreatment cases to be significantly higher, as generally observed,19 this could be expected to produce only 27 secondary cases of MDR tuberculosis annually.
The rates described here represent the lowest values reported in sub-Saharan Africa up to 2011.3 Neighbouring Mozambique identified multidrug resistance in 3.5% of new tuberculosis cases and 11.2% of retreatment cases in 2007. In 2009, Swaziland reported corresponding values of 7.7% and 33.9%, respectively.5 During our survey, the Central Reference Laboratory successfully isolated M. tuberculosis from the sputum samples from 88.8% of new cases and 75.7% of retreatment cases. Although the sample transit times recorded during our survey were disappointing, long transit times were not associated with isolation failures. Mycobacteria could not be grown from 49 of 86 samples from treatment failures, probably because the bacilli in the 49 samples were dead. Mycobacteria other than M. tuberculosis were cultured from six treatment failures. The proportion of sputum samples from retreatment cases that were found culture-positive for M. tuberculosis was significantly lower than the corresponding value for the new cases. This difference is partly explained by (i) the low isolation rate from treatment failures; (ii) the fact that samples from retreatment cases were relatively more likely to grow mycobacteria other than M. tuberculosis; and (iii) the fact that sputum samples from retreatment cases are relatively more likely to be collected from patients who have already begun treatment for their current episode of tuberculosis.
Since the results recorded by Malawi’s Central Reference Laboratory were associated with a 36.7% false-positivity rate and a 0.9% false-negativity rate, the prevalences of MDR tuberculosis that we recorded in Malawi – although low – could overestimate the true values. Given the laboratory’s limited capacity and the observation that resistance patterns probably do not vary between smear-positive and smear-negative cases of tuberculosis,20 we did not investigate the drug resistance of any M. tuberculosis isolates from smear-negative tuberculosis patients.
We found HIV prevalence among new smear-positive cases of pulmonary tuberculosis to be 48.2%. The HIV prevalences reported among all tuberculosis cases by Malawi’s National Tuberculosis Programme in 2010 and 2011 were higher, at 63% and 60%, respectively.21 The programme’s observations indicate that HIV prevalence among smear-negative cases of pulmonary tuberculosis exceeded 65% in 2010–2011. By focusing on smear-positive cases, we probably limited the extent to which we could explore associations between HIV and MDR tuberculosis. Although we found no association between HIV and MDR tuberculosis, it is possible that such an association exists in the overall population of people with tuberculosis. The existence of such a link remains a matter of controversy3,5,22,23 but concomitant HIV infection certainly poses some unique challenges in the management of tuberculosis.10
Although we collected samples from different areas of Malawi at different times of the year, a retrospective analysis of new tuberculosis case notifications between 1999 and 2007 suggested that there was little variation in the number of new cases occurring in each quarter of the year (James Mpunga, Malawi National Tuberculosis Control Programme, personal communication, 2010).
The low prevalence of MDR tuberculosis that we recorded may be attributable to the success of Malawi’s tuberculosis control programme. The frequencies of success in the treatment of tuberculosis in Malawi – 88% for new cases and 85% for retreatment cases – are among the highest recorded in sub-Saharan Africa.4 We recorded higher prevalences of streptomycin resistance than of rifampicin or isoniazid resistance, perhaps because streptomycin was included in the recommended first-line treatment for tuberculosis in Malawi until 1992.
We detected no XDR tuberculosis but did observe some resistance to second-line drugs. Since all of our isolates tested for resistance to ethionamide were found positive, the currently recommended 24-month regimen for the treatment of MDR tuberculosis in Malawi needs to be revised. Resistance to the second-line injectables was detected but not resistance to ofloxacin. At the time of the survey, Malawi’s Central Reference Laboratory relied entirely upon the South African Supranational Reference Laboratory for the identification of Malawian cases of XDR tuberculosis.8
Treatment failure – frequently a forewarning for the development of drug-resistant tuberculosis24 – was associated with a 29.0% risk of MDR tuberculosis in our survey. The initiation of a standard retreatment regimen while awaiting the results of drug susceptibility testing may amplify resistance in cases with pre-existing MDR tuberculosis.25,26 Although use of an empirical MDR treatment regimen has been suggested as a replacement for the standard retreatment regimen for all treatment failures,24,27,28 such a change in Malawi would expose most treatment failures – i.e. those who do not have MDR tuberculosis – to a more toxic and less effective therapy. During our survey, all patients with MDR tuberculosis who were diagnosed by phenotypic testing at the Central Reference Laboratory – including the 11 cases classified as drug sensitive when their sputum samples were investigated in South Africa – were managed with the nationally recommended second-line regimen. This was because (i) the phenotypic results were seen as more predictive of clinical response; (ii) the South African results became available several months after the patients had started second-line therapy; and (iii) it was felt that any changes to treatment made after the South African results became available would be confusing to patients.
Conclusion
The prevalence of MDR tuberculosis is currently low in Malawi – probably as the result of a strong tuberculosis control programme – whereas HIV-coinfection, which has been associated with high mortality in the presence of drug-resistant tuberculosis, is common. Almost a third of the treatment failures we investigated had MDR tuberculosis. Given the discovery of ethionamide resistance in all 18 of the MDR tuberculosis isolates investigated for such resistance, ethionamide should be replaced with an alternative drug in Malawi’s current MDR tuberculosis treatment regimen. Given an increasing prevalence of drug resistance in some neighbouring countries and the recent introduction of unsupervised rifampicin into tuberculosis treatment regimens in Malawi, we recommend repeating this survey within three years.
Acknowledgements
We thank colleagues in Malawi’s National Tuberculosis Control Programme and Central Reference Laboratory. We also thank the MDR tuberculosis Technical Advisory Group, the recruitment teams and tuberculosis patients.
Funding:
Supported by the Tuberculosis Control Assistance Programme (the United States Agency for International Development), under the terms of agreement GHS-A-00–05–00019–00.
Competing interests:
None declared.
References
- 1.Pablos-Méndez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998;338(23):1641–9. [DOI] [PubMed] [Google Scholar]
- 2.Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: World Health Organization; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf [cited 2014 Aug 26].
- 3.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ. 2012;90(2):111–119D. 10.2471/BLT.11.092585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global tuberculosis control 2011. Geneva: World Health Organization; 2011. Available from: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf [cited 2014 Aug 26].
- 5.Sanchez-Padilla E, Dlamini T, Ascorra A, Rüsch-Gerdes S, Tefera ZD, Calain P, et al. High prevalence of multidrug-resistant tuberculosis, Swaziland, 2009–2010. Emerg Infect Dis. 2012;18(1):29–37. 10.3201/eid1801.110850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacombe RJ, Samuti G, Dambe I, Mundy C, Suarez PG, Squire SB, et al. Addressing challenges in preparing the TB Central Reference Laboratory, Malawi, for a national drug resistance survey. In: 41st Union World Conference on Lung Health; 2010 Nov 11–15; Berlin, Germany. Paris: The International Union Against Tuberculosis and Lung Disease; 2010. [Google Scholar]
- 7.Warndorff DK, Yates M, Ngwira B, Chagaluka S, Jenkins PA, Drobniewski F, et al. Trends in antituberculosis drug resistance in Karonga District, Malawi, 1986–1998. Int J Tuberc Lung Dis. 2000;4(8):752–7. [PubMed] [Google Scholar]
- 8.Malawi National TB Control Programme. Manual of the National Tuberculosis Control Programme in Malawi. Lilongwe: Ministry of Health and Population; 2007. [Google Scholar]
- 9.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364(9441):1244–51. 10.1016/S0140-6736(04)17141-9 [DOI] [PubMed] [Google Scholar]
- 10.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(s1) Suppl 1:S86–107. 10.1086/518665 [DOI] [PubMed] [Google Scholar]
- 11.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–80. 10.1016/S0140-6736(06)69573-1 [DOI] [PubMed] [Google Scholar]
- 12.Treatment of tuberculosis guidelines. 4th ed. Geneva: World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf?ua=1 [cited 2014 Aug 26].
- 13.Technical guide. Sputum examination for tuberculosis by direct microscopy in low income countries. 5th ed. Paris: International Union Against Tuberculosis and Lung Disease; 2000. Available from: http://www.uphs.upenn.edu/bugdrug/antibiotic_manual/IUATLD_afb%20microscopy_guide.pdf [cited 2014 Aug 26].
- 14.De Kantor NI, Kim SJ, Frieden T, Laszlo A, Luelmo F, Norval P, et al. Laboratory services in tuberculosis control. WHO/TB/98.258. Geneva: World Health Organization; 1998. [Google Scholar]
- 15.Abe C, Hirano K, Tomiyama T. Simple and rapid identification of the Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodies. J Clin Microbiol. 1999;37(11):3693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent PT, Kubica GP. Public health mycobacteriology. A guide for the level III laboratory. Atlanta: Centers for Disease Control and Prevention; 1985. [Google Scholar]
- 17.Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5(1):62. 10.1186/1471-2334-5-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rüsch-Gerdes S, Domehl C, Nardi G, Gismondo MR, Welscher HM, Pfyffer GE. Multicenter evaluation of the mycobacteria growth indicator tube for testing susceptibility of Mycobacterium tuberculosis to first-line drugs. J Clin Microbiol. 1999;37(1):45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlali RE, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5(10):887–93. [PubMed] [Google Scholar]
- 20.Guidelines for surveillance of drug resistance in tuberculosis. 4th ed. Geneva: World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241598675_eng.pdf [cited 2014 Aug 26].
- 21.Global Tuberculosis Report 2013. WHO/HTM/TB/2013.11. Geneva: World Health Organization; 2013. Available from http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf [cited 2014 Aug 26]. [Google Scholar]
- 22.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS ONE. 2009;4(5):e5561. 10.1371/journal.pone.0005561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean AS, Zignol M, Falzon D, Getahun H, Floyd K. HIV and multidrug-resistant tuberculosis: overlapping epidemics. Eur Respir J. 2014;44(1):251–4. 10.1183/09031936.00205413 [DOI] [PubMed] [Google Scholar]
- 24.Andrews JR, Shah NS, Weissman D, Moll AP, Friedland G, Gandhi NR. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS ONE. 2010;5(12):e15735. 10.1371/journal.pone.0015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox HS, Niemann S, Ismailov G, Doshetov D, Orozco JD, Blok L, et al. Risk of acquired drug resistance during short-course directly observed treatment of tuberculosis in an area with high levels of drug resistance. Clin Infect Dis. 2007;44(11):1421–7. 10.1086/517536 [DOI] [PubMed] [Google Scholar]
- 26.Matthys F, Rigouts L, Sizaire V, Vezhnina N, Lecoq M, Golubeva V, et al. Outcomes after chemotherapy with WHO category II regimen in a population with high prevalence of drug resistant tuberculosis. PLoS ONE. 2009;4(11):e7954. 10.1371/journal.pone.0007954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinal MA. Time to abandon the standard retreatment regimen with first-line drugs for failures of standard treatment. Int J Tuberc Lung Dis. 2003;7(7):607–8. [PubMed] [Google Scholar]
- 28.Tabarsi P, Chitsaz E, Tabatabaei V, Baghaei P, Shamaei M, Farnia P, et al. Revised Category II regimen as an alternative strategy for retreatment of Category I regimen failure and irregular treatment cases. Am J Ther. 2011;18(5):343–9. 10.1097/MJT.0b013e3181dd60ec [DOI] [PubMed] [Google Scholar]