Abstract
The prevalence of type 2 diabetes mellitus (T2DM) is rising in Australia. Sodium glucose co-transporter 2 (SGLT2) inhibitors are an emerging treatment for T2DM. SGLT2 inhibitors offer a novel approach to lowering hyperglycaemia by suppressing renal glucose reabsorption and increasing urinary glucose excretion. The increased urinary glucose excretion has also been associated with caloric loss and osmotic diuresis. Dapagliflozin and canagliflozin are the SGLT2 inhibitors that are approved for clinical use in the US, the European Union (EU), and Australia. Their use results in reductions in HbA1c and body weight across a broad range of patient populations ranging from drug-naive patients to those who require additional therapy due to inadequate glycaemic control on their existing treatment. In addition, reductions in blood pressure (BP), particularly systolic BP, have also been noted. SGLT2 inhibitors are generally well tolerated with low rates of adverse events. Episodes of hypoglycaemia were mostly classified as minor, with low and balanced rates of severe hypoglycaemia across studies. The proportions of patients with genital infections and urinary tract infections were higher with dapagliflozin and canagliflozin versus their comparators. However, these infections were generally mild-to-moderate in intensity, treated with standard antimicrobial therapies, and rarely led to discontinuation. No dosage adjustments for dapagliflozin and canagliflozin are recommended for normal-to-mild renal impairment. Dapagliflozin and canagliflozin are not recommended for use in patients with eGFR<60 and <45mL/min/1.73m2, respectively. Overall, SGLT2 inhibitors have shown the potential to become an important addition to the treatment armamentarium for effective management of patients with T2DM.
Keywords: Type 2 diabetes, SGLT2 inhibitors, management, renal
What this review adds:
-
What is known about this subject?
The prevalence of type 2 diabetes (T2DM) is rising in Australia and there is an increasing need for new glucose-lowering drugs with benefits beyond glucose control.
-
What new information is offered in this review?
Data available from clinical trials of dapagliflozin and canagliflozin have shown the efficacy and safety of these drugs across a spectrum of patients with T2DM.
-
What are the implications for research, policy, or practice?
With the approval of dapagliflozin and canagliflozin in Europe, the US, and Australia, these drugs have the potential to become an important treatment option for effective and well-tolerated management of diabetes.
Background
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease associated with considerable morbidity and premature mortality. The prevalence of T2DM is rising worldwide; 382 million people were living with diabetes in 2013, and the incidence is expected to increase to 592 million by 2035.1 About one-fifth of adults with diabetes live in Southeast Asia, accounting for 72.1 million people with the disease. This number is expected to increase to 123 million by 2035. The prevalence of diabetes in Australia has more than doubled from 1.5 per cent in 1989 to 4.1 per cent in 2008, as reported in a National Health Survey.2
Many existing glucose-lowering therapies have limitations, including insulin-dependent mechanisms of action, which means that these agents lose efficacy over time as both endogenous insulin secretion and insulin sensitivity decrease.3 Furthermore, a number of glucose-lowering drugs are associated with side effects such as hypoglycaemia and weight gain.4 Weight gain and hypertension are of particular significance given the high prevalence of obesity and cardiovascular diseases in patients with T2DM.5,6 Therefore, there is an increasing interest in new glucose-lowering drugs that act independently of insulin, have benefits beyond glucose-lowering actions, and provide improved tolerability compared with traditional medications for T2DM.
Glucose control by the kidney and SGLT2 inhibition as an emerging target in diabetes
The kidneys play a key role in glucose homeostasis. They generate glucose through gluconeogenesis, use glucose as an energy substrate, and reabsorb filtered glucose in the proximal renal tubules. However, until very recently, the kidney was not widely considered as a potential target for therapeutic intervention in the management of diabetes. Approximately 180g of glucose per day is filtered by the kidneys.7 Almost all of the filtered glucose is reabsorbed by the sodium glucose co-transporter 2 (SGLT2) in healthy individuals.
SGLT2 is selectively expressed in the kidney whereas sodium glucose co-transporter 1 (SGLT1) is highly expressed in the gastrointestinal tract, where it is responsible for both glucose and galactose reabsorption, and in the liver, lung, and kidney. SGLT2 is a low-affinity, high-capacity transporter located in the S1 segment of the proximal renal tubule, which reabsorbs 90 per cent of the filtered glucose.8 The remaining 10 per cent is reabsorbed through the high affinity/low capacity SGLT1 transporter in the S3 segment of the proximal tubule leaving minimal or no glucose in the urine of healthy individuals (Figure 1). This state of minimal or no glucosuria is maintained in healthy individuals until the renal threshold of 11mmol/L is exceeded.9 However, in T2DM, expression of the SGLT2 transporter is up-regulated and the renal threshold is increased, meaning that glucosuria is not observed in patients until the plasma glucose levels increase to substantially greater than 11mmol/L.9 In this way, SGLT2 transport works counterproductively to maintain raised plasma glucose levels in T2DM. Inhibition of SGLT2 transport by therapeutic intervention in such patients resets the system by lowering the reabsorption threshold for urinary glucose, thereby leading to correction of hyperglycaemia.
Figure 1. Mechanism of action of SGLT2 inhibitors in the treatment of T2DM*.
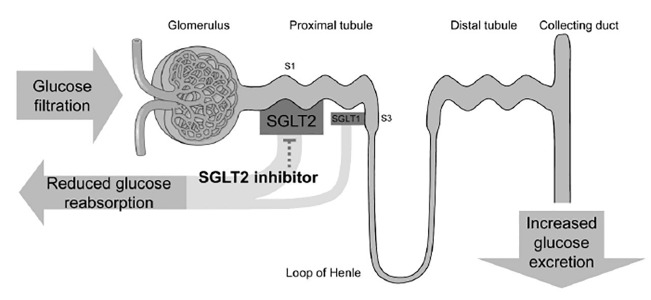
SGLT2=sodium glucose co-transporter 2; T2DM=type 2 diabetes mellitus
* Adapted from Dapagliflozin Food and Drug Administration background document.47
Suppressing renal glucose reabsorption and effectively increasing urinary glucose excretion via SGLT2 inhibition is emerging as a novel insulin-independent mechanism to lower elevated plasma glucose levels in patients with T2DM. Given that SGLT2 is only expressed in the kidney, the effects of SGLT2 inhibitors are limited to glycosuria and associated salt and water losses. The long-term tolerability of glucosuria (albeit in the absence of hyperglycaemia) has been demonstrated in patients with familial renal glucosuria, a condition caused by genetic mutations in SGLT2. Such patients have reported normal kidney function with no increase in the incidence of diabetes, urinary tract infection (UTI), or renal impairment.10,11
SGLT2 inhibitors
Highly selective, orally available SGLT2 inhibitors, including dapagliflozin, canagliflozin, ipragliflozin, empagliflozin, and ertugliflozin have been developed and/or are currently undergoing clinical trials. Dapagliflozin and canagliflozin have now been approved for clinical use in patients with T2DM in the US, the European Union (EU), and Australia.12–18
SGLT2 inhibitors lower blood glucose by increasing urinary glucose excretion. The magnitude of this effect is dependent on the concentration of glucose in the blood and the glomerular filtration rate. As blood glucose levels decrease following initiation of treatment with SGLT2 inhibitors, the glucose load filtered by the kidney reduces and further urinary glucose excretion becomes limited. In this way, SGLT2 inhibitors can be viewed as having a self-regulating effect on blood glucose levels, which is reflected in a low intrinsic propensity to cause hypoglycaemia.19 In addition, glucosuria associated with SGLT2 inhibition has been associated with caloric elimination leading to weight loss, and osmotic diuresis and natriuresis leading to a reduction in blood pressure (BP; noted in the dapagliflozin and canagliflozin studies discussed in this review).20
Efficacy of SGLT2 inhibitors
This review focuses on the key findings from the two approved SGLT2 inhibitors: dapagliflozin and canagliflozin. In Australia, dapagliflozin 10mg is Therapeutic Goods Administration (TGA) approved for use as monotherapy, initial combination therapy with metformin, fixed dose combination with metformin extended-release, and add-on combination therapy with other anti-hyperglycaemic agents.13,14 It has been recommended by the Pharmaceuticals Benefits Scheme (PBS) as a dual oral combination therapy with metformin or a sulfonylurea.13,21
Canagliflozin 100 and 300mg doses have been TGA approved for use as monotherapy and add-on combination therapy with other anti-hyperglycaemic agents including insulin. Canagliflozin has also been recommended by the PBS for dual oral combination therapy with metformin or a sulfonylurea.12
Glycaemic efficacy of SGLT2 inhibitors
Dapagliflozin and canagliflozin have demonstrated efficacy in lowering glucose across a broad range of patient populations ranging from drug-naive patients at an early stage of T2DM, to those who require additional therapy due to inadequate glycaemic control on their existing oral antidiabetic drugs (OADs) or insulin therapy.
A head-to-head comparison of dapagliflozin with glipizide in patients with T2DM inadequately controlled on metformin demonstrated similar changes in HbA1c at 52 weeks (−0.52 vs. −0.52% for dapagliflozin vs. glipizide, respectively) (Table 1). Significant reductions in HbA1c were noted at 24 weeks when dapagliflozin 10mg was administered in patients inadequately controlled on metformin, glimepiride, or insulin ± OADs. Changes in HbA1c ranged from −0.82 to −0.96 per cent for dapagliflozin 10mg vs. −0.13 to −0.39 per cent for placebo; p<0.001 (Table 1).
Table 1. Changes in HbA1c (%) according to SGLT2 inhibitor therapy and patient population.
| Dapagliflozin studies | Canagliflozin studies | ||||
|---|---|---|---|---|---|
| DAPA 10mg | PBO | CANA 300mg | CANA 100mg | PBO | |
| Add-on to MET42,45 | |||||
| Study duration, weeks | 24 | 24 | 26 | 26 | 26 |
| n | 135 | 137 | 360 | 365 | 181 |
| Baseline, % | 7.92 | 8.11 | 7.95 | 7.94 | 7.96 |
| Adjusted Δ from BL, % | -0.84 | -0.30 | -0.94 | -0.79 | -0.17 |
| (95% CI) | (-0.98, -0.70) | (-0.44, -0.16) | (-0.91, -0.64)† | (-0.76, -0.48)† | |
| p-value vs placebo | <0.0001 | <0.0001 | <0.0001 | ||
| Add-on to SU*30,34 | |||||
| Study duration, weeks | 24 | 24 | 26 | 26 | 26 |
| n | 151 | 145 | 156 | 157 | 156 |
| Baseline, % | 8.07 | 8.15 | 8.1 | 8.1 | 8.1 |
| Adjusted Δ from BL, % | -0.82 | -0.13 | -1.06 | -0.85 | -0.13 |
| (95% CI) | (-0.86, -0.51)† | (-1.11, -0.73)† | (-0.90, -0.52)† | ||
| p-value vs placebo | <0.0001 | <0.001 | <0.001 | ||
| Add-on to INS +/- OADs23,35 | |||||
| Study duration, weeks | 24 | 24 | 18 | 18 | 18 |
| n | 194 | 193 | 587 | 566 | 565 |
| Baseline, % | 8.57 | 8.47 | 8.3 | 8.3 | 8.2 |
| Adjusted Δ from BL, % | -0.96 | -0.39 | -0.72 | -0.63 | 0.01 |
| (95% CI) | (-0.72, -0.42)† | (-0.81, -0.65)† | (-0.73, -0.56)† | ||
| p-value vs placebo | <0.001 | <0.001 | <0.001 | ||
| DAPA 10mg | GLIP (up to 20mg) | CANA 300mg | CANA 100mg | GLIM (up to 8mg) | |
| SGLT2 inhibitor vs SU29,33 | |||||
| Study duration, weeks | 52 | 52 | 52 | 52 | 52 |
| n | 400 | 401 | 485 | 483 | 482 |
| Baseline, % | 7.69 | 7.74 | 7.80 | 7.80 | 7.80 |
| Adjusted Δ from BL, % | -0.52 | -0.52 | -0.93 | -0.82 | -0.81 |
| (95% CI) | (-0.60, -0.44) | (-0.60, -0.44) | (-0.22, -0.02)† | (-0.11, 0.09)† | |
BL=baseline; CANA=canagliflozin; CI=confidence interval; DAPA=dapagliflozin; GLIM=glimepiride; GLIP=glipizide; INS=insulin; LOCF=last observation carried forward; MET=metformin; OADs=oral antidiabetic drugs; PBO=placebo; SGLT2=sodium glucose co-transporter 2; SU=sulphonylurea.
DAPA data are adjusted mean change from baseline derived from analysis of covariance and include last observation carried forward (LOCF) and exclude data after rescue medication was initiated except for the add-on to INS study that includes data after INS uptitration.
CANA data are least square mean change from baseline (LOCF) and last post-baseline value prior to the initiation of rescue therapy was included for patients who received rescue therapy.
Patients in the DAPA study were receiving SU monotherapy (GLIM 4mg) at baseline. Patients in the CANA study were receiving stable doses of SU+MET at baseline.
95% CI of difference vs PBO.
Results from the DAPA and CANA studies should not be directly compared due to their different study designs.
The reductions in HbA1c achieved at 24 weeks were maintained over 1–4 years in the head-to-head comparison of dapagliflozin with glipizide administered to patients inadequately controlled on metformin and in the dapagliflozin studies in patients inadequately controlled on metformin, sulphonylurea, or insulin.22–27 These HbA1c decreases maintained over time suggest that dapagliflozin may be useful for long-term glycaemic control.
Clinical trials have also provided encouraging data for the efficacy of canagliflozin 100 or 300mg in a range of clinical settings. A head-to-head comparison study of canagliflozin vs. glimepiride in patients with T2DM on background metformin showed non-inferiority of both canagliflozin doses to glimepiride in HbA1c lowering at 52 weeks (−0.82 and −0.93 vs. −0.81% for canagliflozin 100 and 300mg doses vs. comparator, respectively) (Table 1). This non-inferiority was maintained over a period of two years.28 Significant reductions in HbA1c were noted with canagliflozin 100 and 300mg doses vs. placebo in patients inadequately controlled on metformin, metformin plus sulphonylurea or insulin over 18–26 weeks. Changes in HbA1c ranged from −0.63 to −0.85 per cent for canagliflozin 100mg, −0.72 to −1.06 per cent for canagliflozin 300mg vs +0.01 to −0.17 per cent for placebo; p<0.001 (Table 1).
Weight loss with SGLT2 inhibitors
Reductions in body weight have been observed with SGLT2 inhibition. Dapagliflozin was associated with weight loss vs. weight gain with glipizide at 52 weeks in patients inadequately controlled on metformin (−3.2 vs. +1.4kg for dapagliflozin vs. glipizide, respectively; p<0.0001).29 Significant and clinically meaningful reductions in body weight were observed with dapagliflozin 10mg vs. placebo in patients inadequately controlled on metformin, glimepiride, or insulin ± OADs at 24 weeks. Changes in weight ranged from −1.6 to −2.9kg for dapagliflozin 10mg vs. +0.4 to −0.9kg for placebo; p<0.001). The reductions in body weight were maintained over the longer-term.24–26,30–32 The longest available data for an SGLT2 inhibitor to date demonstrate that dapagliflozin maintains weight loss over a period of 208 weeks.27
Likewise, reductions in body weight were also noted in the canagliflozin studies. Both canagliflozin 100 and 300mg doses produced significant body weight reductions vs. weight gain with glimepiride in patients with T2DM on background metformin at 52 weeks (−3.7 and −4.0 vs. +0.7kg for canagliflozin 100 and 300mg vs. comparator, respectively; p<0.001).33 These reductions were sustained up to 104 weeks.28 Significant dose-related decreases in body weight were noted with canagliflozin 100 and 300mg vs. placebo in patients inadequately controlled on metformin plus sulphonylurea34 (−1.9 and −2.5 vs. −0.8kg, respectively; p<0.001) at 26 weeks, and in patients inadequately controlled on insulin ± OADs35 (−1.8 and −2.3 vs. +0.1kg, respectively; p<0.001) at 18 weeks.
BP reduction with SGLT2 inhibitors
In addition to reductions in body weight, treatment with SGLT2 inhibitors across several studies was associated with decreases in BP, particularly systolic BP. Mean reductions from baseline in systolic BP of 3.6–6.7mmHg with dapagliflozin were noted across studies up to 52 weeks, with no notable increases in orthostatic hypotension. Sustained, although somewhat smaller, decreases in systolic BP were noted with dapagliflozin 10mg over 1–4 years.22,24–27,31
Likewise, reductions in systolic BP of 3.3–5.4 and 4.3–6.9mmHg with canagliflozin 100 and 300mg, respectively, were noted across studies up to 52 weeks. Sustained, but somewhat smaller, reductions in BP were noted at 104 weeks in the canagliflozin vs. glimepiride study.28
The reduction in BP is considered to be due to a decrease in intravascular volume associated with osmotic diuresis and natriuresis. The events of hypotension were, therefore, more common in patients particularly at risk of volume depletion, such as elderly patients, patients with renal impairment or those on loop diuretics. However, the proportion of patients with orthostatic hypotension was similar between patients receiving SGLT2 inhibitors and placebo/comparator.
Safety of SGLT2 inhibitors
For the studies discussed in this review, the safety analyses were conducted in all randomised patients who took at least one dose of study medication and data were summarized using descriptive statistics only; no statistical tests for differences between treatments were performed and no p-values were calculated.
SGLT2 inhibitors were well tolerated in clinical trials; adverse event (AE)-related discontinuations were low and balanced across groups (Table 2).
Table 2. Safety of SGLT2 inhibitors.
| n | ≥1 AE | ≥1 SAE | Disc for AEs | Hypoglycaemia | |||
|---|---|---|---|---|---|---|---|
| Overall | Major/Severe† | ||||||
| Add-on to MET45 | Study duration: 24 weeks | ||||||
| DAPA 10mg | 135 | 98 (73) | 4 (3) | 4 (3) | 5 (4) | 0 | |
| PBO | 137 | 88 (64) | 5 (4) | 5 (4) | 4 (3) | 0 | |
| Study duration: 26 week | |||||||
| CANA 300mg | NA | NA | NA | NA | NA | ||
| CANA 100mg | |||||||
| PBO | |||||||
| Add-on to SU*30,34,37 | Study duration: 24 weeks | ||||||
| DAPA 10mg | 151 | 76 (50.3) | 9 (6.0) | 4 (2.6) | 12 (7.9) | 0 | |
| PBO | 146 | 69 (47.3) | 7 (4.8) | 3 (2.1) | 7 (4.8) | ||
| Study duration: 26 weeks | |||||||
| CANA 300mg | 156 | 97 (62.2) | 6 (3.8) | 9 (5.8) | 30.1% | ≤1 | |
| CANA 100mg | 157 | 90 (57.3) | 5 (3.2) | 9 (5.7) | 26.8% | ≤1 | |
| PBO | 156 | 100 (64.1) | 9 (5.8) | 5 (3.2) | 15.4% | ≤1 | |
| Add-on to INS23,35 | Study duration: 48 weeks | ||||||
| DAPA 10mg | 196 | 145 (74.0) | 23 (11.7) | 10 (5.1) | 105 (53.6) | 3 (1.5) | |
| PBO | 197 | 144 (73.1) | 26 (13.2) | 9 (4.6) | 102 (51.8) | 2 (1.0) | |
| Study duration: 18 weeks | |||||||
| CANA 300mg | 587 | 382 (65.1) | 31 (5.3) | 32 (5.5) | 48.6% | 0.027 | |
| CANA 100mg | 566 | 362 (64.0) | 31 (5.5) | 11 (1.9) | 49.3% | 0.018 | |
| PBO | 565 | 334 (59.1) | 36 (6.4) | 12 (2.1) | 36.8% | 0.025 | |
| SGLT2 inhibitor vs SU29,33,46 | Study duration: 52 weeks | ||||||
| DAPA 10mg | 406 | 318 (78.3) | 35 (8.6) | 37 (9.1) | 14 (3.4) | 0 | |
| GLIP (up to 20mg) | 408 | 318 (77.9) | 46 (11.3) | 24 (5.9) | 162 (39.7) | 3 (0.7) | |
| Study duration: 52 weeks | |||||||
| CANA 300mg | 485 | 332 (68.5) | 26 (5.4) | 32 (6.6) | 4.9% | ||
| CANA 100mg | 483 | 311 (64.4) | 24 (5.0) | 25 (5.2) | 5.6% | NA | |
| GLIM (up to 8mg) | 482 | 330 (68.5) | 39 (8.1) | 28 (5.8) | 34.2% | ||
AE=adverse event; CANA=canagliflozin; DAPA=dapagliflozin; Disc=discontinuations; GLIM=glimepiride; GLIP=glipizide; INS=insulin; MET=metformin; NA=not applicable; PBO=placebo; SAE=serious AE; SGLT2=sodium glucose co-transporter 2; SU=sulphonylurea.
Results from the DAPA and CANA studies should not be directly compared due to their different study designs.
Data are n (%).
Major episodes in DAPA studies were defined as symptomatic episodes requiring third-party assistance due to severe impairment in consciousness or behaviour, with a capillary or plasma glucose value <54 mg/dL, and prompt recovery after glucagon administration. Severe hypoglycaemia episodes in CANA studies were defined as episodes requiring the assistance of another individual or resulting in seizure or loss of consciousness.
Patients in the DAPA study were receiving SU monotherapy (GLIM 4mg) at baseline. Patients in the CANA study were receiving stable doses of SU+MET at baseline.
Results from the DAPA and CANA studies should not be directly compared due to their different study designs.
Hypoglycaemia
The vast majority of hypoglycaemic episodes in the dapagliflozin studies were classified as minor, with no major episodes in the add-on to metformin and glimepiride studies at 24 weeks Table 2). In the add-on to insulin study, the number of patients with events of major hypoglycaemia was low and balanced in the dapagliflozin 10mg and placebo groups at 48 weeks (Table 2).
Over the longer term, there were no major episodes of hypoglycaemia with dapagliflozin administered to patients inadequately controlled on metformin over 102 weeks, or those inadequately controlled on glimepiride over 48 weeks.22,24,25 Furthermore, in the head-to-head active comparator study, there were ~10-fold fewer dapagliflozin-treated patients (5.4 per cent) reporting hypoglycaemia vs. glipizide-treated patients (51.5 per cent) over 208 weeks.27
The proportions of patients with severe hypoglycaemia were low and balanced in the canagliflozin studies in patients inadequately controlled on metformin plus sulphonyurea and those inadequately controlled on insulin. No patient reported severe hypoglycaemia when canagliflozin was administered to patients inadequately controlled on metformin monotherapy (Table 2). The head-to-head comparison of canagliflozin with glimepiride showed lower proportions of patients with hypoglycaemia episodes in the canagliflozin 100 and 300mg groups vs. glimepiride (Table 2).
Genital infections and urinary tract infections
High levels of glucosuria induced by SGLT2 inhibitors raise the risk of developing genital infections (mostly candidiasis) and to a relatively lesser extent, urinary tract infections (UTIs) in treated patients. Events suggestive of genital infections were increased with dapagliflozin 10mg (6.6–12.9 per cent) vs. placebo (0.7–5.0 per cent) across studies at 24, 48, and 52 weeks, and were observed more frequently in women than men. However, these events were generally mild-to-moderate in intensity, treated with standard antimicrobial therapies and rarely led to discontinuation. Data over the longer term showed that most events suggestive of genital infections occurred early in the course of treatment (mostly in the first 24 weeks) and that recurrent infections were uncommon.22–25,27 In the head-to-head comparison of dapagliflozin with glipizide in patients inadequately controlled on metformin, the majority of patients with genital infections first presented during year 1 (12.3 vs. 2.7 per cent for dapagliflozin and glipizide, respectively). The events suggestive of genital infections at year 4 were 16.3 vs. 4.2 per cent, respectively.27
Events suggestive of UTI were also higher with dapagliflozin 10mg (5.3–10.8 per cent) vs. placebo (4.0–8.0 per cent) across most studies up to 52 weeks23,25,36 and over the longer term.7,37 These findings were further supported by safety data pooled from 12 dapagliflozin trials that showed higher frequency of UTIs with dapagliflozin 10mg (6.5 per cent) vs. placebo (4.5 per cent); however, it is important to note that the difference in the frequency of UTIs between the two groups was not statistically significant.38 Most UTIs responded to treatment with standard antimicrobial medications and rarely led to discontinuation.
The incidence of genital mycotic infections and UTIs was higher with canagliflozin 100 and 300mg vs. placebo across studies up to 52 weeks (genital infections: 6.2–10.8 and 6.6–11.1 vs. 1.1–3.2 per cent, respectively; UTIs: 2.3–7.2 and 3.4–6.4 vs. 2.1–5.1 per cent, respectively). Similar to the dapagliflozin findings, the AEs of genital infections and UTIs noted with canagliflozin were generally mild-to-moderate in severity, treated with topical and/or oral antifungal therapies, and rarely led to study discontinuation.
Other considerations
Use in patients with impaired renal function
The efficacy of dapagliflozin is dependent on renal function and it is not recommended for use in patients with moderate-to-severe renal impairment [estimated glomerular filtration rate (eGFR) persistently <60 mL/min/1.73m2]. However, no dosage adjustment based on pharmacokinetic analyses is recommended for normal-to-mild renal impairment (eGFR ≥60 mL/min/1.73 m2).13
Renal safety of dapagliflozin was assessed by pooling data from double-blind clinical trials of dapagliflozin in patients with T2DM and normal or mildly impaired renal function.39 Renal AEs were balanced between dapagliflozin 10mg (1.9 per cent) and placebo (1.7 per cent) groups over 102 weeks; discontinuations due to renal AEs were slightly higher in the dapagliflozin group (0.9 per cent) vs. placebo (0.4 per cent) at 102 weeks. AEs of volume depletion were slightly higher in the dapagliflozin (1.5 per cent) vs. placebo (0.8 per cent) group at 102 weeks.
In a special study in patients with T2DM and moderate renal impairment (eGFR 30–59mL/min/1.73m2), dapagliflozin showed improvements in weight and BP despite no significant improvements in glycaemic measures vs. placebo.40 Mean increases in serum creatinine and eGFR were noted at week 1, but remained stable up to 104 weeks.
Canagliflozin is not recommended for use in patients with an eGFR <45mL/min/1.73m2.41 No dose adjustment is needed in patients with mild renal impairment (eGFR≥60 mL/min/1.73m2). However, the dose for canagliflozin is limited to 100mg once daily in patients with moderate renal impairment with an eGFR of 45–≤60mL/min/1.73m2. Low incidences of renal-related AEs (0.6 and 1.7 per cent in the canagliflozin 100 and 300mg groups vs. 0.6 per cent in the placebo group) were observed in the data pooled from canagliflozin studies at 26 weeks. Furthermore, the proportion of patients with renal-related AEs leading to study discontinuation were low, though slightly higher in the canagliflozin groups vs. placebo (0.4 and 0.8 per cent in the canagliflozin 100 and 300mg groups vs. 0.2 per cent in the placebo group).42
Use in the elderly
In a pooled analysis of dapagliflozin studies, the overall incidence of AEs in patients aged ≥65 years was similar to the overall population and in those <65 years.43 Elderly patients are, however, more likely to have impaired renal function and/or to be treated with antihypertensive drugs that may alter renal function. Consistent with the overall population, AEs of renal impairment or failure and volume-related events in patients aged ≥65 years were more frequent in the dapagliflozin 10mg vs. placebo group.13 The most commonly reported renal AE was increased blood serum creatinine, the majority of which was transient and reversible. TGA does not recommend any dose adjustment based on age; however, renal function and risk of volume depletion should be taken into account while prescribing dapagliflozin. Therapeutic experience in patients aged ≥75 years is limited. Therefore, initiation of dapagliflozin therapy is not recommended in this patient population.13
A canagliflozin study in older patients aged 55–80 years inadequately controlled on a range of blood glucose-lowering agents (oral or injectable) showed improvement in HbA1c, weight and BP with canagliflozin, with slightly higher incidence of overall AEs with canagliflozin 100 and 300mg doses vs. placebo (72.2 and 78.0 per cent vs. 73.4 per cent, respectively).44 The recommended starting dose of canagliflozin is 100mg once daily in patients ≥75 years of age. Renal function and risk of volume depletion should also be taken into account while prescribing canagliflozin in this patient population.12
Summary
The unique mechanism of action of SGLT2 inhibitors provides improved glycaemic control, with the added benefits of a low risk of hypoglycaemia, and sustained reductions in body weight and BP compared with existing glucose-lowering therapies. SGLT2 inhibitors were generally well tolerated in clinical trials; however, there were slightly increased incidences of genital infections and to a relatively lesser extent, UTIs with SGLT2 inhibitors vs. placebo.
Overall, data available from clinical trials of dapagliflozin and canagliflozin have shown the efficacy and safety of these drugs across a spectrum of patients with T2DM. With the approval of these drugs in the EU, the US and Australia, SGLT2 inhibitors have the potential to become an important addition to the treatment armamentarium for effective and well-tolerated management of complications of diabetes.
ACKNOWLEDGEMENTS AND FUNDING
The development of the review article was funded by Bristol-Myers Squibb and AstraZeneca; however, the authors did not receive an honorarium for their contribution/authorship. Editorial and writing assistance was provided by Shelley Narula of In Science Communications, Springer Healthcare and funded by Bristol-Myers Squibb.
Footnotes
PEER REVIEW
Not commissioned. Externally peer reviewed.
CONFLICTS OF INTEREST
The authors declare the following conflict of interest. RGM has been involved in, and continues to be involved in, several of the clinical trials for both dapagliflozin and canaglifozin. CP is on advisory boards for both dapagliflozin and canagliflozin. SC is on an advisory board for dapagliflozin and was on an advisory board for canagliflozin.
Please cite this paper as: Moses RG, Colagiuri S, Pollock C. SGLT2 inhibitors: New medicines for addressing unmet needs in type 2 diabetes. AMJ 2014;7(10):405-415.http//dx.doi.org/10.4066/AMJ.2014.2181.
References
- 1.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy for 2013 for the IDF Diabetes Atlas. Diabetes Res Clin Pr. 2014 Feb;103(2):176–85. doi: 10.1016/j.diabres.2013.11.003. doi: 10.1016/j.diabres.2013.11.003. Epub 2013 Dec 1. [DOI] [PubMed] [Google Scholar]
- 2.Australian Government, Australian Institute of Health and Welfare. Prevalence of diabetes [Internet]. 2013. [cited 2014 September 2] Available from: http://www.aihw.gov.au/diabetes/ [Google Scholar]
- 3.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998 Apr 15;(4):297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. oi:10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002 Sep 9;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ. American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007 Jan 2;115(1):114–26. doi: 10.1161/CIRCULATIONAHA.106.179294. Doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 7.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011 Jan;22(1):104–12. doi: 10.1681/ASN.2010030246. doi:10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994 Jan;93(1):397–404. doi: 10.1172/JCI116972. doi:10.1172/JCI116972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008 Sep;14(6):782–90. doi: 10.4158/EP.14.6.782. doi:10.4158/EP.14.6.782. [DOI] [PubMed] [Google Scholar]
- 10.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003 Nov;14(11):2873–82. doi: 10.1097/01.asn.0000092790.89332.d2. [DOI] [PubMed] [Google Scholar]
- 11.Perez Lopez G, Gonzalez Albarran O, Cano Megias M. Sodium-glucose cotransporter type 2 inhibitors (SGLT2): from familial renal glucosuria to the treatment of type 2 diabetes mellitus. Nefrologia. 2010;30(6):618–25. doi: 10.3265/Nefrologia.pre2010.Sep.10494. [DOI] [PubMed] [Google Scholar]
- 12.Australian Government, Department of Health. Canagliflozin tablet, 100 mg and 300 mg, Invokana®: Public Summary Document [Internet] 2013 [cited 2014 September 2]. Available from: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2013-07/canagliflozin. [Google Scholar]
- 13.Australian Government, Department of Health Therapeutic Goods Administration. FORXIGA® dapagliflozin propanediol monohydrate: Product Information [Internet] 2012 [cited 2014 September 2]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2012-PI-02861-1. [Google Scholar]
- 14.Australian Government, Department of Health Therapeutic Goods Administration. XIGDUO® XR dapagliflozin propanediol monohydrate/metformin hydrochloride: Product Information [Internet]. 2014 [cited 2014 September 2]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2014-PI-02269-1. [Google Scholar]
- 15.US Food and Drug Administration. FDA news release: FDA approves Invokana to treat type 2 diabetes [Internet] 2013 [cited 2014 September 2]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm. [Google Scholar]
- 16.Europeans Medicines Agency. Summary of product characteristics. Canagliflozin [Internet] Canagliflozin [Internet] [cited 2014 September 2]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002649/WC500156456.pdf. [Google Scholar]
- 17.European Commission, Public Health. Community register of medicinal products for human use. Invokana [database on the Internet] 2013 [cited 2013 December 3]. Available from: http://ec.europa.eu/health/documents/community-register/html/h884.htm#EndOfPage. [Google Scholar]
- 18.US Food and Drug Administration. FDA news release: FDA approves Farxiga to treat type 2 diabetes [Internet] 2014 [cited 2014 September 2]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829.htm. [Google Scholar]
- 19.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009 May;85(5):513–9. doi: 10.1038/clpt.2008.250. doi:10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 20.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011 Mar;(120):S20–7. doi: 10.1038/ki.2010.512. doi:10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 21.Dapagliflozin, tablet, 10 mg, Forxiga®–July 2013. 2013 [cited 2014 February 20]. Available from: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2013-07/dapagliflozin. [Google Scholar]
- 22.Strojek K, Hruba V, Elze M, Langkilde AM, Parikh S. Efficacy and safety of dapagliflozin as an add-on to glimepiride in T2DM inadequately controlled with glimepiride alone over 48 weeks. International Diabetes Federation; Dubai: Oral presentation #0622; 2011 [Google Scholar]
- 23.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012 Mar 20;156(6):405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 24.Woo V, Tang W, Salsali A, List JF. Long-term efficacy of dapagliflozin monotherapy in patients with type 2 diabetes mellitus. International Diabetes Federation 2011 World Diabetes Congress; December 4–8, 2011; Dubai, United Arab Emirates. Poster #PD62; 2010. [Google Scholar]
- 25.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013 Feb 20;11(1):43. doi: 10.1186/1741-7015-11-43. doi:10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes and Metab. 2014 Feb;16(2):124–36. doi: 10.1111/dom.12187. doi:10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 27.Del Prato S, Nauck MA, Duran-Garcia S, Rohwedder K, Theuerkauf A, Langkilde AM. et al. Durability of dapagliflozin vs glipizide as add-on therapies in T2DM inadequately controlled on metformin: 4-year data. American Diabetes Association; Chicago: Poster #62-LB. 2013 Jun 21-25; [Google Scholar]
- 28.Cefalu WT, Leiter LA, Yoon KH, Langslet G, Arias P, Xie J. et al. Canagliflozin demonstrates durable glycemic improvements over 104 weeks versus glimepiride in subjects with type 2 diabetes mellitus on metformin. 73rd Scientific Sessions of the American Diabetes Association Chicago, Illinois:Poster #65-LB; 2013 Jun 21-25; [Google Scholar]
- 29.Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011 Sep;34(9):2015–22. doi: 10.2337/dc11-0606. doi:10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strojek K, Yoon KH, Hruba V, Elze M, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011 Oct;13(10):928–38. doi: 10.1111/j.1463-1326.2011.01434.x. doi:10.1111/j.1463-1326.2011.01434. [DOI] [PubMed] [Google Scholar]
- 31.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014 Feb;16(2):124–36. doi: 10.1111/dom.12187. doi:10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Del Prato S, Rohwedder K, Theuerkauf A, Langkilde AM, Parikh SJ. Long-term efficacy and safety of add-on dapagliflozin vs add-on glipizide in patients with T2DM inadequately controlled with metformin: 2-year results. 71st Scientific Sessions of the American Diabetes Association (ADA) 2011 Jun 24-28; San Diego, CA: Poster #40-LB; 2011. [Google Scholar]
- 33.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013 Sep 14;382(9896):941–50. doi: 10.1016/S0140-6736(13)60683-2. doi:10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 34.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013 Dec;67(12):1267–82. doi: 10.1111/ijcp.12322. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews DR, Fulcher G, Perkovic V, de Zeeuw D, Mahaffey KW, Rosenstock J. et al. Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy +/- oral agents in type 2 diabetes. European Association of Diabetes (EASD); Poster #764; 2012 Oct 1-5; Berlin, Germany 2012. [Google Scholar]
- 36.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010 Oct;33(10):2217–24. doi: 10.2337/dc10-0612. doi:10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilding J, Mathieu C, Vercruysse F, Usiskin K, Deng L, Canovatchel W. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves glycemic control and reduces body weight in subjects with type 2 diabetes inadequately controlled with metformin and sulfonylurea. 72nd Scientific Sessions of the American Diabetes Association (ADA); Philadelphia, PA, USA: Poster #1022. 2012 Jun;:8–12. [Google Scholar]
- 38.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013 Sep-Oct;27(5):473–8. doi: 10.1016/j.jdiacomp.2013.05.004. doi:10.1016/j.jdiacomp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Ptaszynska A, Chalamandaris A-G, Sugg J, Johnsson KM, Parikh SJ, List JF. Effect of dapagliflozin on renal function. 72nd Scientific Sessions of the American Diabetes Association Philadelphia, PA, USA. 2012 Jun 8-12; [Google Scholar]
- 40.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014 Apr;85(4):962–71. doi: 10.1038/ki.2013.356. doi:10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Australian Government, Department of Health Therapeutic Goods Administration. INVOKANA (canagliflozin) Product Information [Internet] 2013 [cited 2014 September 2]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-02171-1. [Google Scholar]
- 42.US Food and Drug Administration. FDA Briefing document. Invokana (canagliflozin) tablets. Endocrinologic and Metabolic Drugs Advisory Committee Meeting [Internet]. 2013 [cited 2014 September 2]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf 2013; Accessed on 2 September 2014. [Google Scholar]
- 43.Ptaszynska A, Johnsson K, Parikh S, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Safety. 2014 Aug 6; doi: 10.1007/s40264-014-0213-4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44.Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract (1995) 2013 Apr;41(2):72–84. doi: 10.3810/hp.2013.04.1020. doi:10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 45.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Jun 26;375(9733):2223–33. doi: 10.1016/S0140-6736(10)60407-2. doi:10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 46.Niskanen L, Cefalu WT, Leiter LA, Xie J, Balis D, Canovatchel W. et al. Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with glimepiride in patients with type 2 diabetes on background metformin. European Association for the Study of Diabetes. 2012 Oct 1-5; Berlin, Germany: Poster #763; [Google Scholar]
- 47.US Food and Drug Administration. FDA Background document. Dapagliflozin. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 2011 [Google Scholar]


