Summary
Bcl-3 is an atypical member of the IκB family that modulates transcription in the nucleus via association with p50 (NF-κB1) or p52 (NF-κB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFNγ- and GM-CSF, and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less pathogenic Th17-like cells, in part via mechanisms involving expression of the RORγt transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
Introduction
Bcl-3 is a partner in recurrent translocations in some B cells tumors, and high amounts of Bcl-3 are found in a number of solid tumors (Maldonado and Melendez-Zajgla, 2003; Ohno et al., 1990; Soma et al., 2006). Bcl-3 is a member of the IκB transcription factor family, but unlike the classical NF-κB-inhibitory members, Bcl-3 readily enters nuclei to modulate NF-κB activity via association with DNA-bound p50 (NF-κB1) or p52 (NF-κB2) homodimers. Bcl-3 may either promote or inhibit NF-κB-target gene expression, dependent on context and by mechanisms not well understood (Bours et al., 1993; Franzoso et al., 1992; Fujita et al., 1993; Hinz et al., 2012; Palmer and Chen, 2008). Nevertheless, studies with Bcl-3-deficient mice have revealed the profound physiologic impact of this protein, particularly in immune responses: Bcl-3 is essential for effective adaptive and innate immune defenses against certain pathogens, and contributes to germinal center reactions, central tolerance, and prevention of autoimmune diabetes (Franzoso et al., 1997; Kreisel et al., 2011; Pene et al., 2011; Ruan et al.; Zhang et al., 2007). However, the critical cell-specific functions controlled by Bcl-3 in these settings have remained elusive.
The transfer of naive CD4+ T cells into Rag1−/− mice induces colitis associated with wasting disease and is a commonly used model of inflammatory bowel disease (IBD) (Powrie et al., 1994a). In this transfer model, development of colitis requires both spontaneous proliferation of T cells driven by microbiota-derived innate signals, as well as antigen-specific proliferation of T cells (Feng et al.). Regarding the fate of the transferred T cells, most studies have focused on the role of the IL-12 -Th1 cell and IL-23-Th17 cell axes (Powrie et al., 1994b; Yen et al., 2006). IFNγ-deficient and T-bet-deficient [Au: We avoid the jargon “KO”] T cells failed to induce colitis (Ito and Fathman, 1997; Neurath et al., 2002) and blocking IFNγ greatly ameliorated disease (Powrie et al., 1994b). In addition to IL-12, IL-23 has also been shown to be essential for disease development (Yen et al., 2006), but rather than specifically drive expression of only IL-17, IL-23 may, over time, promote conversion of Th17 cells into more colitogenic, IFNγ-expressing Th1-like cells (Feng et al., 2011; Morrison et al., 2013). A similar conversion has also been observed in experimental autoimmune encephalomyelitis (Hirota et al., 2011). IL-23 may furthermore drive the pathogenicity of auto-reactive T cells by inducing production of GM-CSF (Codarri et al., 2011; El-Behi et al., 2011).
In the present study we identify Bcl-3 as a physiologically relevant regulator of Th1 cell plasticity and pathogenicity. We showed that loss of Bcl-3 in T cells blocked T cell transfer-induced colitis as well as experimental autoimmune encephalomyelitis, which correlated with diminished numbers of Th1 cells and enhanced numbers of Th17 cells. Although loss of Bcl-3 did not impair Th1 cell differentiation, it allowed for the conversion of pathogenic GM-CSF-expressing Th1 cells into non-pathogenic Th17-like cells. We also provide evidence that Bcl-3 stabilized the Th1 cell phenotype in part by controlling expression of the Th17 cell transcription factor RORγt.
RESULTS
Loss of Bcl-3 in T Cells Greatly Ameliorates T Cell Transfer-Induced Colitis
CD4+ CD45RBhi CD25− naive T cells were isolated from 8-12-week-old healthy wild-type (WT) and Bcl-3-deficient (Bcl3−/−) mice by flow cytometry sorting, and then injected into Rag1−/− mice. Recipients of WT T cells steadily lost weight, beginning as early as 4 weeks post transfer while recipients of Bcl3−/− [Au: Global change, please.] T cells gained some weight during the course of the experiment; they were healthy at the time recipients of WT T cells had to be euthanized (Figure 1A). H&E staining of colon cross-sections revealed severe inflammation in recipients of WT T cells, characterized by profound cellular infiltration and by damaged colon structures, while recipients of Bcl3−/− T cells were largely protected (Figures 1B and S1A). Crypt abscesses and submucosal inflammation were readily apparent in colons of WT T cell recipients, but essentially absent in recipients of Bcl3−/− T cells.
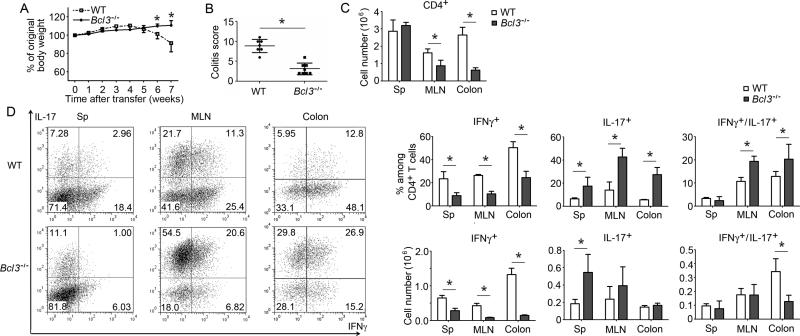
Figure 1.
Loss of Bcl-3 in T cells protects against T cell transfer-induced colitis and is associated with a shift from Th1 to Th17. (A) CD4+ CD45RBhi CD25− naïve wild-type (WT) and Bcl-3-deficient (Bcl3−/−) T cells were injected i.p. into Rag1−/− mice and recipients were weighed weekly and sacrificed 7 weeks post transfer when some mice were losing 20% of body weight (n=6 mice/group). (B) Colitis scores of WT or Bcl3−/− T cell recipients as in (A). (C) Total numbers of CD4+ T cells recovered form spleen (Sp), mesenteric lymph nodes (MLN) and colon (n=6). (D) Representative flow cytometric analysis of indicated cytokine expression of CD4+ T cells recovered from WT and Bcl3−/−T cell recipients; summary of percentages and absolute numbers shown on the right (n=5 mice/group). Results similar to those shown in A-D were obtained in a second experiment with n=5 mice/group). Data represent means ± SD; * indicates p < 0.05.
To examine the phenotype of transferred cells, CD4+ T cells were recovered from spleens, mesenteric lymph nodes (MLNs) and lamina propria of colons 7 weeks after transfer and analyzed for cytokine production. More CD4+ T cells were present in MLNs and especially colons (but not spleens) in mice that had received WT compared to Bcl3−/− T cells, correlating with and likely in consequence of increased local inflammation in the gut (Figure 1C) (see below). The percentage of IFNγ-expressing T cells (Th1 cells) was much reduced, while that of IL-17-expressing T cells (Th17 cells) was much increased if T cells lacked Bcl-3 (Figure 1 D). This shift from a primarily Th1 to a Th17 cell phenotype was particularly pronounced in Bcl3−/− T cells isolated from MLNs and colons, likely due to local conditions favoring the generation and/or maintenance of Th17 cells. We observed only scant T regulatory (Treg) cells (Treg) and their percentage was similar in transferred WT and Bcl3−/− T cells (Figure S1B). We also performed transfers with naive CD4+ CD62L+ CD25− T cells and obtained similar results (Figures S1C-E). Thus transfer of Bcl3−/− T cells resulted in an unexpected skewing from a predominantly Th1 to a predominantly Th17-like cell phenotype.
The Th1 to Th17 cell Shift in Transferred Bcl3−/− T Cells is Cell-Autonomous
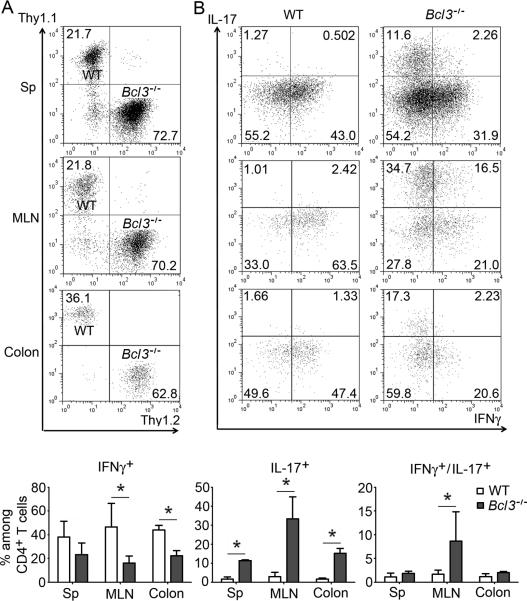
To address whether Bcl-3 functioned not only cell-intrinsically but also cell-autonomously (independent of trans-acting factors), we co-transferred Thy1.1-marked WT and Thy1.2-marked Bcl3−/− naïve CD4+ T cells into Rag1−/− mice. The relative ratio of WT to Bcl3−/− T cells recovered 4 weeks after transfer was similar throughout spleen, mesenteric lymph node and lamina propria and in line with the ratio at the time of transfer, indicating that Bcl-3 did not selectively affect localization and/or local expansion of T cells (Figure 2A). Nevertheless, we observed far fewer IFNγ-producing Th1 cells and many more IL-17-producing Th17 cells among the progeny of the transferred Bcl3−/− T cells compared to their WT counterparts, especially in MLNs and colon (Figures 2B). We obtained similar results upon co-transfer of Ly5.1-marked WT and Ly5.2-marked Bcl3−/− naïve CD4+ T cells (Figure S2). These findings indicate a T cell-autonomous role of Bcl-3 in determining the predominant helper phenotype of transferred naïve T cells. As there was no genotype-specific difference in recovery of T cells in these co-transfers, these results also suggest that the pronounced gut inflammation and associated high levels of IFNγ seen upon transfer of only WT T cells in Figure 1 was likely responsible for the higher numbers of T cells present in gut-associated tissues when compared to transfer of only Bcl3−/− T cells.
Figure 2.
Bcl-3 functions cell-autonomously to control T helper cell phenotype. (A, B) Representative flow cytometric analyses of T cells recovered from Rag1−/−mice 4 weeks after co-transfer of WT Thy1.1 and Bcl3−/− Thy1.2 naïve CD4+ T cells, showing (A) relative amounts of Bcl3−/− and WT T cells recovered from indicated sites and (B) expression of indicated cytokines, with summary of 6 independent co-transfers shown below. Data represent means ± SD; * indicates p < 0.05.
Bcl3−/− Th1 Cells Convert to Less-Colitogenic Th17-Like Cells
To determine whether Bcl-3 controlled the initial differentiation of naïve T cells into either Th1 or Th17 cells, we cultured naïve WT and Bcl3−/− CD4+ T cells for two rounds under standard Th1 and Th17 cell conditions, as well as under ‘enhanced’ Th17 cell conditions (standard conditions plus IL-1, IL-21 and IL-23). Flow cytometry analysis for signature cytokine expression under these conditions did not reveal any differences between Bcl3−/− and Bcl-3-sufficient T cells (Figures 3A). We also differentiated co-cultures of Thy1.1 WT and Thy1.2 Bcl3−/− naïve CD4+ T cells under Th1, Th17 or enhanced Th17 cell conditions. The ratio of WT to Bcl3−/− T cells did not change during the course of these experiments, and WT and mutant T cells differentiated to similar extents (Figures S3A and S3B). Therefore Bcl-3 did not inherently alter the potential of naïve T cells to differentiate into either Th1 or Th17 cells in vitro.
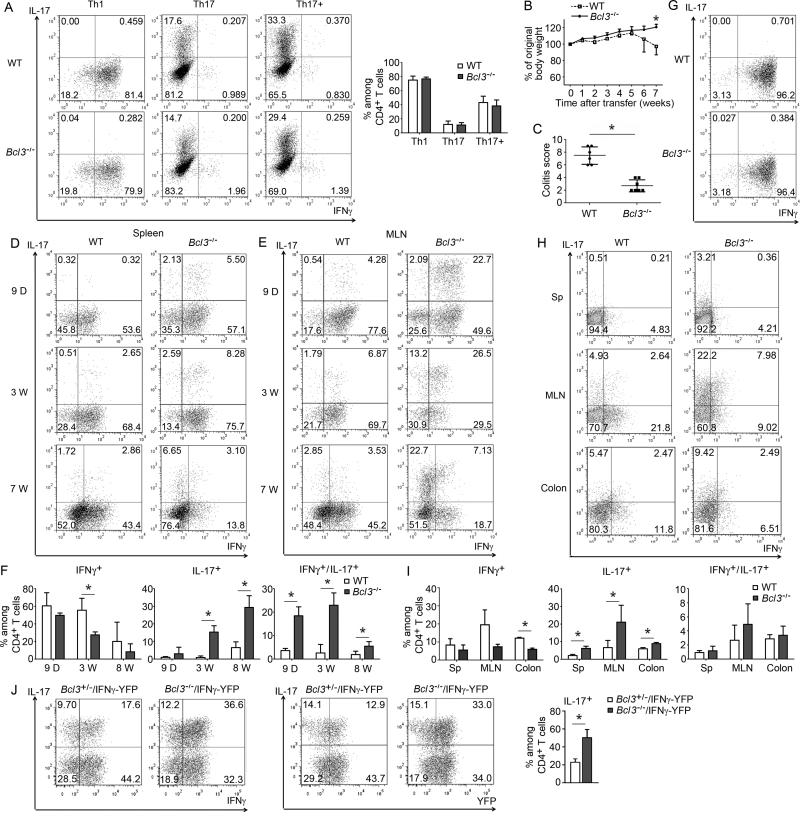
Figure 3.
Bcl3−/− Th1 cells convert to non-pathogenic Th17 cells upon transfer. (A) Representative flow cytometric analyses of WT and Bcl3−/−naïve CD4+ T cells cultured under Th1, Th17 or enhanced Th17 (Th17+) conditions in vitro and analyzed for expression of indicated cytokines. Summary of percentages of differentiated T cells from 5 independent experiments shown on the right. (B) In vitro differentiated WT and Bcl3−/− Th1 cells were injected i.p. into Rag1−/− mice, recipients were weighed weekly and sacrificed 7 weeks post transfer (n=6 mice/group). (C) Colitis scores of WT or Bcl3−/− Th1 cell recipients as in (B); results similar to those shown in (B,C) were obtained in a second experiment with n=6 mice/group). Representative cytometric analyses for indicated cytokine expression of T cells recovered from spleen (D) and MLNs (E) at indicated times post transfer. (F) Summary of 3 independent experiments as in (E) (total n=9 mice/group). (G) Representative flow cytometric analyses for indicated cytokine expression of highly differentiated WT and Bcl3−/− Th1 cells. (H) Representative flow cytometric analyses for indicated cytokine expression in T cells recovered from indicated sites 4 weeks after transfer of highly differentiated Th1 cells into Rag1−/− mice. (I) Summary of 4 independent experiments as in (H). (J) Representative flow cytometric analyses for indicated cytokine and YFP expression of CD4+ T cells from MLNs of Rag1−/− mice 4 weeks after receiving FACS-sorted YFP+ Th1 cells that had been isolated from spleens of Rag1−/− 4 weeks after initial transfer with naïve Bcl3−/− and control (Bcl-3+/−) CD4+ T cells carrying the IFNγ-YFP reporter. Summary of 3 independent experiments for IL-17 shown on the right. Data represent means ± SD; * indicates p < 0.05.
This finding suggested that Bcl-3 might instead affect the behavior of already differentiated T cells. We differentiated WT and Bcl3−/− CD4+ T cells into Th1 cells in vitro (two rounds) and then adoptively transferred these cells into Rag1−/− mice. Transfer of Th1-differentiated WT cells successfully induced colitis and weight loss, although this was not as severe and slightly delayed when compared to transfer of naïve WT cells. By contrast, transfer of Th1-differentiated Bcl3−/− T cells failed to induce any weight loss or colon inflammation, even though these cells produced just as much IFNγ as WT Th1 cells at time of transfer (Figures 3B, 3C and S3C). We recovered CD4+ T cells from recipient animals 9 days, 3 weeks and 7 weeks post transfer to assess production of IFNγ and IL-17. T cells isolated from spleens and MLNs of recipients of WT Th1s maintained this phenotype and continued to produce IFNγ at all three times tested, with only scant production of IL-17; by contrast, many of the T cells isolated from recipients of Bcl3−/− Th1 cells started to co-produce IL-17 together with IFNγ as early as 9 days after transfer, especially in MLNs, and by 7 weeks most cells had progressed to produce IL-17 exclusively (Figures 3D-F). The shift in production of only IFNγ to IFNγ+IL-17 (double producers), and finally, to only IL-17 suggests a gradual conversion from a Th1 to a Th17 phenotype. Because up to almost 20% of the in vitro differentiated Th1 cells did not actively express IFNγ at time of transfer, it remained theoretically possible that IL-17-producers might have been derived from a less-differentiated population, although this still would not explain the progression through double cytokine-producing to just IL-17-producing T cells in vivo. To address this issue further we optimized in vitro differentiation conditions, such that more than 95% of the CD4+ T cells produced IFNγ(Figure 3G). 4 weeks after transfer of these cells we observed as much of a shift from a Th1 to a Th17-like cell phenotype in Bcl3−/− T cells as before, but not in WT T cells, again most evident in MLNs and colons (Figures 3H and 3I). In a second approach we generated Bcl3+/− heterozygous (control) and Bcl3−/− IFNγ-YFP reporter mice, isolated naïve CD4+ T cells and flow cytometry-sorted for YFP+ Th1 cells after in vitro differentiation (above 98% purity) (Figure S3D). Upon transfer YFP+ Bcl3−/− Th1 cells again more readily converted into IL-17-producers than their WT counterparts (Figure S3E) (Bcl3+/− control T cells exhibited an increased tendency to convert compared to WT, but were still significantly more stable than Bcl3−/− cells). In a third approach we also tested whether Th1 cells generated in vivo would undergo a shift to Th17 cells after re-transfer. Naïve CD4+ T cells were isolated from Bcl3+/− (control) and Bcl3−/− IFNγ-YFP reporter mice, adoptively transferred into Rag1−/− mice and 4 weeks later, YFP+ cells were flow cytometry-sorted from spleens and then re-transferred into Rag1−/− mice. Such in vivo generated YFP+ Th1 cells again exhibited more plasticity in the absence of Bcl-3, producing notably more IL-17, mostly as double-producers at this relatively early stage after transfer (Figure 3J).
IL-17-producing Bcl3−/− T cells isolated from MLNs 4 weeks after transfers of in vitro differentiated Th1 cells also showed significant co-expression of IL-22, and to a lesser degree, IL-17F, two additional cytokines associated with the Th17 phenotype. Interestingly, these cells expressed very little GM-CSF, a cytokine recently reported to be critical for pathogenicity of auto-reactive T cells (Figure S3F). We also detected notably increased RORγt protein expression and reduced amounts of T-bet, consistent with a conversion of Th1 cell-differentiated Bcl3−/− T cells to a Th17-like cell phenotype (Figure S3G).
We investigated whether Bcl3−/− Th1 cells might preferentially convert to Treg cells. However, neither WT nor Bcl3−/− Th1 cells expressed significant amounts of Foxp3 after exposure to Treg cell-skewing conditions in vitro or after transfer in vivo (Figures S3H and S3I). To rule out the possibility that CD4+ T cells isolated from Bcl3−/− mice may bear a developmental defect caused by lack of Bcl-3 in other cells, we generated mice conditionally ablated of Bcl-3 in T cells (via Lck-Cre) (Figure S3J). Naïve CD4+ T cells from these mice also failed to induce significant pathology upon transfer and they produced much less IFNγ and GM-CSF and more IL-17 than control Bcl-3-heterozygous T cells (Figures S3K and S3L) (Bcl3+/− controls exhibited a partially increased potential to convert; see above). We further confirmed that naïve T cells isolated from mice with T cell-specific ablation of Bcl-3 did not differ from heterozygous controls in expression of IL-17, IFNγ and GM-CSF after in vitro differentiation under either Th1 or Th17 cell conditions (Figure S3M). Finally, T cells isolated from the conditionally ablated mutant mice and differentiated into Th1 cells in vitro also much more readily converted to Th17-like cells upon transfer than controls and they produced less GM-CSF (Figure S3N). Thus Bcl3−/− Th1 cells converted to less-colitogenic Th17-like cells in vivo.
Conversion of Bcl3−/− Th1 Cells depends on antigen and cytokine stimulation
To determine whether the conversion of Bcl3−/− Th1 cells into Th17-like cells was cell-autonomous, we co-transferred Ly5.1-marked WT together with Ly5.2-marked Bcl3−/− Th1-differentiated cells into Rag1−/− mice. The ratio of these two populations was maintained 9 days after transfer in both spleen and mesenteric lymph node (Figure 4A). Bcl3−/− Th1 cells also preferentially converted to produce IL-17, primarily in the gut (MLNs), and at this early stage, IFNγ was still co-produced, while the co-transferred WT Th1 cells continued to produce IFNγalmost exclusively (Figure 4B). Similar results were obtained upon co-transfer of Thy1.1-marked WT with Thy1.2-marked Bcl3−/− Th1-differentiated cells (Figures S4A and S4B).
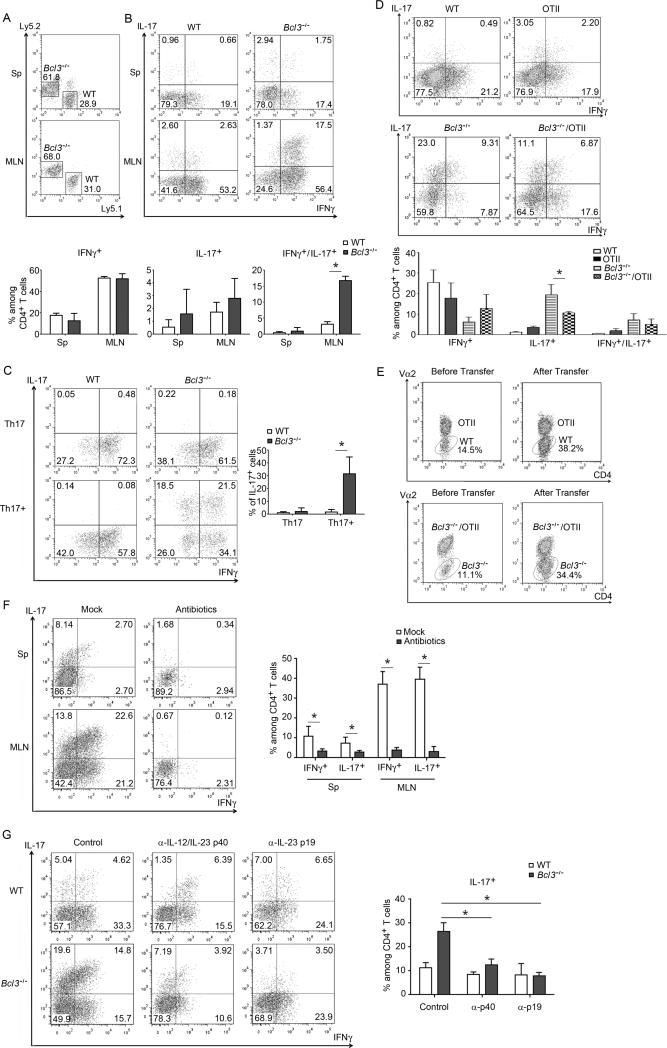
Figure 4.
Bcl-3 functions cell-autonomously to control Th1 plasticity. Representative flow cytometric analyses of T cells recovered from Rag1−/− mice 9 days after co-transfer of Ly5.1 WT and Ly5.2 Bcl3−/− Th1 cells, showing (A) relative amounts of Bcl3−/− and WT T cells recovered from indicated sites and (B) expression of indicated cytokines in recovered Bcl3−/− and WT T cells, with summary of 3 independent co-transfers shown below. (C) Representative flow cytometric analyses of indicated cytokine expression of in vitro generated Th1 cells after re-differentiation under Th17 or Th17+ conditions for 3 weeks, with summary of 3 independent experiments on the right. (D) Representative flow cytometric analyses of T cells recovered from MLNs of Rag1−/− mice 3 weeks after transfer of WT, OTII, Bcl3−/− or Bcl3−/−/OTII Th1 cells for expression of indicated cytokines, with summary of 4 independent experiments shown below. (E) Representative flow cytometric analyses of relative amounts of OTII and WT, Bcl3−/−/OTII and Bcl3−/− T cells before and after transfer. (F) Rag1−/− mice were treated with antibiotics for three weeks or not treated prior to transfer of Bcl3−/− Th1 cells. Shown are representative flow cytometric analyses of T cells recovered from spleen and MLN 3 weeks after transfer, with summary of 3 independent experiments on the right. (G) Rag1−/− mice adoptively transferred with WT and Bcl3−/− Th1 cells were injected i.p. with indicated neutralizing antibodies. Shown are representative flow cytometric analyses of T cells recovered from MLN 3 weeks after transfer, with summary of 3 independent experiments on the right. Data represent means ± SD; * indicates p < 0.05.
We sought to convert previously differentiated Bcl3−/− Th1 cells into Th17-like cells under in vitro standard and ‘enhanced’ Th17 cell-skewing conditions. Standard Th17 cell differentiation conditions were largely ineffective in converting Bcl3−/− Th1 into Th17 cells, but ‘enhanced’ conditions did allow for significant conversion, with some cells now able to produce IL-17 alone or together with IFNγ; by contrast, WT Th1 cells could not be converted at all under any conditions (Figure 4C). We also tested whether loss of Bcl-3 might prevent the ability of Th17-differentiated cells to convert to Th1 cell under appropriate skewing conditions in vitro, as such conversion has been well documented (Lee et al., 2009). However, both WT and Bcl3−/− Th17 cells could be similarly converted to Th1-like cells (Figure S4C).
To test whether conversion of Bcl3−/− Th1 to Th17 cells might depend on (microbial) antigen recognition, we generated Bcl3−/− OTII mice (ovalbumin-specific TCR), subjected naïve CD4+ T cells isolated from these and from Bcl3−/− mice to Th1 cell differentiation and co-transferred both Th1 cell populations into Rag1−/− mice. T cells isolated from MLNs 3 weeks after transfer already revealed a lower conversion in Bcl3−/− OTII TCR-bearing T cells compared to Bcl3−/− T cells with a diverse TCR repertoire (Figure 4D). Of note, more of the latter cells were recovered, likely due to antigen-specific expansion not available to the OTII T cells (shift in ratios; Figure 4E). To query the role of microbiota in the fate of Bcl3−/− Th1 cells, Rag1−/− mice were pre-treated with antibiotics prior to transfer. This greatly reduced expression of IL-17 and IFNγ, suggesting that microbiota were required to maintain and/or expand and convert Th1 cells (Figure 4F). To determine whether conversion of Bcl3−/− Th1 cells depended on IL-23 or both IL-23 and IL-12, we administered neutralizing antibodies. Blocking IL-23 p19 eliminated conversion to IL-17 production, whereas blocking IL12 (IL23) p40 additionally reduced IFNγ (Figure 4G). Thus conversion of Bcl3−/− Th1 cells into Th17-like cells was dependent on TCR stimulation and IL-23.
Bcl-3 controls plasticity and pathogenicity of Th1 cells in experimental autoimmune encephalomyelitis (EAE)
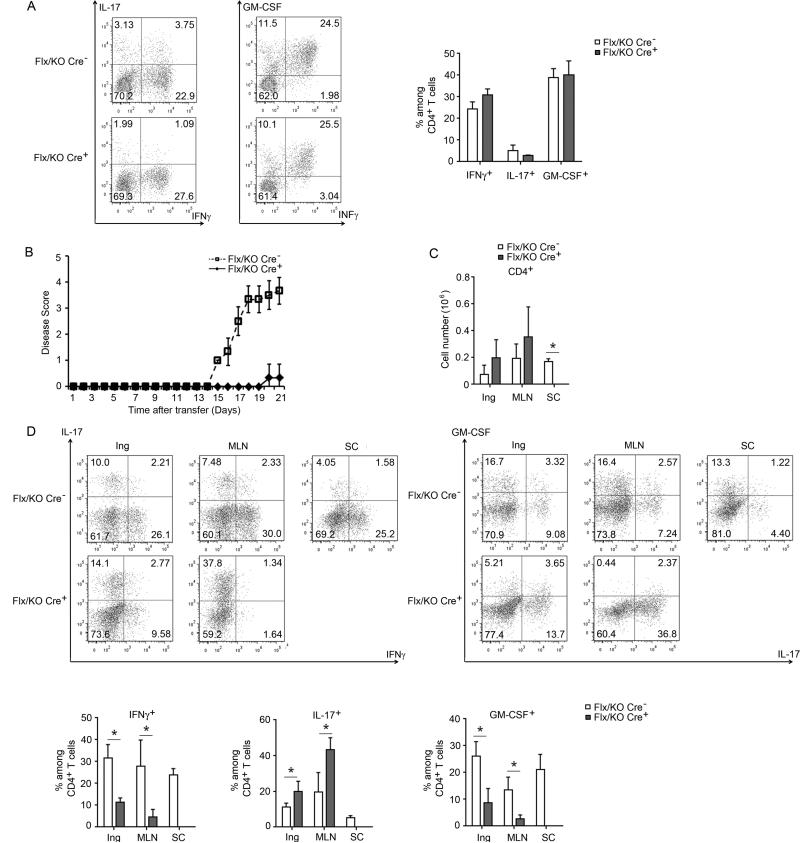
To determine whether Bcl-3's role in controlling plasticity and pathogenicity of Th1 cells in colitis might extend to other autoimmune conditions, we tested an EAE model. Bcl-3 control heterozygous mice and mice with T cell-specific ablation of Bcl-3 (heterozygous background) were immunized with MOG peptide, draining lymph nodes were isolated and re-stimulated in vitro with MOG under Th1 cell conditions. Analysis of T cells showed equivalent production of IFNγ and GM-CSF (with little IL-17 expression) in Bcl3−/− and control CD4+ T cells (Figure 5A). Upon transfer into Rag1−/− mice and booster immunization, recipients of control MOG-primed Th1 cells developed severe symptoms of EAE, while recipients of Bcl3−/− MOG-primed Th1 cells were fully protected from disease (Figures 5B and S5A). In contrast to recipients of control T cells, only scant CD4+ T cells could be recovered from spinal cords of recipients of Bcl3−/− T cells, even though we observed no difference in the numbers of CD4+ T cells in other organs (Figure 5C). Analogous to what we observed in the colitis model, the Bcl3−/− Th1 cells produced more IL-17 and less IFNγ, especially in MLNs, but also elsewhere, and most remarkably, they produced much less GM-CSF, consistent with an important role of this cytokine in EAE pathogenesis (Figure 5D).
Figure 5.
Bcl-3 controls Th1 cell plasticity and pathogenicity in experimental autoimmune encephalomyelitis. Control mice (Bcl-3Flx/KO) and mice with T cells specific ablation of Bcl-3 (Bcl-3Flx/KO Lck-Cre+) were immunized with MOG. Ten days later, cells from draining lymph node and spleen were isolated and re-stimulated in vitro under Th1 conditions. (A) Representative flow cytometric analyses of re-stimulated T cells for indicated cytokine expression, with summary of 3 independent experiments on the right. (B) Th1 cells from (A) were transferred into Rag1−/− mice and immunized with MOG. Disease score of the recipients were monitored daily (n=6 mice/group). (C) Total numbers of CD4+ T cells recovered from inguinal lymph node (Ing), MLN and spinal cord (SC) at end of experiment as in (B) (n=5 mice/group). (D) Representative flow cytometric analyses for expression of indicated cytokines in T cells recovered from indicated sites at end of experiments (B,C), with summary of data shown below (n=5 mice/group). Results similar to those in B-D were obtained in a separate set of experiments, n=3 mice/group. Data represent means ± SD; * indicates p < 0.05.
We also induced EAE directly via immunization with MOG peptide followed by a booster. Consistent with the transfer model, mice conditionally ablated of Bcl-3 in T cells were protected from EAE, while Bcl3+/− controls developed typical disease symptoms (Figure S5B and C). Also, spinal cords of control mice were infiltrated with T cells, while those of conditional gene deletion were not; furthermore, compared to controls, T cells from draining lymph nodes of conditional gene deletion mice exhibited a clear shift from Th1 to Th17 cells and a decrease in GM-CSF production (Figure S5D; as expected, percentage of cytokine-producers was lower in this EAE model). Thus Bcl3−/− Th1 cells converted to less-pathogenic Th17-like cells in the context of EAE.
Bcl-3 May Control Th1 Cell Plasticity via an RORγt-Dependent Mechanism
Since Bcl-3 can regulate gene expression via association with p50 (NF-κB1) and p52 (NF-κB2) homodimers, we asked whether in vitro differentiated Th1 cells from NF-κB1 or NF-κB2-deficient mice might also show increased plasticity upon transfer. NF-κB1-deficient Th1 cells were as stable as WT Th1 cells, but some NF-κB2-deficient Th1cells did convert into IL-17-producing cells, especially in MLNs (Figure S6A). While this might suggest a role for p52 (NF-κB2) in mediating Bcl-3 activity, the fact that both p50 (NF-κB1) and p52 (NF-κB2) are also central to overall NF-κB activity precludes definitive conclusions; nevertheless these findings do further implicate NF-κB in control of plasticity. We also assessed mRNA levels of Bcl-3 in differentiated Th1 and Th17 cells, but did not find a significant difference, suggesting that Bcl-3's activity was likely context-dependent (Figure S6B).
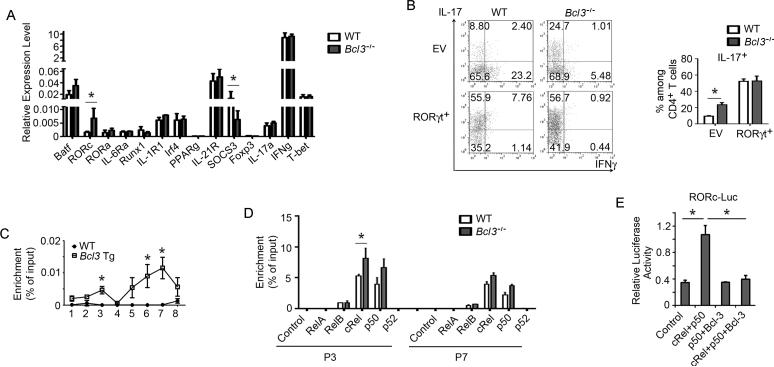
To determine whether Bcl-3 might be involved in direct control of IL-17 transcription, we made use of an IL-17 promoter and enhancer-driven luciferase reporter in Jurkat cells, but were unable to find any evidence for a role of NF-κB (Figure S6C). We next surveyed Th1-differentiated cells for expression levels of genes known to be relevant for the differentiation of naïve T cells into either Th1 or Th17 cells. Although both WT and Bcl3−/− in vitro-differentiated Th1 cells expressed equal levels of IFNγ and no IL-17, the Bcl3−/− Th1 cells could already be distinguished from their WT counterparts by notably higher amounts of the Th17 phenotype-associated major regulator RORγt and lower amounts of the signal-regulator SOCS3, even in the absence of Th17 cell-skewing conditions (Figure 6A). Bcl3−/− (as opposed to WT) Th1 cells thus appeared to be less ‘fixed’ into the Th1-state, even after two rounds of Th1 cell-skewing differentiation regimens, and consequently potentially more likely to switch to Th17 cell with appropriate stimuli. To determine whether increased RORγt expression might be sufficient to allow Th1 cells to switch to Th17, we over-expressed RORγt via retroviral-transduction in both Bcl3−/− and WT Th1 cells, and then subjected them to Th17 cell differentiation conditions. Over-expressed RORγt was able to break the constraints on plasticity even in WT Th1 cells, as we now observed IL-17 expression in both WT and Bcl3−/− Th1 cells under Th17-skewing, and especially under ‘enhanced’ Th17-skewing conditions, although Bcl3−/− cells still expressed more IL-17 (Figure S6D). We also transferred RORγt-over-expressing Th1cells into Rag1−/− mice, and under these in vivo conditions WT and Bcl3−/− Th1 cells now produced equally high levels of IL-17, suggesting that RORγt levels were critical for the ability to convert to a Th17 phenotype in vivo (Figure 6B).
Figure 6.
Mechanisms involved in constraining Th1 plasticity. (A) Relative expression levels (RT-PCR) of indicated genes of in vitro generated WT and Bcl3−/− Th1 cells (n=3/group; each separately differentiated). (B) Representative flow cytometric analyses of GFP+ T cells from MLNs for indicated cytokine expression of in vitro generated WT and Bcl3−/− Th1 cells transduced with a GFP-RORγt retrovirus or an empty retrovirus vector (EV) and transferred into Rag1−/− mice for 3 weeks. Summary (IL-17) of 3 independent experiments shown on the right. (C) ChIP analysis of tagged-Bcl-3 binding to Rorc promoter regions 1 through 8 in WT and tagged-Bcl-3 transgenic (Tg) Th1 cells (n=3 mice/group). (D) ChIP analysis of indicated NF-κB subunits binding to Rorc promoter regions 3 and 7 in WT and Bcl3−/− Th1 cells (n=3). (E) Rorc promoter luciferase assays in Jurkat cells transfected with expression constructs for cRel, p50 and Bcl-3 as indicated (n=3). Two additional repeats of each experiment in A, C-E yielded similar results. Data represent means ± SD; * indicates p < 0.05.
To provide further support for the notion that Bcl-3 regulates expression of Rorc, we made use of transgenic (Tg) mice that over-expressed Bcl-3 specifically in T cells. Naïve CD4+ T from these transgenic and from WT mice were differentiated into Th1 and Th17 cells and assessed for Rorc expression. Over-expressed Bcl-3 inhibited Rorc expression in Th17 cells and further reduced already low expression in Th1 cells (Figure S6E). To determine whether Bcl-3 might directly regulate expression of Rorc, we performed Chromatin Immuo-Preciptiation (ChIP) assays with Bcl3 Tg and WT Th1 cells, making use of the Tag on Tg Bcl3 to allow unambiguous detection. We scanned the Rorc regulatory region by dividing it into 8 segments (Lazarevic et al., 2011), and detected an association of Bcl-3 with segments 3 and 6/7 (Figure 6C), regions previously identified as binding sites of c-Rel- and RelA-containing NF-κB complexes important for induced expression of Rorc during Th17 cell differentiation (Ruan et al., 2011). Chip analysis of Th1-differentiated cells still revealed the presence of c-Rel and p50 in both segments, and, importantly, increased association of c-Rel especially in segment 3 in the absence of Bcl-3 (Figure 6D). To confirm that Bcl-3 may directly inhibit c-Rel-p50-mediated expression of Rorc, we made use of an Rorc promoter and enhancer-driven luciferase reporter assay in Jurkat cells. We confirmed that expression vectors for RelA and c-Rel could induce this reporter (Figure S6F). Exogenously introduced c-Rel together with p50/NF-κB1 also stimulated the Rorc-driven reporter, and this was completely abolished in the presence of exogenously introduced Bcl-3 (Figure 6E).
DISCUSSION
The research presented describes a physiologically critical role for the oncoprotein Bcl-3, a modulator of NF-κB-dependent gene transcription. We demonstrate that Bcl-3 constrains the plasticity of Th1 cells and assures their pathogenicity in the setting of autoimmune conditions. Bcl-3 acted cell-autonomously to maintain a pathogenic Th1 cell phenotype and prevent conversion to a Th17-like cell, non-pathogenic phenotype. Thus, in contrast to naïve WT T cells, their Bcl3−/− counterparts failed to induce colitis or weight loss upon transfer into Rag1−/− recipient mice, shifting from pathogenic, IFNγ-producing, to non-pathogenic, IL-17-producing cells. Initial differentiation into either Th1 or Th17 cells was not altered in the absence of Bcl-3. However, when T cells lacking Bcl-3 were fully differentiated into IFNγ-producing Th1 cells, either in vitro or in vivo, they failed to maintain their phenotype upon transfer and converted to Th17-like cells in the context of both the colitis and EAE models. This conversion was also associated with notably decreased production of GM-CSF, a cytokine linked to pathogenicity in both models (Codarri et al., 2011; El-Behi et al., 2011; Griseri et al., 2012). Bcl-3 functioned, at least in part, by repressing expression of the Th17-associated regulator RORγt in Th1 cells. Thus Bcl3−/− Th1 cells exhibited notable expression of RORγt, making them more susceptible to conversion into Th17 cells upon encounter with appropriate skewing signals. Bcl-3 bound to the Rorc regulatory domain, inhibiting c-Rel-NF-κB association and consequent Rorc expression. These findings reveal an unexpected level of regulation of T cell plasticity, and they provide a molecular explanation for why Th1 cells do not readily convert to Th17 cells, while the reverse conversion from Th17 to Th1-like cells has been noted in various pathogenic settings. Targeting Bcl-3 could therefore provide a new path to control plasticity and pathogenicity of T cells.
Inflammatory Bowel Disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and includes Crohn's disease and ulcerative colitis. Inappropriate activation of the mucosal immune system in response to microbial products plays a key role in disease progression and, in the case of Crohn's disease, is associated with activation of in particular Th1 and Th17 cells, the type of IBD most similar to T cell transfer induced colitis (Bene et al., 2011). The role of the Th17 cell signature cytokine IL-17 in Crohn's disease remains controversial though, as initial clinical trials aimed at neutralizing IL-17 worsened the disease, suggestive of an overriding protective rather than inflammatory role for this cytokine in this disease (Marwaha et al., 2012). Furthermore, while blocking Th1 cell responses was quite effective in ameliorating colitis in mice (Ito and Fathman, 1997; Neurath et al., 2002; Uhlig et al., 2006), loss of IL-17 in transferred T cells had the opposite effect (O'Connor et al., 2009). Curiously, IL-23, a cytokine known to drive Th17 cell responses, proved to be critical in various autoimmune disease settings, including IBD and EAE; however, it now appears that this cytokine may over time drive Th17 cells to convert into more pathogenic, IFNγ-expressing Th1-like cells in chronic inflammatory settings (Ahern et al., 2010; Feng et al., 2011; Hirota et al., 2011; Morrison et al., 2013). In our studies, loss of Bcl-3 in the transferred T cells led to the preferential loss of pathogenic IFNγ-producing cells, which converted to IL-17-producing, non-pathogenic cells with significantly reduced expression of GM-CSF, both in the colitis and EAE models. In the latter model, Bcl3−/− T cells failed to accumulate in the CNS, the site of their antigen-driven pathogenic activity (this was also observed when EAE was induced directly upon immunization of naïve mice, without transfer of T cells). Recent evidence suggests that T cell production of GM-CSF in particular may be the primary pathogenic driver in the EAE model, rather than IFNγ or IL-17. Based on these findings, the Bcl-3 pathway could be a potential target for therapeutic intervention in Crohn's disease and multiple sclerosis. Patients with Crohn's disease were found to exhibit elevated levels of Bcl-3 mRNA (O'Carroll et al., 2013). On the other hand, genome-wide association studies identified a risk variant of Bcl-3 in Crohn's disease that appeared to be correlated with lower expression (Fransen et al., 2010). The significance of these observations in patients remains to be determined, especially since Bcl-3 may have divergent functions depending on cell type, and since it can be regulated by multiple mechanisms, not just levels of expression (Hinz et al., 2012; Palmer and Chen, 2008).
The transcription factor RORγt is required for expression of a fully differentiated Th17 cell phenotype (Ivanov et al., 2006). This so-called ‘master regulator’ of Th17 cells is essentially not expressed in Th1-polarized WT T cells, but as shown here, is expressed in Bcl3−/− Th1 cells, albeit at a level well below that in Th17 cells. Upon transfer into recipients, Bcl3−/− Th1s, but not WT Th1s converted to a Th17-like cells, associated with greatly increased expression of RORγt and reduced levels of T-bet. The increased expression of RORγt in Bcl3−/−, as opposed to WT Th1 cells appeared to be biologically relevant, since even WT Th1 cells transduced with RORγt became susceptible to conversion upon exposure to strong Th17 polarizing conditions, while non-transduced cells were resistant. ChIP analyses identified two Bcl-3-associated regions in the Rorc regulatory domain, both of which contain NF-κB binding sites reported to be critical for c-Rel- and RelA-induced expression of RORγt during Th17 differentiation (Ruan et al., 2011). Bcl3−/− Th1 cells exhibited increased association of c-Rel with the Rorc regulatory region, consistent with higher expression, and addition of Bcl-3 abolished c-Rel/p50-induced expression of an Rorc-promoter/enhancer-driven reporter in Jurkat T cells. Bcl-3 appears to help ‘fix’ the pathogenic Th1 phenotype by suppressing expression of RORγt in these cells, but this does not rule out other, yet unknown targets of Bcl-3 that could potentially be involved in this process.
T helper cells are not fully locked into their originally adapted fate (Zhu and Paul, 2010). The potential to convert into a different T helper type, i.e. their plasticity, is reflected in the mixed epigenetic marks – positive and negative – present in all so-called ‘master regulatory’ gene loci, regardless of the Th type analyzed and consistent with the notion that any of these major regulators could potentially be activated in any given Th type, if given the right signals (Kanno et al., 2012). Although many such instances of conversion have been documented, most especially from Th17 to an apparently more pathogenic Th1-like cells in the context of disease progression, including T cell transfer-induced colitis and EAE, the reverse, i.e. conversion from Th1 to Th17 does not appear to occur readily (although it is not totally blocked, as shown here), but the mechanisms underlying the apparent stability of Th1s have not previously been explored (Feng et al., 2011; Lee et al., 2009; Mukasa et al., 2010; Zhu and Paul, 2010). The present study is the first to report that Th1 stability and pathogenicity can be quite readily broken in the absence of Bcl-3, allowing conversion into Th17-like cells associated also with lower expression of GM-CSF, especially at anatomical sites where Th17-polarizing conditions prevail, including the presence of IL-23 and antigens to expand T cells. Whether T helper cells adopt a stable phenotype or exhibit plasticity and how this relates to pathogenicity is of great interest clinically. The identification of Bcl-3 as a positive regulator of pathogenic Th1 cells may open new avenues to ameliorate inflammatory disease pathology.
EXPERIMENTAL PROCEDURES
Mice
Bcl3−/−, Nfkb2−/−, Bcl3 transgenic and conditional Bcl-3−/− mice have been described previously (Franzoso et al., 1998; Franzoso et al., 1997; Tassi et al., 2014; Zhang et al., 2013).. C57BL/6, OTII, Ly5.1 and Rag1−/− mice were purchased from Taconic, Thy1.1, Lck-Cre, Nfkb1−/− and IFNγ-YFP reporter mice from Jackson. Mice were housed in NIAID facilities, and all experiments were done with approval of the NIAID Animal Care and Use Committee and in accordance with all relevant institutional guidelines.
T cell transfer induced colitis
5×105 FACS-sorted CD4+ CD45RBhi CD25− naive T cells from 8-12 weeks-old healthy mice or 1×106 cells Th1 cells were transferred into Rag1−/− mice. All donors and recipients were males. Recipients were weighed weekly and sacrificed about 7 weeks post transfer, when some of mice had lost 20% of their original body. Colon inflammation scores were assessed as described (Izcue et al., 2008).
Experimentally-induced autoimmune encephalomyelitis
200 μg of MOG peptide (35-55) in CFA were injected s.c. into BL/6 mice and 200 ng of pertussis toxin (PT) was delivered i.p.. Auxiliary and inguinal lymph nodes and spleens were harvested 10 days post-immunization and cell suspensions were cultured with 25 μg/mL MOG and 20 ng/mL IL-12 for one week and live cells were transferred into Rag1−/− mice in amounts containing 1×106 CD4+ T cells. The recipients were boosted with 200 μg MOG/CFA/PT on the same day. To induce EAE directly, naive mice were immunized and boosted one-week later with MOG peptide (with CFA and PT as above).
Immunohistochemistry
Colon tissue samples were fixed with 10 ml of 10% formalin buffer and embedded in paraffin. Sections (5 μm) were cut and stained (H&E).
Flow cytometry
100 μm filters were used to prepare single cell suspensions from spleen and lymph node samples Dead cells were removed by gradient centrifugation with lymphocyte M (Cedarlane) and live cells stained with surface antibodies for FACS flow cytometry analysis. To isolate lymphocytes from colon, colon tissue cut into less than 0.1 cm pieces was digested at 37 °C with 3 mg/mL dispase II (Gibco), 1 mg/mL collagenase D (Roche) and 0.1 mg/mL DNase I (Roche) for 1 h.. Digested tissue was passed through 100 μm filters, followed by centrifugation on Percoll gradients.
For intracellular staining, cells were stimulated with PMA, ionomycin and Golgi stop for 4 hours, stained with antibodies for surface markers, permeabilized overnight and finally stained with antibodies for intracellular proteins for 30 min. Data were collected with a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). Antibodies to the following markers were used: CD4 (RM4-5, eBioscience), CD3 (145-2C11, Biolegend), TCRβ (H57-597, BD Biosciences), TCR Vα2 (B20.1, BD Biosciences), IL-22 (Poly5164, Biolegend), IL-17F (O79-289 BD Biosciences), RORγt (AFKJS-9, eBioscience), T-bet (eBio4B10, eBioscience), Thy1.1 (OX-7, BD Biosciences), Thy1.2 (53-2.1, BD Biosciences), Ly5.1 (A20, BD Biosciences), Ly5.2 (104, BD Biosciences), CD25 (PC61, BD Biosciences), Foxp3(FJK-16s, eBioscience), IL-17 (eBio17B7, eBioscience), and IFNγ( XMG1.2, eBioscience).
T cell differentiation
Naïve CD4+ T cells were isolated from 8 weeks-old healthy mice with the Miltenyi T cell Isolation Kit II and seeded at a concentration of 1×105 cells per well in 96-well plates coated with 1 μg/mL anti-CD3 (145-2C11). Anti-CD28 (37.51) was added to the media at 2 μg/mL and for Th1 differentiation also 10 ng/mL IL-12 and 10 μg/mL anti-IL-4 (11B11); for Treg, 100 U/mL IL-2, 2 ng/mL TGFβ, 10 μg/mL anti-IL-12 (C18.2), 10 μg/mL anti-IFNγXMG1.2) and 10 μg/mL anti-IL-4; for Th17, 20 ng/mL IL-6, 5 ng/mL TGFβ, 10 μg/mL anti-IL-12 (C18.2), 10 μg/mL anti-IFNγXMG1.2) and 10 μg/mL anti-IL-4; for Th17+, in addition, IL-1 (10 ng/mL), IL-21 (50 ng/mL) and IL-23 (20 ng/mL). Mitomycin C treated splenocytes were added at 5 × 105 cells per well along with 1 μg/mL anti-CD3 and 2 μg/mL anti-CD28 for enhanced Th1, and for Th17 and Th17+ conditions. Media were changed after 4 days and cells subjected to a second round of differentiation. For Th1 to Th17 or Treg conversion experiments, Th1 cells were subjected to Th17 or Treg conditions for 3 additional weeks, remaining in the same well with media changed every 4 days. Th1 cells were transduced with a GFP-RORγt retrovirus in the presence of 8μg/mL polybrene, as described (Lazarevic et al., 2011). All cytokines and antibodies noted above were purchased from PeproTech and BioXCell, respectively, except anti-IL-12 (C18.2) (eBioscience). In some experiments, Rag1 KO mice were treated for 4 weeks with ampicillin (1 g/L), vancomycin (500 mg/L), neomycin sulfate (1 g/L), and metronidazole (1 g/L) in drinking water for 4 weeks prior to Th1 transfers. Also, in some transfers, recipients were injected i.p. with 0.2 mg of anti-IL23 p19 (BD Biosciences) or anti-IL12/IL23 p40 (BioXCell) neutralizing antibodies/mouse every 3 days.
Real time PCR
RNA was isolated using the RNeasy (Qiagen) according to the manufacturer's instructions. cDNA was synthesized with Superscript III (Invitrogen). Gene expression was quantified with the TaqMan real time PCR primers (Applied Biosystems). The results were normalized against β-actin.
Chromatin-Immunopreciptiation (ChIP)
Naïve T cells, differentiated twice under Th1 conditions and stimulated with plate-bound anti-CD3 for 2h were analyzed for association of Bcl-3 with the Rorc promoter region after precipitation of streptavidin binding peptide-tagged transgenic Bcl-3 with streptavidin resin, followed by real-time PCR with primers corresponding to Rorc promoter regions 1-8, as reported (Lazarevic et al., 2011). To detect association of these regions with subunits of NF-κB in WT and Bcl-3 KO Th1 cells, ChIP grade antibodies as detailed in the experiments were purchased from Santa Cruz Biotechnology.
Statistical Analysis
All data are expressed as the mean ± SD from at least three independent experiments. Differences between groups were evaluated using unpaired Student's t test. p values were considered to be statistically significant when less than 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. L. Glimcher and V. Lazarevic for providing the Rorc-luciferase reporter and the GFP-RORγt retrovirus, E. Shevach for advice with the EAE model, H. Takayanagi and K. Okamoto for the IL-17-driven luciferase reporter and BD Biosciences for α-p19 antibodies. Research was supported by the Intramural Research Program of NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bene L, Falus A, Baffy N, Fulop AK. Cellular and molecular mechanisms in the two major forms of inflammatory bowel disease. Pathology oncology research : POR. 2011;17:463–472. doi: 10.1007/s12253-011-9397-4. [DOI] [PubMed] [Google Scholar]
- Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen K, Visschedijk MC, van Sommeren S, Fu JY, Franke L, Festen EA, Stokkers PC, van Bodegraven AA, Crusius JB, Hommes DW, et al. Analysis of SNPs with an effect on gene expression identifies UBE2L3 and BCL3 as potential new risk genes for Crohn's disease. Human molecular genetics. 2010;19:3482–3488. doi: 10.1093/hmg/ddq264. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, Grinberg A, Tran T, Scharton-Kersten T, Anver M, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Scharton-Kersten T, Shores EW, Epstein S, Grinberg A, Tran T, Shacter E, Leonardi A, Anver M, et al. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479–490. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37:1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Arslan SC, Scheidereit C. It takes two to tango: IkappaBs, the multifunctional partners of NF-kappaB. Immunol Rev. 2012;246:59–76. doi: 10.1111/j.1600-065X.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fathman CG. CD45RBhigh CD4+ T cells from IFN-gamma knockout mice do not induce wasting disease. J Autoimmun. 1997;10:455–459. doi: 10.1016/s0896-8411(97)90152-9. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Vahedi G, Hirahara K, Singleton K, O'Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annual review of immunology. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel D, Sugimoto S, Tietjens J, Zhu J, Yamamoto S, Krupnick AS, Carmody RJ, Gelman AE. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121:265–276. doi: 10.1172/JCI42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado V, Melendez-Zajgla J. Role of Bcl-3 in solid tumors. Mol Cancer. 2003;10:152. doi: 10.1186/1476-4598-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 Cells in Autoimmunity and Immunodeficiency: Protective or Pathogenic? Frontiers in immunology. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PJ, Bending D, Fouser LA, Wright JF, Stockinger B, Cooke A, Kullberg MC. Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal immunology. 2013;6:1143–1156. doi: 10.1038/mi.2013.11. [DOI] [PubMed] [Google Scholar]
- Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll C, Moloney G, Hurley G, Melgar S, Brint E, Nally K, Nibbs RJ, Shanahan F, Carmody RJ. Bcl-3 deficiency protects against dextran-sodium sulphate-induced colitis in the mouse. Clinical and experimental immunology. 2013;173:332–342. doi: 10.1111/cei.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Palmer S, Chen YH. Bcl-3, a multifaceted modulator of NF-kappaB-mediated gene transcription. Immunol Res. 2008;42:210–218. doi: 10.1007/s12026-008-8075-4. [DOI] [PubMed] [Google Scholar]
- Pene F, Paun A, Sonder SU, Rikhi N, Wang H, Claudio E, Siebenlist U. The IkappaB family member Bcl-3 coordinates the pulmonary defense against Klebsiella pneumoniae infection. J Immunol. 2011;186:2412–2421. doi: 10.4049/jimmunol.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994a;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994b;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Zheng SJ, Palmer S, Carmody RJ, Chen YH. Roles of Bcl-3 in the pathogenesis of murine type 1 diabetes. Diabetes. 59:2549–2557. doi: 10.2337/db10-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma LA, Gollin SM, Remstein ED, Ketterling RP, Flynn HC, Rajasenan KK, Swerdlow SH. Splenic small B-cell lymphoma with IGH/BCL3 translocation. Human pathology. 2006;37:218–230. doi: 10.1016/j.humpath.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Tassi I, Claudio E, Wang H, Tang W, Ha HL, Saret S, Ramaswamy M, Siegel R, Siebenlist U. The NF-kappaB Regulator Bcl-3 Governs Dendritic Cell Antigen Presentation Functions in Adaptive Immunity. J Immunol. 2014 doi: 10.4049/jimmunol.1401505. Epub: 2014/09/24, 10.4049/jimmunol.1401505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Paun A, Claudio E, Wang H, Siebenlist U. The tumor promoter and NF-kappaB modulator Bcl-3 regulates splenic B cell development. J Immunol. 2013;191:5984–5992. doi: 10.4049/jimmunol.1300611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27:438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4+ T cell plasticity-Th2 cells join the crowd. Immunity. 2010;32:11–13. doi: 10.1016/j.immuni.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.