Abstract
The local anaesthetic lidocaine is known to block voltage-gated Na+ channels (VGSCs), although at high concentration it was also reported to block other ion channel currents as well as to alter lipid membranes. The aim of this study was to investigate whether the clinical regional anaesthetic action of lidocaine could be accounted for solely by the block of VGSCs or whether other mechanisms are also relevant. We tested the recovery of motor axon conduction and multiple measures of excitability by ‘threshold-tracking’ after ultrasound-guided distal median nerve regional anaesthesia in 13 healthy volunteers. Lidocaine caused rapid complete motor axon conduction block localized at the wrist. Within 3 h, the force of the abductor pollicis brevis muscle and median motor nerve conduction studies returned to normal. In contrast, the excitability of the motor axons at the wrist remained markedly impaired as indicated by a 7-fold shift of the stimulus–response curves to higher currents with partial recovery by 6 h and full recovery by 24 h. The strength–duration properties were abnormal with markedly increased rheobase and reduced strength–duration time constant. The changes in threshold during electrotonus, especially during depolarization, were markedly reduced. The recovery cycle showed increased refractoriness and reduced superexcitability. The excitability changes were only partly similar to those previously observed after poisoning with the VGSC blocker tetrodotoxin. Assuming an unaltered ion-channel gating, modelling indicated that, apart from up to a 4-fold reduction in the number of functioning VGSCs, lidocaine also caused a decrease of passive membrane resistance and an increase of capacitance. Our data suggest that the lidocaine effects, even at clinical ‘sub-blocking’ concentrations, could reflect, at least in part, a reversible structural impairment of the axolemma.
Key points
We tested the recovery of motor axon conduction and multiple measures of excitability by ‘threshold-tracking’ after ultrasound-guided distal median nerve regional anaesthesia by lidocaine.
Lidocaine caused a transient conduction failure that recovered completely by 3 h, whereas excitability recovered only partially by 6 h and fully by 24 h.
The up to 7-fold increase in threshold after complete recovery of conduction was associated with excitability changes that could only partially be explained by block of the voltage-gated Na+ channel (VGSC).
Mathematical modelling indicated that, apart from a reduction in the number of functioning VGSCs, lidocaine also caused a decrease of passive membrane resistance and an increase of capacitance.
Our data suggest that lidocaine, even at clinical ‘sub-blocking’ concentrations, could cause a reversible structural impairment of the axolemma.
Introduction
Axons are complex structures specialized in reliable conduction of action potentials. Various hydrophilic natural toxins and pharmaceutical compounds with high affinity for specific ion channel proteins in the axonal membranes have been found to selectively decrease the voltage-dependent Na+ currents, increase the K+ currents or reduce the inward rectifier currents (Huang 1997). As such, they can reduce the axonal ‘safety factor’ for conduction (Tasaki, 1953) precipitating conduction failure (Hodgkin & Huxley, 1952).
The local anaesthetic lidocaine, synthesized under the name xylocaine by the Swedish chemist Nils Löfgren in 1943, is thought to cause axonal conduction failure (Nathan & Sears, 1961) by impairing the function of the voltage-gated Na+ channel (VGSC) (Sheets & Hanck, 2007). Although various VGSC mutations were found to alter the susceptibility to local anaesthetics (Onizuka et al. 2012), lidocaine was also reported to affect other membrane currents, such as voltage-gated K+ currents (Komai & McDowell, 2001; Panigel & Cook, 2011) and the inward rectifier currents (Raymond, 1992). This apparent lack of specificity of lidocaine, in contrast to, for example, the VGSC block caused by toxins (Stevens et al. 2011), questions its direct actions on ion channels altogether.
Lidocaine, like other ‘caine’-type local anaesthetics consisting of a benzene ring linked to an amine group, dissolves in the lipids of the axolemma (Gokin et al. 2001; Scholz, 2002). If the Meyer–Overton law described over a century ago for general anaesthetics is also applicable to local anaesthetics, anaesthesia could occur when a critical lidocaine concentration is reached in the axolemma (Strichartz, 1973; Butterworth & Strichartz 1990). Such a ‘membrane fluidization’ by lidocaine would not only directly alter its electrical properties (Tabatabai & Booth, 1990; Nau & Wang, 2004) but also could lead to conformational changes in the membrane ion channel proteins indirectly affecting their function (Courtney, 1975). Disentangling the relative importance of effects of lidocaine on the function of voltage-gated ion channels from structural changes of the axolemma of the clinically relevant local anaesthetic actions remains, however, methodologically challenging.
Clues about the membrane biophysical properties of peripheral myelinated axons can be obtained using excitability testing by a computer-controlled ‘threshold tracking’ technique, in which the threshold, based on the stimulus–response curve, is defined as the current required to activate 40–50% of the maximal response (Bostock et al. 1998). A standardized protocol investigating in sequence the threshold changes associated with stimulus duration, electrotonic polarization and after a conditioning response (Kiernan et al. 2000) has been used during the last decade in a wide range of physiological and pathological conditions (Burke et al. 2001; Nodera & Kaji, 2006; Krarup & Moldovan, 2009) allowing interpretation by comparison. Furthermore, a space-clamped ion channel-based mathematical membrane model, referred to hereafter as the ‘Bostock’ model (Bostock et al. 1991), was able to predict the changes in multiple measures of excitability following accidental tetrodotoxin (TTX) poisoning in humans (Kiernan et al. 2005a) and in mutations of VGSC genes (Kiernan et al. 2005b), and can therefore be of aid in interpretation.
The aim of this study was to investigate by nerve excitability testing whether the clinical regional anaesthetic action of lidocaine could be accounted for only by block of VGSC or whether other mechanisms are also relevant. Specifically, we investigated the recovery of conduction and multiple measures of excitability (Bostock et al. 1998) of the motor axons to the abductor pollicis brevis muscle (APB) following ultrasound-guided distal median nerve regional anaesthesia.
Methods
Subjects and experimental design
The study was conducted according to the Helsinki declaration using a protocol approved by the Regional Ethical Committee of the Capital Region of Denmark (protocol no. H-4-2011-075). After informed consent, the non-dominant hand was investigated in 13 healthy volunteers (seven men), 28 ± 3 (mean ± SEM) years old, 182 ± 6 cm tall and 78 ± 6 kg in body weight. The first three subjects were used as methodological controls. Exclusion criteria were a history of peripheral nerve disease including carpal tunnel syndrome and polyneuropathy, diabetes, or a history of adverse reaction to anaesthetics.
Our experimental setup is illustrated in Fig. 1. After baseline median nerve motor conduction and excitability tests prior to application of lidocaine, we carried out a distal ulnar nerve block, followed by a median nerve block at the wrist. The ulnar nerve block was performed to ensure that strong stimulation currents at the wrist would not evoke responses in ulnar nerve innervated muscles and thus contaminate the compound muscle action potential (CMAP) recorded from the APB. The lidocaine injected around the ulnar nerve did not appear to significantly influence the measurements from the median nerve at the wrist through a possible ‘spill-over’ effect (data not shown). Electrophysiological recovery was monitored for 6 h after the median nerve block. Clinical recovery of muscle strength was evaluated using the Medical Research Council scale for muscle strength. Four subjects were also examined 24 h after the block to ascertain the completeness of recovery and reproducibility of findings. Two subjects were recalled for an additional control experiment using only lidocaine vehicle (protocol no. H-2-2014-006).
Figure 1. Experimental setup.

A, photograph of the experimental setup. Compound muscle action potentials (CMAP) were recorded from the abductor pollicis brevis (APB), the abductor digiti quinti (ADQ) and the flexor digitorum (FD) muscles. The median nerve was stimulated at the wrist and elbow whereas the ulnar nerve was stimulated only at the elbow (proximal to the medial epicondyle). B, diagram of the experimental setup indicating the ulnar nerve anaesthesia followed by the median nerve anaesthesia. C, ultrasound image during lidocaine injection (arrow) near the median nerve. The spread of lidocaine (arrow) over several centimetres along the nerve is depicted in the three-dimensional reconstruction to the right.
Ultrasound-guided median and ulnar nerve blocks
We used an ultrasound system with a high frequency linear ultrasound transducer (probe 8870, 1202 Flex Focus 500, BK Medical ApS, Herlev, Denmark) to ensure good nerve visualization during the injection of lidocaine close to the ulnar and median nerves. Concentrations of lidocaine similar to those used in routine peripheral nerve blocks were used. Continuous monitoring included electrocardiography and pulse oximetry. An intravenous catheter was inserted into a superficial cubital vein of the contralateral arm.
Ulnar nerve block
We localized the ulnar nerve in cross-section as close to the wrist as possible as a typical hyper-echoic nerve structure running just ulnar to the ulnar artery, and after disinfection with ethanol/chlorhexidine (83 and 0.5%, respectively) we inserted a 35 mm insulated nerve stimulation needle (Stimuplex D 25 G 35 mm, 15O; B.Braun Melsungen AG, Melsungen, Germany) using an in-plane technique, and placed the needle tip as close to the nerve as possible. We confirmed ulnar nerve identity by visible contractions of hypothenar muscles in response to 2–3 electric impulses (2 Hz, 1.5 mA, 0.1 ms) generated by a nerve stimulator (Stimuplex HNS 12 Peripheral Nerve Stimulator, B.Braun Melsungen AG). After confirmation we aspirated ensuring a non-intravascular needle tip position and injected 1.5 ml of lidocaine 20 mg ml−1 with adrenaline 5 μg ml−1 (Lidokain-Adrenalin ‘SAD’, SAD, Copenhagen, Denmark) and observed perineurial spread of the local anaesthetic on the ultrasound system screen.
Median nerve block
Median nerve block was performed distally in the forearm at two distinct injection sites, each separated by a distance of approximately 5 cm. First, we identified the median nerve in cross-section by performing a dynamic ultrasound scan in proximal–distal direction of the forearm. We performed the distal median nerve block as close to the wrist as possible using a similar ultrasound-guided technique as described above. Median nerve identity was confirmed by eliciting visible contractions of the APB. The time of the distal median block was considered time zero of the experiment. Thereafter, we performed a median nerve block 5 cm proximal to the distal block. At each site we injected 5–6 ml of lidocaine 13 mg ml−1 (obtained by diluting 6.7 ml Lidokain 20 mg ml−1 ‘SAD’, SAD, Copenhagen, Denmark with 3.3 ml isotonic saline) (Fig. 1C).
Vehicle control
To control for the effect of the injection fluid itself, we obtained a lidocaine vehicle solution for human use from the Pharmacy of the Capital Region (Dansk Region Hovedstadens Apotek). Care was taken that the solution was titrated to the same pH as the standard lidocaine solution (Maurer et al. 2012). Serial ultrasound scans (see Fig. 6) were captured from four consecutive anatomical levels ∼2 cm apart starting distal to the stimulation site at wrist (Fig. 6B). Hourly measurements were carried out from all levels up to 3 h after the injection (Fig. 6C).
Figure 6. Effect of lidocaine vehicle.

The effect of lidocaine vehicle (Veh.) was compared to the effect of lidocaine (Lid. + Veh.) in two subjects re-examined for this control experiment. A, mean excitability measures (charge–duration (upper left), recovery cycle (upper right), threshold electrotonus (lower left) and the current–threshold relationships (lower right)) were measured before injection (PRE, full lines), immediately after injection (0H, open circles) and at 3 h (3H, filled circles). The vehicle measurements are shown in grey. Note that the measurements at immediately after injection could only be obtained for the vehicle injection. B, photograph showing four consecutive anatomical levels of injection from distal to proximal from the stimulation site. C, corresponding ultrasound scans (approx. 2 cm in width/1.5 cm in height) for one subject. White arrows indicate the fluid volume around the median nerve just after injection.
Electrophysiological setup and conduction studies
The subjects rested comfortably with the arm extended on a stand covered by hydrophobic cotton (Fig. 1A) under a custom-made rectangular heating lamp thermostatically controlled to maintain the temperature of the stimulated site at 37–38°C, minimizing the effect of temperature changes induced by the regional anaesthetic block (Lange et al. 2011). CMAPs were recorded from the median nerve innervated APB, the ulnar nerve innervated abductor digiti quinti (ADQ) as well as from the median nerve innervated forearm flexor digitorum (FD) muscles (Fig. 1A, B). The CMAPs were recorded through surface electrodes in a tendon–belly configuration (20 Hz–10 kHz, EMG amplifier type 15 C 01, DISA-Dantec, Copenhagen Denmark) using a custom-made data acquisition system (Nikolic & Krarup, 2011). The CMAP amplitude at submaximal and supramaximal stimulation was measured peak-to-peak and the latency of the fastest fibres was measured at the first deflection from baseline (Fig. 2A). The median nerve was stimulated at wrist and elbow whereas the ulnar nerve was stimulated only at elbow (proximal to the epicondylus). Stimulation was carried out via single-use pre-gelled, non-polarizable Ag/AgCl electrodes (Blue Sensor, Ambu, Ballerup, Denmark) with the cathode placed over the nerve at the wrist or elbow and the anode at a distance of 10 cm over the radial forearm or upper arm. A single-use ground electrode was placed on the dorsum of the hand.
Figure 2. Recovery of CMAPs during the first 2 h after lidocaine injection at the median nerve.

A, abductor pollicis brevis (APB) CMAPs evoked after stimulation of the median nerve at the wrist (Wr) and elbow (Elb) and flexor digitorum (FD) muscles CMAPs evoked after stimulation of the median nerve at Elb. B and C, the mean time course of recovery of maximal CMAP amplitude (B) and shortest latency (C) was averaged in three subjects in whom the maximal CMAP recovery was studied in details. Error bars represent SEM.
During threshold tracking, current stimuli were delivered via two linear constant current stimulators each of 50 mA maximal output (DS5, Digitimer Ltd, Welwyn Garden City, UK), connected in parallel. This setup was designed to ensure that adequate stimulation was obtained during high threshold conditions. In control experiments, the two-stimulator setup was found to perform indistinguishably from the single stimulator setup (data not shown). For conduction studies we used negative rectangular current pulses with duration up to 1 ms, repeated with a frequency of 2 Hz or less.
Multiple measures of excitability
Peripheral nerve excitability was assessed using QtracS stimulation software (version 23/10-2012, © Professor Hugh Bostock) and the TRONDNF multiple measures of excitability protocol (Bostock et al. 1998; Kiernan et al. 2000; Tomlinson et al. 2010a). At a sampling rate of 10 kHz the amplified signal was digitized online by computer (PC) that was also used to control the stimulator with an analog-to-digital (A/D) board (NI-6221, National Instruments, Austin, TX, USA).
First, the stimulus–response curves were obtained using test stimuli of 1 ms duration to establish the maximal CMAP (100%) to supramaximal nerve stimulation (Fig. 3A, B). Then, the ‘threshold’ current necessary to evoke a submaximal target potential set to 40–50% of the maximum CMAP could be automatically tracked by trial and error computer feedback. Although threshold is a global index of excitability (i.e. an increase in threshold corresponds to a decrease in excitability), the absolute value of threshold yields little information about the underlying membrane function (Bostock et al. 1998). Instead, relative changes in threshold in various experimental settings were determined using a multiple excitability sequence: strength–duration relationship, threshold electrotonus, current–threshold relationship and recovery cycle. These excitability measures and their derived excitability indices are illustrated in Figs. 3 and 4.
Figure 3. Recovery of motor response threshold from 2 h (2H) to 24 h (24H) after lidocaine as compared to measures prior to lidocaine (PRE).

A, stimulus–response relationships from the wrist (filled circles) and elbow (Elb, triangles) to APB are presented for a single subject before lidocaine injection and at 2 h. Note that in spite of the large right shift in threshold at the wrist, measurements from the elbow remained unchanged. B, mean stimulus–response relationships at the wrist are shown for all subjects at the different time points before and after lidocaine injection. The stimulus current axis is presented on a log scale to facilitate the display of the large changes in threshold. Error bars represent SEM. C–F, dot plots presenting the changes over time of the peak CMAP amplitude (C), threshold latency (D), threshold stimulus (E) and rheobase (F). Hourly means are indicated.
Figure 4. Recovery of motor axon excitability measures after lidocaine block.

A–D, mean changes in threshold electrotonus (A), current–threshold relationship (B), recovery cycle (C) and charge–duration relationship (D). Error bars represent SEM. Measurements are presented prior to lidocaine (PRE, grey line), and at 2 h (2H, open circles), 4 h (4H, open triangles) and 24 h (24H, filled circles). E–H, dot plots presenting the changes over time after lidocaine injection of the corresponding measurements in strength–duration time constant, SDTC (E), threshold reduction during depolarizing threshold electrotonus, TEd(90–100 ms) (F), superexcitability of the recovery cycle at 5 ms (G) and threshold reduction during hyperpolarizing threshold electrotonus, TEh(20–40 ms) (H). Hourly means are indicated.
The strength–duration properties, reflecting primarily the function of nodal membrane (Mogyoros et al. 1996, 2000), were determined by measuring the thresholds for test stimuli with duration of 0.2 to 1 ms. Rheobase (Fig. 3F) and the strength–duration time constant (SDTC, Fig. 4E) were derived from the corresponding linear charge (current strength × duration)–duration relationship using Weiss's law (Bostock, 1983): rheobase from the slope and SDTC from the negative intercept on the duration axis (Fig. 4D).
Accommodation to prolonged subthreshold polarization was ascertained by measuring changes in threshold in response to subthreshold depolarization and hyperpolarization during threshold electrotonus (Fig. 4A) and current–threshold relationship (Fig. 4B). Threshold electrotonus was measured for 100 ms depolarizing and hyperpolarizing currents set to 40% of the control threshold current. The threshold reduction during depolarization was quantified at 90–100 ms, referred to as TEd(90–100 ms) (Fig. 4A, F). The threshold increase (negative threshold reduction) during hyperpolarization was quantified at 20–40 ms, referred to as TEh(20–40 ms) (Fig. 4A, H). Accommodation to even longer polarization, reflecting inward rectification, was investigated at the end of 200 ms current pulses from 50% to −100% of the current–threshold relationship, a threshold analogue of the I–V relationship (Kiernan et al. 2000).
Changes in threshold following a single supramaximal stimulus were tested at intervals from 1.5 to 200 ms during recovery cycle (Fig. 4C). In myelinated axons, the initial increase in threshold a few milliseconds after the conditioning stimulus (relative refractoriness) is followed by a superexcitable (a smaller than normal threshold) and a late subexcitable (larger than normal threshold) period (Barrett & Barrett, 1982). Here we quantified the superexcitability after 5 ms (Fig. 4G).
Modelling of myelinated axons
Using Hodgkin–Huxley type differential equations (Hodgkin & Huxley, 1952), the ‘Bostock’ model (Bostock et al. 1991) accounts for the transient and persistent Na+ currents, the slow and fast K+ currents, the inward rectifier current, the Na+/K+ pump current and the passive leak currents distributed in distinct nodal and internodal capacitive membrane compartments linked via a Barrett–Barrett resistance (Barrett & Barrett, 1982). Here we used the equations and parameters from a recently revised version of the model that accounts also for the subtle biophysical differences between myelinated motor and sensory axons (Howells et al. 2012).
The model optimization was provided by MEMFIT, part of QTRACP (version 15/4-2013, © Professor Hugh Bostock). The software allows for 1, 2 or 3 of 66 parameters to be automatically changed, simultaneously or in turn, so that the discrepancy (measured in % change in error) between the model output and the measured multiple excitability measures is reduced.
Data analysis
Quantitative changes are given as mean ± SEM. Analysis of multiple measures of nerve excitability was carried out using QtracP software (version 15/4-2013, © Professor Hugh Bostock). Due to the relatively small number of samples per group, the normality of the distributions could not be reliably ascertained. As such, distribution-free non-parametric statistical comparison tests were preferred. Difference levels of P < 0.05 were considered significant.
Results
The transient conduction failure
In all subjects, lidocaine caused complete conduction failure and APB paralysis when tested at 30 min (Fig. 2). No measurable APB CMAP could be recorded when stimulating the median nerve at the wrist (anaesthetized region) up to the maximum stimulator output of 80–90 mA at a stimulus duration of 1 ms (Fig. 2A, B). In addition, CMAP could also not be recorded from the APB when stimulating the median nerve at the elbow (un-anaesthetized region) at an intensity that evoked a maximal CMAP at the FD (Fig. 2A, B).
From 2 h after lidocaine, a CMAP at the APB could again be evoked by stimulating the median nerve at the wrist, whereas the recovery from the elbow was ∼1 h further delayed (Fig. 2A, B). Of note, the early submaximal APB responses that could be evoked from the elbow had prolongation of latency (conduction slowing) of more than 25% (Fig. 2A, C).
Recovery of threshold
After 2–3 h, the CMAP amplitude and latency and force of the APB were normal. The excitability of the median nerve at the wrist remained, however, markedly impaired as indicated by the shift of the stimulus–response curves to higher currents (Fig. 3A, B). Note that the stimulus–response curves (Fig. 3A) and excitability measures (data not shown) from the elbow were normal. At the wrist, the stimulus threshold (for a 1 ms pulse) was increased from 8.6 ± 2 to 36.7 ± 3 mA (Fig. 3E; P < 0.01) measured as a 7-fold increase in rheobase (Fig. 3F; P < 0.01). These changes in threshold recovered slowly during the next few hours (Fig. 3B, E, F), whereas the peak response (Fig. 3C) and threshold latency (Fig. 3D) remained unchanged.
Recovery of the multiple measures of excitability
The deviations in excitability were largest at 2 h following lidocaine (Fig. 4). Recording of some excitability measures, typically the current–threshold relationship, had to be skipped at 2 h to avoid discomfort at high stimulus currents. At 3 h, there was a shortening of the SDTC from 415 ± 30 to 145 ± 8 μs (Fig. 4D, E; P < 0.01). The changes during electrotonic depolarization (Fig. 4A) were markedly abnormal. After 90 ms of 40% subthreshold depolarization, the threshold reduction normally accommodated to plateau of TEd(90–100 ms) ∼40% of threshold (Fig. 4A, F). At 3 h after lidocaine, TEd(90–100 ms) dropped to nearly 0 (0.9 ± 5% of threshold, P < 0.01). In parallel, the changes during electrotonic hyperpolarization, measured at TEh(20–40 ms), indicated a 37% smaller than normal threshold increase upon hyperpolarization after 30 ms (Fig. 4A, H; P < 0.01). The recovery cycle was also abnormal, indicating a larger increase in threshold at short inter-stimulus intervals (Fig. 4C). This ‘increased refractoriness’ occurred at the expense of the superexcitable period, as indicated by a decrease in superexcitability at 5 ms inter-stimulus interval by 22% of threshold (Fig. 4G; P < 0.01).
The excitability measures recovered slowly and were the same at 24 h as before the injection of lidocaine (Fig. 4). The longest lasting abnormality was the change during electrotonic depolarization, as seen as an inward shift on the depolarizing threshold electrotonus (Fig. 4A) and a left shift of the depolarizing (upper half) current–threshold relationship. Thus, at 6 h after lidocaine, TEd(90–100 ms) remained significantly reduced to 35.1 ± 3 % of threshold (Fig. 4F; P < 0.05).
Mathematical electrochemical modelling of the deviations in excitability measures
The mean of the control measurements (PRE) corresponded to the amended ‘Bostock’ model (Howells et al. 2012) after increasing the temperature (Tabs) by 3°C (Fig. 5A) in accordance with our temperature control at 37°C (see Methods).
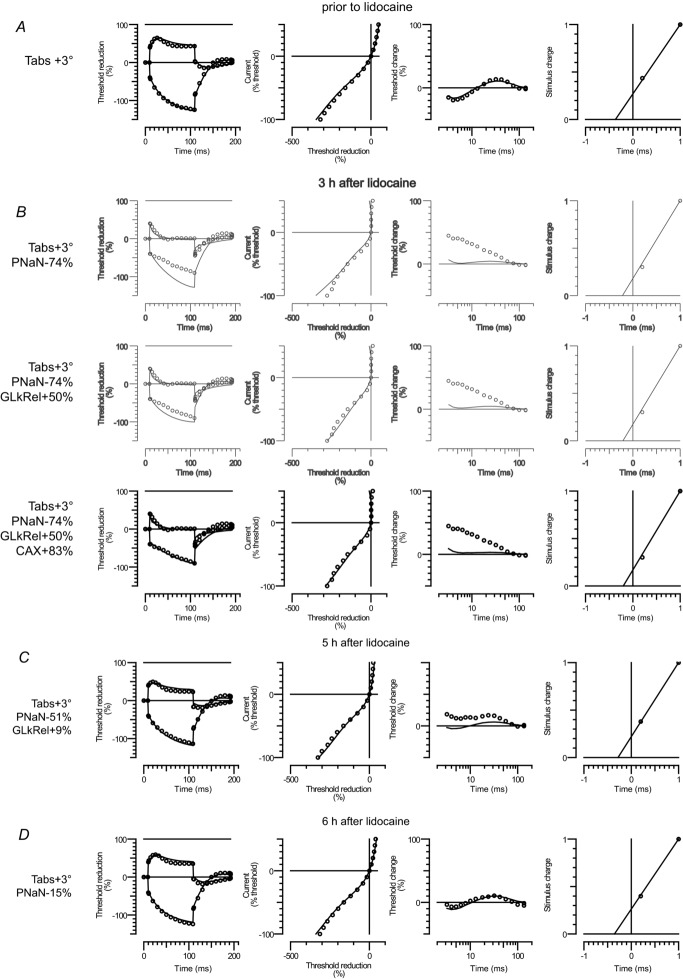
Figure 5. Interpretation of lidocaine effects using the ‘Bostock’ mathematical model.
The four corresponding excitability measures are presented in rows from left to right: threshold electrotonus, current–threshold relationship, recovery cycle and charge–duration relationship. Open circles indicate mean excitability measurements recorded prior to lidocaine (A) and at 3 h after lidocaine (B), 5 h after lidocaine (C) and 6 h after lidocaine (D). Note that the first two rows in B are shown in grey as they reflect unsatisfactory intermediate fits. The modelled rheobase is scaled down to the single axon by setting the charge for a 1 ms pulse as 1. The continuous line indicates the modelled excitability measures. The changed parameters from the reference model (Howells et al. 2012) are indicated to the left: absolute temperature (Tabs), VGSC permeability (PNaN), relative leak conductance (GLkRel) and axonal capacitance (CAX).
A limitation of the current software implementation of the Bostock model is that the stimulus charge for a 1 ms stimulus is pre-scaled to 1 for all investigated conditions (Fig. 5, right column). We therefore weighted the model optimization on threshold changes during electrotonic depolarization TEd(90–100 ms) that showed the largest relative change after lidocaine (Fig. 4F). We first attempted to fit the model to the recordings at 3 h (mean and SD) by changing a single parameter (data not shown). This pointed towards a reduction in VGSC permeability (PNaN); however, the reduction in discrepancy remained unsatisfactory. Varying the gating properties of the modelled VGSC in addition to PNaN did not add a significant improvement either (data not shown). We therefore attempted to improve the fit by optimizing three parameters (two in addition to PNaN) while leaving the PNaN gating unmodified.
Modelling indicated that at 3 h after lidocaine (Fig. 5B) there was a 74% reduction in VGSC permeability (PNaN), which recovered to about half by 5 h (Fig. 5C). At 6 h, all excitability deviations could be reasonably explained by a 15% reduction in PNaN (Fig. 5D). Nevertheless, at earlier time points (less lidocaine wash-out), modelling indicated an additional alteration in passive electrical membrane properties: an increase in axonal conductance (GLkRel) by up to 50% and an increase in axonal capacitance (CAX) by up to 83% (Fig. 5B, C). Note that the model, although predicting the measured reduction in superexcitability, underestimated the extent of the progressive increase in threshold at short inter-stimulus intervals (Fig. 5B, C).
No confounding effect of injection ‘fluid’
The fact that the modelling indicated a change in the passive electrical properties in addition to a reduction in VGSC permeability raised the possibility that such changes could reflect electrical alterations in the near-nerve environment rather than true axonal changes. To test this possibility directly we carried out an additional set of control experiments, monitoring the effect of lidocaine vehicle (Fig. 6). We found that at 3 h, when the described effects of lidocaine were maximal, the excitability after vehicle was unchanged (Fig. 6A) and no fluid around the nerve could be detected by ultrasound (Fig. 6C). Furthermore, in the first hour after injection when the fluid around the stimulation site was maximal (Fig. 6B, C), excitability measures remained unchanged (Fig. 6A). We therefore concluded that the injection fluid did not confound the effects of lidocaine.
Discussion
Longitudinal studies in normal subjects of motor axon function during and after regional lidocaine anaesthesia showed a marked discrepancy between the complete recovery of conduction and force while recovery of motor axon excitability lagged hours behind the amplitude and conduction velocity of the CMAP. Assuming an unaltered ion-channel gating, modelling indicated that the lidocaine effects were not limited to reduction of the VGSC currents but that lidocaine also increased passive membrane conductance and capacitance. This indicates that effects of lidocaine, even at clinical ‘sub-blocking’ concentrations, may reflect, at least in part, a transient structural impairment of the axolemma.
Transient conduction block after lidocaine
With increasing concentrations of anaesthetics, previous electrophysiological studies of ‘-caines’ (Gasser & Erlanger, 1929; Gasser & Grundfest, 1939) found a progressive decrease in amplitude, slowing of conduction and increase in threshold of the compound nerve action potential (CNAP). As CNAPs reflect the summated electrical field of axons with different conduction velocities and stimulation thresholds (Gasser & Erlanger, 1927), it remained possible that the progressive conduction failure reflected merely the progressive inability of the stimulation to overcome the anaesthetic-induced increase in threshold (Nathan & Sears, 1962; Kassahun et al. 2010). Nevertheless, experimental studies of single fibre preparations indicated that there is in fact a ‘critical dose’ of local anaesthetic for which all or none action potential generation is no longer possible, the susceptibility to anaesthetic conduction block being different between neuronal populations according to their functional specializations (Butterworth & Strichartz, 1990; Park et al. 2012) consistent with differences in their ‘safety factor’ for conduction (Tasaki, 1939; Franz & Perry, 1974; Gordh, 2010; Moldovan et al. 2013).
In our study we tracked the effect of lidocaine on the CMAP of the APB because: (1) the CMAP size depends exclusively on summation of all or none activated single motor units (the motor fibres innervated by a single motor axons); (2) the investigated fibre population consists of only a few hundred functionally homogeneous myelinated axons (Neuwirth et al. 2011); and (3) the ‘Bostock’ model could reliably be used to aid interpretation (Bostock et al. 1991; Howells et al. 2012).
No CMAP could be recorded from the APB by electrical stimulation of the median nerve at the wrist (anaesthetized region) or elbow (un-anaesthetized region) within 30 min following lidocaine, whereas the forearm FD CMAP evoked at the elbow remained maximal, consistent with an anaesthetic conduction block (Fig. 2). Moreover, the recovery of APB response from the elbow was delayed as compared to the wrist (Fig. 2B), probably due to the spatial spread of lidocaine along the nerve (Fig. 1C). The action potentials originating at the elbow had to propagate through a longer region with reduced ‘safety factor’ of conduction than from the wrist (Nakamura et al. 2003), as indicated by the greater increase in latency (Fig. 2C).
Impaired excitability after lidocaine in the ‘sub-blocking’ range
Within 3 h after lidocaine, the APB force as well as median nerve conduction studies returned to normal. In contrast, even at these ‘sub-blocking’ concentrations, median nerve excitability at the wrist (the region exposed directly to lidocaine) remained markedly abnormal, then recovered incompletely by 6 h and completely by 24 h. Lidocaine shifted the stimulus–response curves to higher currents with an increase in rheobase (Fig. 3). Such a shift (increase in threshold) could be related to a direct VGSC block, as was found in TTX poisoning (Kiernan et al. 2005a) as well as in mutations of specific VGSCs (Kiernan et al. 2005b). Nevertheless, membrane hyperpolarization (Kiernan & Bostock, 2000; Moldovan & Krarup, 2004), mutations in voltage-gated K+ currents (Tomlinson et al. 2010b), decreased axonal calibre (Yang et al. 2000) and even physical alterations of the lipid membrane by inert gases (Kassahun et al. 2010) were also found to shift the stimulus–response curves. As such, the increase in axonal threshold per se was not informative about the cause of reduced excitability.
Clues about the mechanism underlying abnormal nerve excitability can be identified when there are concordant changes in multiple measures of axonal excitability (Burke et al. 2001; Krarup & Moldovan, 2009). The combination of increased rheobase with reduced strength–duration time constant, smaller threshold decrease during tonic depolarization and reduced superexcitable period of the recovery cycle encountered at 6 h following lidocaine (Fig. 3) was, to our knowledge, encountered only in TTX poisoning (Kiernan et al. 2005b) which supported a lidocaine block of the VGSC. Nevertheless, at earlier time points (higher lidocaine concentrations), there was a smaller than normal threshold increase during hyperpolarizing electrotonus and the current–threshold relationship (Fig. 4), which was opposite to that observed in TTX-poisoned patients (Kiernan et al. 2005b). This raised the suspicion that, with increasing lidocaine concentrations, other effects beside VGSC block aggravated the impairment in excitability.
Interpretation of the excitability changes after lidocaine through the ‘Bostock’ model
The simple ‘Bostock’ mathematical model was able to identify a reduction in VGSC permeability (PNaN) by about 50% as the main mechanism responsible for the excitability changes at 21 h after poisoning with TTX (Kiernan et al. 2005a). Using an updated version of the model (Howells et al. 2012), we found that at 6 h following lidocaine, excitability deviations could also be reasonably explained by a 15% reduction in PNaN (Fig. 5D). At 3 h, close to the time at which conduction completely recovered, the model indicated a reduction in PNaN by 74% (Fig. 5B). The magnitude of this estimated ‘sub-blocking’ VGSC permeability change fitted remarkably with the ‘safety factor’ predictions, i.e. that VGSC permeability had to drop by more than 80% for conduction failure to occur in myelinated axons (Tasaki, 1953). Nevertheless, at these higher lidocaine concentrations, decreasing PNaN failed to account for the smaller than normal threshold increase after lidocaine during hyperpolarizing electrotonus (Fig. 5B, C). Furthermore, the model underestimated the extent of the progressive increase in threshold at short inter-stimulus intervals during the recovery cycle (Fig. 5B, C).
Earlier studies of lidocaine derivatives showed an additional progressive decrease of the peak Na+ currents at high frequency impulse repetition (Strichartz, 1973), referred to as use-dependent block or ‘phasic block’ of VGSC to differentiate it from ‘tonic block’ occurring at low stimulation frequencies (Starmer et al. 1984). In our experiments, the baseline stimulation rate for conduction and excitability studies was below 2 Hz, which places our lidocaine-induced conduction block well within the ‘tonic block’ paradigm (Butterworth & Strichartz, 1990; Gokin et al. 2001; Park et al. 2012). Nevertheless, during recovery cycle measurements higher stimulation rates were attained and we cannot exclude that the large increase in threshold at short inter-stimulus intervals was confounded by a ‘phasic block’ phenomenon, which was not modelled. Regardless, varying the standard time and voltage gating properties of the VGSC included in the ‘Bostock’ model (Howells et al. 2012) did not improve the model prediction beyond the simple PNaN reduction (data not shown). Although the software model implementation did not allow accurate estimation of the magnitude of the rheobase changes, it was also theoretically unlikely that a reduction in VGSC currents alone, whatever the gating alterations, could lead to the measured 7-fold change in threshold (Noble, 1966). Thus, modelling indicated that, with increasing lidocaine concentrations (less washout), mechanisms other than reduced VGSC currents were physiologically important.
Assuming unaltered ion-channel gating properties, the discrepancy between the modelled and recorded excitability changes at high lidocaine concentrations could be greatly reduced by changing, in addition to PNaN, the passive (non-voltage-dependent) properties of the axolemma, namely an increase in the membrane leak conductance (GLkRel) and an increase in axonal capacitance (CAX). These passive changes could not be attributed to changes in the near nerve environment, i.e. due to the ‘fluid accumulation’ around the nerve (Fig. 6). Furthermore, a change in the passive electrical properties of the axonal membrane was not experimentally unrealistic as a reduced membrane resistance (increase in conductance) of similar magnitude was previously measured after lidocaine (Tabatabai & Booth, 1990; Nau & Wang, 2004), as well as an increased membrane capacitance (Tabatabai & Booth, 1990). The change in three parameters from the 66 ‘Bostock’ model parameters (including various ion channel gating properties) could also not be regarded as simple ‘parameterization’. In fact, our data indicate that changes in voltage-gated K+ currents (Komai & McDowell, 2001; Panigel & Cook, 2011), inward rectifier currents (Raymond, 1992) and membrane potential (Tabatabai & Booth, 1990), previously reported to be altered with high concentrations of lidocaine, did not appear to contribute decisively to the alterations in excitability in the investigated clinical situation.
In conclusion, mathematical modelling suggested that the reduction of excitability after lidocaine, at concentrations that do not block conduction of action potentials, could not be explained solely by a reduction in VGSC currents. In addition, there was a change of two voltage-independent parameters (axonal resistance and capacitance), both reflecting axolemma structure.
Relevance of effects of lidocaine on the axolemma
The current wisdom is that lidocaine impairs excitability to the extent of conduction failure by interacting with the VGSC and reducing the regenerative Na+ influx. Although the local anaesthetic binding site has not been elucidated completely, several lines of evidence suggest that it is located on the inner pore of the VGSC, and that it is more accessible in the open channel state (Fozzard et al. 2011). To reach the binding site, however, lidocaine has to diffuse through the axolemma in its uncharged (base) form and then concentrate inside the membrane as a charged (protonated) form (Sano et al. 1999). This long recognized ‘membrane solubility’ of lidocaine (as well as other local anaesthetics) (Ritchie & Greengard, 1966) fuelled the alternative view that the presence of lidocaine in the axolemma could also indirectly affect the VGSC gating (Lee, 1976). More recent studies using X-ray scattering techniques confirmed that lidocaine and other related local anaesthetics alter the membrane structure by increasing its thickness/stiffness (Mateu et al. 1997; Luzzati et al. 1999) to an extent that it can impair the VGSC function (Hendry et al. 1985).
The reduction in Na+ current predicted by our study could not distinguish between a direct and an indirect effect of lidocaine on the VGSC. Nevertheless, it is tempting to suspect that the predicted increase in capacitance reflected the structural alteration of the membrane by lidocaine (Fernandez et al. 1983; Hendry et al. 1985). Furthermore, the structural membrane alterations could also be responsible for the predicted increase of the voltage-independent membrane conductance either directly by altering the electrical properties of the axolemma or indirectly by increasing the conductance of some voltage-independent ‘leak’ channels, such as the two-pore domain K+ channel family that was reported to be opened by anaesthetics (Patel et al. 1999). Taken together, both the increase in capacitance and the leakiness of the membrane induced by lidocaine aggravate the excitability impairment induced by VGSC block, further reducing the safety factor for conduction and thus enhancing the anaesthetic effect.
Since the description of the cauda equina syndrome occurring after a lidocaine overdose (Rigler et al. 1991), it has become apparent that, at high concentrations, lidocaine is neurotoxic, leading to irreversible conduction block (Lambert et al. 1994) by exerting a detergent-like disruption of the membrane structure (Kitagawa et al. 2004). A similar structural disruption could also be responsible for the reported antibacterial properties of lidocaine (Tustin et al. 2014). Nevertheless, the dogma remained that, at low but clinically effective lidocaine concentrations, the alterations in axolemma structure are of little consequence (Strichartz, 1973; Putrenko & Schwarz, 2011). In contrast, our data suggest that even at concentrations that do not prevent conduction of action potentials, lidocaine causes reversible structural axolemma alterations that aggravate the excitability impairment due to the reduction of Na+ currents. Recognizing the relevance of these structural effects at sub-anaesthetic concentrations could be important for understanding lidocaine actions beyond VGSC block, such as the reported analgesic effectiveness of both systemic lidocaine (Mao & Chen, 2000) and topical lidocaine (Argoff, 2013) for the management of chronic pain.
Acknowledgments
Brief reports of these studies were presented at the 26th Nordic Congress of Clinical Neurophysiology, Lund, Sweden, in May 2012 and at the 15th Clinical Neurophysiology Workshop of the Australian and New Zealand Association of Neurologists (Festschrift for David Burke)’, Gold Coast, Australia, in September 2013.
Glossary
- ADQ
abductor digiti quinti
- APB
abductor pollicis brevis
- CMAP
compound muscle action potential
- CNAP
compound nerve action potential
- FD
flexor digitorum
- SDTC
strength–duration time constant
- TTX
tetrodotoxin
- VGSC
voltage-gated Na+ channel
Additional information
Competing interests
None declared.
Author contributions
C.K., N.V.O. and M.M. designed the study. K.H.W.L. and N.J.A.A. carried out the ultrasound-guided lidocaine injection. All authors contributed to the lidocaine and control experiments. M.M. analysed the data and wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final submission.
Funding
The project was supported by the Lundbeck Foundation and the Danish Medical Research Council (C.K.) and The Research Foundation of University of Copenhagen (N.V.O.).
References
- Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013;88:195–205. doi: 10.1016/j.mayocp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H. The strength–duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. J Physiol. 1983;341:59–74. doi: 10.1113/jphysiol.1983.sp014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M, Reid G. Changes in excitability of human motor axons underlying post-ischaemic fasciculations: evidence for two stable states. J Physiol. 1991;441:537–557. doi: 10.1113/jphysiol.1991.sp018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol. 2001;112:1575–1585. doi: 10.1016/s1388-2457(01)00595-8. [DOI] [PubMed] [Google Scholar]
- Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Courtney KR. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- Fernandez JM, Taylor RE, Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983;82:331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard HA, Sheets MF, Hanck DA. The sodium channel as a target for local anesthetic drugs. Front Pharmacol. 2011;2:68. doi: 10.3389/fphar.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Perry RS. Mechanisms for differential block among single myelinated and non-myelinated axons by procaine. J Physiol. 1974;236:193–210. doi: 10.1113/jphysiol.1974.sp010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, Erlanger J. The role played by the sizes of the constituent fibers of a nerve trunk in determining the form of its action potential wave. Am J Physiol. 1927;80:522–547. [Google Scholar]
- Gasser HS, Erlanger J. The role of fiber size in the establishment of a nerve block by pressure or cocaine. Am J Physiol. 1929;88:581–591. [Google Scholar]
- Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Physiol. 1939;127:393–414. [Google Scholar]
- Gokin AP, Philip B, Strichartz GR. Preferential block of small myelinated sensory and motor fibers by lidocaine: in vivo electrophysiology in the rat sciatic nerve. Anesthesiology. 2001;95:1441–1454. doi: 10.1097/00000542-200112000-00025. [DOI] [PubMed] [Google Scholar]
- Gordh T. Lidocaine: the origin of a modern local anesthetic. Anesthesiology. 2010;113:1433–1437. doi: 10.1097/ALN.0b013e3181fcef48. [DOI] [PubMed] [Google Scholar]
- Hendry BM, Elliott JR, Haydon DA. Further evidence that membrane thickness influences voltage-gated sodium channels. Biophys J. 1985;47:841–845. doi: 10.1016/S0006-3495(85)83988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells J, Trevillion L, Bostock H, Burke D. The voltage dependence of Ih in human myelinated axons. J Physiol. 2012;590:1625–1640. doi: 10.1113/jphysiol.2011.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, Thalhammer JG, Raymond SA, Strichartz GR. Susceptibility to lidocaine of impulses in different somatosensory afferent fibers of rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:802–811. [PubMed] [Google Scholar]
- Kassahun BT, Murashov AK, Bier M. A thermodynamic mechanism behind an action potential and behind anesthesia. Biophys Rev Lett. 2010;5:35–41. [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123:2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Isbister GK, Lin CS, Burke D, Bostock H. Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann Neurol. 2005a;57:339–348. doi: 10.1002/ana.20395. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Krishnan AV, Lin CS, Burke D, Berkovic SF. Mutation in the Na+ channel subunit SCN1B produces paradoxical changes in peripheral nerve excitability. Brain. 2005b;128:1841–1846. doi: 10.1093/brain/awh520. [DOI] [PubMed] [Google Scholar]
- Kitagawa N, Oda M, Totoki T. Possible mechanism of irreversible nerve injury caused by local anesthetics: detergent properties of local anesthetics and membrane disruption. Anesthesiology. 2004;100:962–967. doi: 10.1097/00000542-200404000-00029. [DOI] [PubMed] [Google Scholar]
- Komai H, McDowell TS. Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology. 2001;94:1089–1095. doi: 10.1097/00000542-200106000-00025. [DOI] [PubMed] [Google Scholar]
- Krarup C, Moldovan M. Nerve conduction and excitability studies in peripheral nerve disorders. Curr Opin Neurol. 2009;22:460–466. doi: 10.1097/WCO.0b013e3283304c9d. [DOI] [PubMed] [Google Scholar]
- Lambert LA, Lambert DH, Strichartz GR. Irreversible conduction block in isolated nerve by high concentrations of local anesthetics. Anesthesiology. 1994;80:1082–1093. doi: 10.1097/00000542-199405000-00017. [DOI] [PubMed] [Google Scholar]
- Lange KH, Jansen T, Asghar S, Kristensen PL, Skjonnemand M, Norgaard P. Skin temperature measured by infrared thermography after specific ultrasound-guided blocking of the musculocutaneous, radial, ulnar, and median nerves in the upper extremity. Br J Anaesth. 2011;106:887–895. doi: 10.1093/bja/aer085. [DOI] [PubMed] [Google Scholar]
- Lee AG. Model for action of local anaesthetics. Nature. 1976;262:545–548. doi: 10.1038/262545a0. [DOI] [PubMed] [Google Scholar]
- Luzzati V, Mateu L, Marquez G, Borgo M. Structural and electrophysiological effects of local anesthetics and of low temperature on myelinated nerves: implication of the lipid chains in nerve excitability. J Mol Biol. 1999;286:1389–1402. doi: 10.1006/jmbi.1998.2587. [DOI] [PubMed] [Google Scholar]
- Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87:7–17. doi: 10.1016/S0304-3959(00)00229-3. [DOI] [PubMed] [Google Scholar]
- Mateu L, Moran O, Padron R, Borgo M, Vonasek E, Marquez G, Luzzati V. The action of local anesthetics on myelin structure and nerve conduction in toad sciatic nerve. Biophys J. 1997;72:2581–2587. doi: 10.1016/S0006-3495(97)78901-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer K, Bostock H, Koltzenburg M. Protons regulate the excitability properties of rat myelinated sensory axons in vitro through block of persistent sodium currents. J Peripher Nerv Syst. 2012;17:102–111. doi: 10.1111/j.1529-8027.2012.00381.x. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D. Strength–duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Lin CS, Kuwabara S, Cappelen-Smith C, Burke D. Strength–duration properties and their voltage dependence as measures of a threshold conductance at the node of Ranvier of single motor axons. Muscle Nerve. 2000;23:1719–1726. doi: 10.1002/1097-4598(200011)23:11<1719::aid-mus8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Alvarez S, Romer Rosberg M, Krarup C. Axonal voltage-gated ion channels as pharmacological targets for pain. Eur J Pharmacol. 2013;708:105–112. doi: 10.1016/j.ejphar.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Mechanisms of hyperpolarization in regenerated mature motor axons in cat. J Physiol. 2004;560:807–819. doi: 10.1113/jphysiol.2004.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Popitz-Bergez F, Birknes J, Strichartz GR. The critical role of concentration for lidocaine block of peripheral nerve in vivo: studies of function and drug uptake in the rat. Anesthesiology. 2003;99:1189–1197. doi: 10.1097/00000542-200311000-00028. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Sears TA. Some factors concerned in differential nerve block by local anaesthetics. J Physiol. 1961;157:565–580. doi: 10.1113/jphysiol.1961.sp006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Sears TA. Differential nerve block by sodium free and sodium-deficient solutions. J Physiol. 1962;164:375–394. doi: 10.1113/jphysiol.1962.sp007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C, Wang GK. Interactions of local anesthetics with voltage-gated Na+ channels. J Membr Biol. 2004;201:1–8. doi: 10.1007/s00232-004-0702-y. [DOI] [PubMed] [Google Scholar]
- Neuwirth C, Nandedkar S, Stalberg E, Barkhaus PE, Carvalho M, Furtula J, Dijk JP, Baldinger R, Castro J, Costa J, Otto M, Sandberg A, Weber M. Motor Unit Number Index (MUNIX): a novel neurophysiological marker for neuromuscular disorders; test–retest reliability in healthy volunteers. Clin Neurophysiol. 2011;122:1867–1872. doi: 10.1016/j.clinph.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Krarup C. EMGTools, an adaptive and versatile tool for detailed EMG analysis. IEEE Trans Biomed Eng. 2011;58:2707–2718. doi: 10.1109/TBME.2010.2064773. [DOI] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin–Huxley equations to excitable tissues. Physiol Rev. 1966;46:1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]
- Nodera H, Kaji R. Nerve excitability testing and its clinical application to neuromuscular diseases. Clin Neurophysiol. 2006;117:1902–1916. doi: 10.1016/j.clinph.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Tamura R, Yonaha T, Oda N, Kawasaki Y, Shirasaka T, Shiraishi S, Tsuneyoshi I. Clinical dose of lidocaine destroys the cell membrane and induces both necrosis and apoptosis in an identified Lymnaea neuron. J Anesth. 2012;26:54–61. doi: 10.1007/s00540-011-1260-y. [DOI] [PubMed] [Google Scholar]
- Panigel J, Cook SP. A point mutation at F1737 of the human Nav1.7 sodium channel decreases inhibition by local anesthetics. J Neurogenet. 2011;25:134–139. doi: 10.3109/01677063.2011.629702. [DOI] [PubMed] [Google Scholar]
- Park JS, Jung TS, Noh YH, Kim WS, Park WI, Kim YS, Chung IK, Sohn UD, Bae SK, Bae MK, Jang HO, Yun I. The effect of lidocaine. HCl on the fluidity of native and model membrane lipid bilayers. Korean J Physiol Pharmacol. 2012;16:413–422. doi: 10.4196/kjpp.2012.16.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Putrenko I, Schwarz SK. Lidocaine blocks the hyperpolarization-activated mixed cation current, Ih, in rat thalamocortical neurons. Anesthesiology. 2011;115:822–835. doi: 10.1097/ALN.0b013e31822ddf08. [DOI] [PubMed] [Google Scholar]
- Raymond SA. Subblocking concentrations of local anesthetics: effects on impulse generation and conduction in single myelinated sciatic nerve axons in frog. Anesth Analg. 1992;75:906–921. doi: 10.1213/00000539-199212000-00008. [DOI] [PubMed] [Google Scholar]
- Rigler ML, Drasner K, Krejcie TC, Yelich SJ, Scholnick FT, DeFontes J, Bohner D. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281. doi: 10.1213/00000539-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Greengard P. On the mode of action of local anesthetics. Annu Rev Pharmacol. 1966;6:405–430. doi: 10.1146/annurev.pa.06.040166.002201. [DOI] [PubMed] [Google Scholar]
- Sano S, Yokono S, Kinoshita H, Ogli K, Satake H, Kageyama T, Kaneshina S. Intra-axonal continuous measurement of lidocaine concentration and pH in squid giant axon. Can J Anaesth. 1999;46:1156–1163. doi: 10.1007/BF03015526. [DOI] [PubMed] [Google Scholar]
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol. 2007;582:317–334. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer CF, Grant AO, Strauss HC. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984;46:15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Peigneur S, Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front Pharmacol. 2011;2:71. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai M, Booth AM. Effects of lidocaine on the excitability and membrane properties of the nerve cell soma. Clin Physiol Biochem. 1990;8:289–296. [PubMed] [Google Scholar]
- Tasaki I. The electro-saltatory transmision of the nerve impulse and the effect of narcosis upon the nerve fiber. Am J Physiol. 1939;127:211–227. [Google Scholar]
- Tasaki I. Nervous Transmission. Springfield, IL: Charles C. Thomas; 1953. [Google Scholar]
- Tomlinson S, Burke D, Hanna M, Koltzenburg M, Bostock H. In vivo assessment of HCN channel current (Ih) in human motor axons. Muscle Nerve. 2010a;41:247–256. doi: 10.1002/mus.21482. [DOI] [PubMed] [Google Scholar]
- Tomlinson SE, Tan SV, Kullmann DM, Griggs RC, Burke D, Hanna MG, Bostock H. Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain. 2010b;133:3530–3540. doi: 10.1093/brain/awq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustin A, Kim SJ, Chomsky A, Hubbard GB, 3rd, Sheng J. Antibacterial properties of 2% lidocaine and reduced rate of endophthalmitis after intravitreal injection. Retina. 2014;34:935–942. doi: 10.1097/IAE.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kaji R, Hirota N, Kojima Y, Takagi T, Kohara N, Kimura J, Shibasaki H, Bostock H. Effect of maturation on nerve excitability in an experimental model of threshold electrotonus. Muscle Nerve. 2000;23:498–506. doi: 10.1002/(sici)1097-4598(200004)23:4<498::aid-mus7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]