Abstract
8-Prenylnaringenin (8-PN) is a phytoestrogen with the highest estrogenic activity. The objective of the present study was to confirm the superiority of 8-PN on bone metabolisms and the estrogen receptor (ER) subtype mediating effects of 8-PN. The osteoblast MC3T3-E1 and osteoclast-like cell line RAW264.7 were treated with 17β-estradiol (10−8 mol/L), genistein (10−5 mol/L), daidzein (10−5 mol/L), 8-PN (10−5 mol/L) alone or in the presence of ERα antagonist MPP (10−7 mol/L) and ERβ antagonist PTHPP (1.5 × 10−7 mol/L). It has been found that 8-PN did not affect osteoblast proliferation, and that 8-PN increased alkaline phosphatase (ALP) activity, osteocalcin (OCN) concentrations, and the mineralized nodules. 8-PN inhibited RAW264.7 differentiating into osteoclasts and reduced the pit area of bone resorption. 8-PN could also inhibit the protein and mRNA expression of receptor activator of nuclear factor-κB ligand (RANKL) in osteoblasts, and conversely promote the expression of osteoprotegerin (OPG). These effects of 8-PN were mainly inhibited not by PTHPP but by MPP and they were weaker than estrogen's effects but stronger than those of genistein and daidzein. In conclusion, the effects of 8-PN on promoting osteoblastic bone formation and inhibiting osteoclastic bone resorption were mediated by ERα instead of ERβ and the efficacy was more potent than that of the two classic phytoestrogens: genistein and daidzein.
Keywords: 8-Prenylnaringenin, daidzein, estrogen receptor, genistein, osteoblast, osteoclast
Introduction
Osteoporosis, usually arising from estrogen deficiency in postmenopausal women, is a common illness in elderly women. Estrogen deficiency results in an imbalance in bone remodeling, with a substantial relative increase in osteoclastic bone resorption compared to osteoblastic bone formation (Li et al. 2009; Hu et al. 2011; Khosla et al. 2012). Estrogen replacement therapy has been well established in the treatment of postmenopausal osteoporosis for decades until recent past users of hormonal replacement therapy (HRT) were found having a higher risk of cardiovascular diseases, breast cancer, etc. (Santen et al. 2010). More and more studies emphasized on exploring alternative medicines and agents for estrogen replacement therapy such as phytoestrogens (Lagari and Levis 2012).
Phytoestrogens are a group of biological activity substances extracted from plants and seeds and they have chemical structures similar to physiological estradiol. This structural similarity accounts for these compounds binding to estrogen receptor (ER) and exerting various estrogenic and antiestrogenic effects. There are three main families of phytoestrogens: isoflavones, lignans, and coumestans. As estrogen-mimic compounds, phytoestrogens have been considered as a therapeutic option for osteoporosis. In this regard, the effects of phytoestrogens on bone metabolism have been examined in a number of cellular, animal, and clinical trials, but with inconsistent results. Most of the related reviews seem to come to the conclusion that current evidences are not sufficient to make recommendations regarding the effects of phytoestrogens on bone health. Variable results may be due to the differences in study design, duration, populations studied, and also the insufficient potential of estrogenic activities of classic isoflavoid such as genestein and daidzein (Patisaul and Jefferson 2010).
Recently, 8-Prenylnaringenin (8-PN), 8-isoprene-4,5-7-hydroxy flavanone, a demethylating fulvic phenol isomer, has aroused much interest as it is considered to be by far the most potent phytoestrogen with higher estrogenic bioactivities than others (Schaefer et al. 2003; Heyerick et al. 2006). 8-PN is an active constituent isolated from the female flowers of Humulus lupulus L. (hop) (Cannabaceae) which is well known for the usage of bitterness, preservative, and flavoring in beer brewing. There have been several studies performed that demonstrated the potential of 8-PN to alleviate climacteric symptoms like osteoporosis, vasomotoric complaints, and sexual motivation (Zanoli and Zavatti 2008; Keiler et al. 2013). The promising potential of 8-PN on postmenopause osteoporosis has been shown in a few cellular and animal experiments (Effenberger et al. 2005; Sehmisch et al. 2008; Pedrera-Zamorano et al. 2009; Ming et al. 2012, 2013). However, only limited data have been found about whether the effects of 8-PN on bone turnover are higher than those of other phytoestrogens (Sehmisch et al. 2008). On the other hand, although several studies applying rapid yeast estrogen bioassays stably expressing human ER have showed that 8-PN exhibited higher preference for ERα than for ERβ (Milligan et al. 2002; Bovee et al. 2004; Chadwick et al. 2006), we cannot find any study about ER subtype mediating 8-PN function on bone metabolism. This study aims to compare the effects of 8-PN with genistein and daidzein on the differentiation and functions of osteoblast and osteoclast, and ascertain how the ER subtype mediates the bone effects of 8-PN by applying ERα or -β special antagonist.
Materials and Methods
Reagents and cell culture
Mouse osteoblastic cell line MC3T3-E1 was obtained from Peking Union Medical College Center (Beijing, China), and mouse mononuclear macrophage RAW264.7 was purchased from the Chinese Academy of Sciences (Beijing, China). Culture medium (Dulbecco's Modified Eagle's Medium [DMEM], α-MEM) was obtained from Invitrogen (Auckland, Scotland, UK). Fetal bovine serum (FBS) was from Lanzhou National Hycolone Bio-Engineering (Lanzhou, China). 8-PN, 17β-estradiol, daidaizn, and geneistein were purchased from Sigma (St. Louis, MO), and ERα antagonist methyl-piperidino-pyrazole (MPP) and ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl]phenol (PTHPP) were from Tocris biosciences (Bristol, UK). Receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) were purchased from PeproTech (Rocky Hill, NJ). Osteocalcin (OCN), RANKL, and osteoprotegerin (OPG) ELISA Kit were purchased from USCN (Houston, TX). The primers were synthesized in Shanghai Bio-Engineering Technology Service Co., Ltd. (Shanghai, China). All other reagents used were of analytical grade. MC3T3-E1 cells were cultured in phenol red-free α-MEM medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, 50 mg/L ascorbic acid, and 2 mmol/L sodium β-glycerophosphate. RAW264.7 cells were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, 25 ng/mL M-CSF, and 50 ng/mL RANKL. Both the cell lines were incubated at 37°C with 5% CO2 and saturated humidity. The medium was changed every 3 days. 0.25% trypsin digested and passaged. The cells were treated with 8-PN (10−5 mol/L) alone and in the presence of MPP (10−7 mol/L) or PTHPP (1.5 × 10−7 mol/L), genistein (10−5 mol/L, Gen), daidzein (10−5 mol/L, Dai), and 17β-estradiol (10−8 mol/L, E2) for different periods.
MTT of MC3T3-E1 in proliferation phase
MC3T3-E1 cells were plated into 96-well plates at 104 cells/well in 100 μL culture medium in which FBS was replaced by 10% activated carbon-treated FBS, and cells were treated with drugs the next day. After 48 h, the cell viability was detected with MTT according to the manufacturers' instructions. In brief, each well was added with 10 μL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] solution (5 mg/mL) and incubated for 4 h. Discarded the culture medium, added 100 μL DMSO and shook for 10 min. Then the absorption values were measured at the wavelength of 490 nm. The culture medium with no cells was used as blank control. Cell viability = (absorption values of group treated/absorption values of control group) × 100%.
Alkaline phosphatase (ALP) activity and OCN, RANKL, and OPG ELISA analysis of MC3T3-E1 in differentiation phase
MC3T3-E1 cells were plated into 12-well plates. It almost took 6 days for MC3T3-E1 to differentiate into mature osteoblasts. After 6 days, FBS was replaced by 10% activated carbon-treated FBS for estrogen starvation for 3 days. Subsequently, agents were added into medium 3 days. The cells were treated with drugs on the 9th day. After treating for 48 or 72 h, the culture medium was collected and used for the ELISA analysis of OCN, RANKL, and OPG according to the manufacturer's instructions. Additionally, each well was added with 300 μL of cell lysis buffer and placed on the ice for 30 min. After centrifuged for 10 min at 5000 rpm, the lysate supernatant was collected and ALP activity and total protein content were measured by automatic biochemical analyzer. The total protein content was measured by BCA.
Detection of RANKL and OPG mRNA in MC3T3-E1 in differentiation phase
MC3T3-E1 cells were plated into 6-well plates and followed the same procedure as in section Alkaline phosphatase (ALP) activity and OCN, RANKL, and OPG ELISA analysis of MC3T3-E1 in differentiation phase. Agents were added into medium 3 days before RNA isolation. Total RNA was isolated with Trizol (Invitrogen) according to the manufacturer's instructions. RNA (1 μg, measured photometrically) was transcribed into cDNA by Superscript Kit (Invitrogen). The sequences of specific primers, annealing temperature, PCR cycles used for investigated genes were shown in Table 1. In brief, cDNA synthesis was carried out for 60 min at 37°C in a final volume of 20 μL (containing 0.5 mmol/L of each dNTP, 1 μmol/L of oligo-dT starter, 0.2 U/μL of Omniscript reverse transcriptase, and 0.5 U/μL of RNase inhibitor). The amplification reactions were performed in a total volume of 25 μL containing, apart from cDNA, 2 mmol/L of MgCl2, 0.25 mmol/L of each dNTP, 0.25 μmol/L of each sense and antisense primer, and 0.1 U/μL of Taq DNA Polymerase (Fermentas (MBI), Vilnius, Lithuania). All reactions were performed in a thermal cycler (PE). The amplified fragments were separated in 2% agarose gel by electrophoresis, stained with ethidium bromide and photographed.
Table 1.
PCR condition for investigated genes
| Forward primer(5′–3′) | Reverse primer(5′–3′) | bp | AT (°C) | Cycles | |
|---|---|---|---|---|---|
| β-actin | CTCCATCCTGGCCTCGCTGT | GCTGTCACCTTCACCGTTCC | 436 | 56 | 25 |
| RANKL | CCATCGGGTTCCCATAAAGT | CCCCAAAGTACGTCGCATCT | 403 | 51 | 35 |
| OPG | TGTTCCTACCAAGATTATACCAAAT | CGCTCGATTTGCAGGTCTTT | 338 | 58 | 30 |
AT, annealing temperature; OPG, osteoprotegerin; RANKl, receptor activator of nuclear factor-κB ligand.
Detection of calcified nodules of MC3T3-E1 in mineralization phase
MC3T3-E1 cells were plated into 24-well plates in 500 μL culture medium with 10% activated carbon treated FBS, and then the agents were added into the wells. The culture medium was changed every 3 days. On day 28, the calcified nodules were stained with alizarin red. The plates were washed with phosphate-buffered saline (PBS) for twice and the cells were fixed for 10 min in 95% ethanol. After the cells were washed three times in deionized water, 0.1% alizarin red-tris-HCl (pH 8.3) was added into the well and the plate was incubated for 30 min at 37°C. Then the cells were washed three times with deionized water. After air-dried and mounted, the calcified nodules were observed under the microscope.
Osteoclast identification and count
The RAW264.7 cells were placed into 24-well plates (cells growing on glass coverslips) at 5 × 104 cells/well in 500 μL culture medium with 10% activated carbon-treated FBS and agents were added into the wells. The medium was changed every 3 days. After treated for 6, 9, and 12 days, the coverslips with RAW264.7 cells were taken out and rinsed once with PBS. The coverslips were fixed for 10 min in glutaraldehyde at 4°C, and washed three times with deionized water. Then the coverslips were put into the TRAP staining solution which had been preheated to 37°C, avoiding light and incubating for 1 h. After rinsed with deionized water thrice for 1 min, the coverslips were natural dried and were mounted with neutral resin. The coverslips were observed under the microscope. Cells with multinuclear (≥3) TRAP+ were considered to be osteoclasts. Three coverslips in each group were counted.
Detection of the bone resorption activity of osteoclastic
Fresh bovine femoral compact bone was cross cut with low speed saws slicing machine, and the slices were 80 μm thick and 20 mm × 20 mm size. The slices were then ground to 50 μm thick with frosted glass and washed thrice for 10 min with an ultrasonic cleaning device. The slices were put into 75% alcohol for 24 h and washed five times with culture medium. Then, they were air-dried and exposed to UV light overnight. The RAW264.7 cells were placed into 24-well plates which contained bovine femoral slices at 5 × 104 cells/well. Each well contained 500 μL medium. The culture medium was DMEM supplemented with 10% normal FBS. At the differentiation phase of day 6, the normal FBS was changed into FBS treated with 10% activated carbon. At the differentiation phase of day 9 (estrogen starvation for 3 days), the agents were added into the wells. On day 15, the stain was performed. The bone slices attached with cells were taken out and fixed in the 2.5% glutaraldehyde for 10 min at 4°C. Ultrasonic cleaning was done thrice for 3 min with 0.25 mol/L ammonia hydroxide and washed twice with deionized water. They were air-dried and mounted with neutral resin. The bone pits (in classic shape, such as round, oval, and sausage and shown as blue metachromatic area with clear border) were observed under optical microscope. The area of the bone pits was calculated by the IPP software (version 5; Media Cybernetics, Inc., Rockville, MD). Three slices were used for analysis at each time point.
Data analysis
Data were presented as the mean ± SD. Analysis of variance (ANOVA) for overall comparison was followed by LSD for paired comparisons. All statistical analyses were carried out with the SPSS 11.5 software (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered statistically significant.
Results
Effects on the proliferation of MC3T3-E1 cell in proliferation phase
As shown in Figure 1, on day 2 of the proliferation phase, MC3T3-E1 cells were treated with drugs for 48 h. Cell proliferation was detected by MTT. The results indicated that there were no significant differences among the effects of drugs on the cell proliferation.
Figure 1.
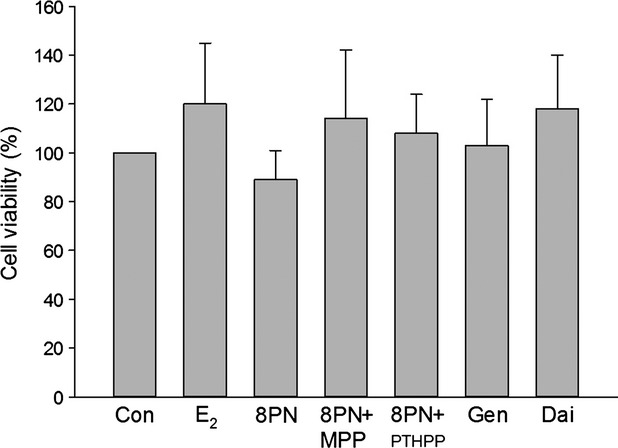
Effects of 48 h treatment of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the number of MC3T3-E1 cells. Con, control group; ER, estrogen receptor.
Effects on the differentiation of MC3T3-E1
The MC3T3-E1 cells were treated with 8-PN and other agents on day 9 of differentiation phase. As shown in Figure 2, 8-PN at 10−5 mol/L increased ALP activity of MC3T3-E1 equivalent to E2 at 10−8 mol/L after 48 or 72 h of administration. MPP inhibited the effects of 8-PN (P < 0.05), whereas PTHPP had no influences on the effects of 8-PN. It was shown that the tendency was enhanced in the 8-PN group compared with in the genistein and daidzein groups at the same concentration (P > 0.05). The changes of OCN level among groups were similar to those of ALP levels (Fig. 3).
Figure 2.
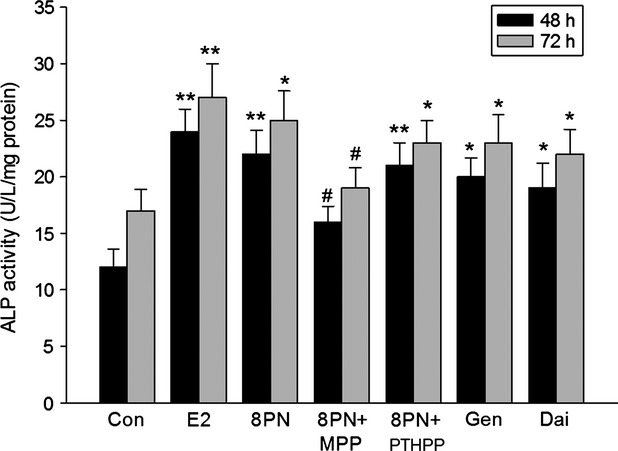
Effects of 48 or 72 h treatment of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN,10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the alkaline (ALP) activity (U/L) of MC3T3-E1. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor; ALP, alkaline phosphatase.
Figure 3.
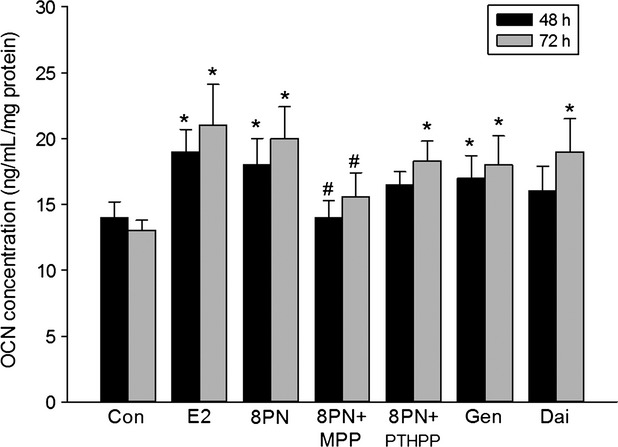
Effects of 48 or 72 h treatment of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the osteocalcin (OCN) concentration (ng/mL) of MC3T3-E1. *P < 0.05 versus control group (Con). #P < 0.05 versus 8PN group. ER, estrogen receptor.
Effects on the calcified nodule of MC3T3-E1 in mineralization
As shown in Figure 4, MC3T3-E1 cells were treated long-term with 8-PN and other agents. On day 28 of mineralization, alizarin red staining was performed for detecting calcified nodule. E2 and phytoestrogens promoted the formation of the calcified nodules. The effects of 8-PN seemed to be inhibited not by PTHPP but by MPP. Additionally, there seemed to be more calcified nodules in 8-PN groups than in genistein and daidzein groups.
Figure 4.
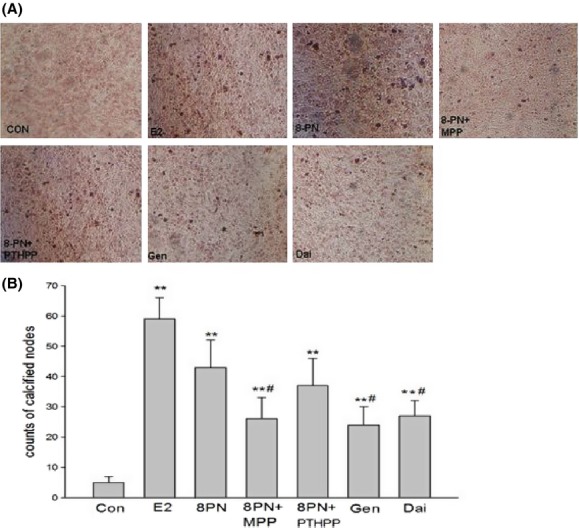
(A) Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on mineralized nodules of MC3T3-E1 demonstrated by alizarin red staining at day 28 (100×). (B) Different counts of calcified nodes among groups. **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Effects on osteoclast differentiation
On the third, sixth, ninth, and twelfth day after the treatment, the RAW264.7 cells were observed under the microscope. On day 3, there were no osteoclasts appeared. As shown in Figure 5, on day 6, there were a few osteoclasts but no significant differences among the groups. On day 9, there were less osteoclasts in the E2 group (P < 0.01), 8-PN group (P < 0.05) than those in the control group. And on day 12, the number of osteoclasts was obviously decreased in the E2 (P < 0.01), 8-PN (P < 0.05), genistein (P < 0.05), and daidzein groups (P < 0.05) when compared with those in the control group. Likewise, the effects of 8-PN were equivalent to those of E2 and significantly stronger than those of Gen and Dai (P < 0.05) and were inhibited not by PTHPP but by MPP (P < 0.05).
Figure 5.
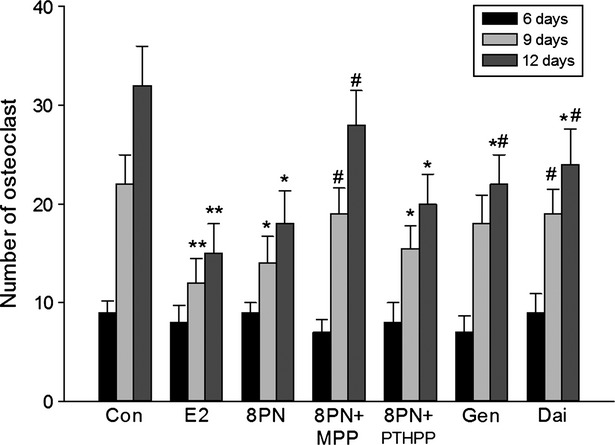
Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the number of osteoclast-like cells characterized via tartrate-resistant acid phosphatase (TRAP) staining from RAW264.7 induced by macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor-κ B ligand (RANKL) for 6, 9, 12 days (×200). *P < 0.01 and **P < 0.01 vs. control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Effects on osteoclast activity
RAW264.7 cells induced osteoclastic differentiation with the stimulation of RANKL and m-CSF. On day 15, after the bone slices were added, the blue bone absorption pits appeared in the round, oval, and sausage shaped (Fig. 6). The borders of the bone pits were clear and the sizes were different. When treated with the compounds, the bone slices coculturing with RAW264.7 were shown differently decreased area of the bone pits. The effects of 8-PN were similar to those of E2 and significantly stronger than those of Gen and Dai (P < 0.05) and were inhibited not by PTHPP but by MPP(P < 0.05) (Fig. 7).
Figure 6.
The characteristics of bone resorption pits on bone slices as shown by arrows (400×).
Figure 7.
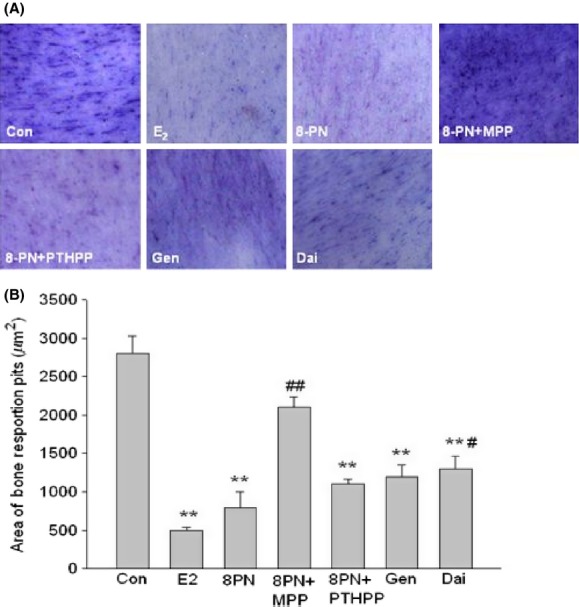
(A) Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro- methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on bone resorption pits on bone slice observed under optical microscope (100×). (B) Different area of bone resorption pits calculated by the IPP software among groups. **P < 0.01 versus control group (Con), #P < 0.05 and ##P < 0.01 versus 8PN group. ER, estrogen receptor.
Effects on the expressions of RANKL and OPG in MC3T3-E1 cells
As shown in Figures 8–10, all of the compounds decreased the expression of RANKL protein in MC3T3-E1 cells. The effects of 8-PN were stronger than those of genistein and daidzein at the same concentration (P < 0.05) and were almost abolished by MPP but not by PTHPP (P < 0.05). All agents increased the secretion of OPG protein when compared with the control(P < 0.05)and there were no significant differences among the treated groups. The results of the RANKL/OPG ratio were similar to the secretion of RANKL protein. The effect of drugs on the expressions of RANKL and OPG mRNA were similar to the protein secretion (Fig. 11).
Figure 8.
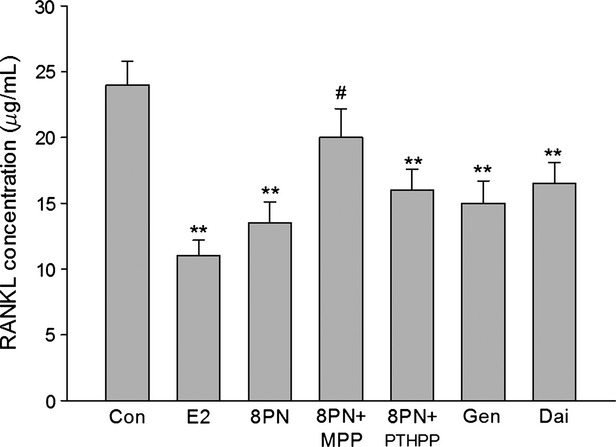
Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the secretion of receptor activator for nuclear factor-κ B ligand (RANKL) protein in MC3T3-E1 cells. **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Figure 10.
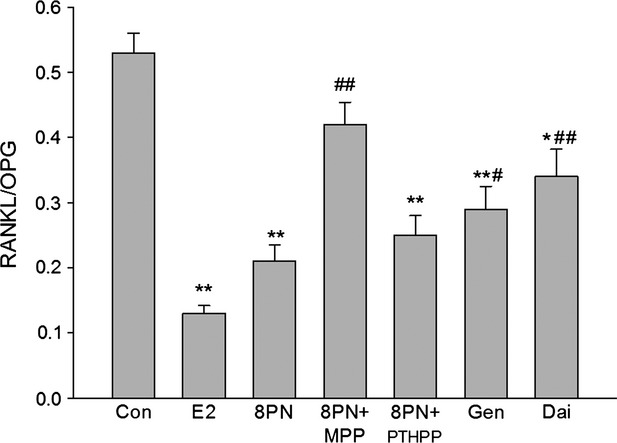
Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the relative value of secreting protein of receptor activator for nuclear factor-κB ligand (RANKL) to osteoprotegerin (OPG) in MC3T3-E1 cells. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 and ##P < 0.01 versus 8PN group. ER, estrogen receptor.
Figure 11.
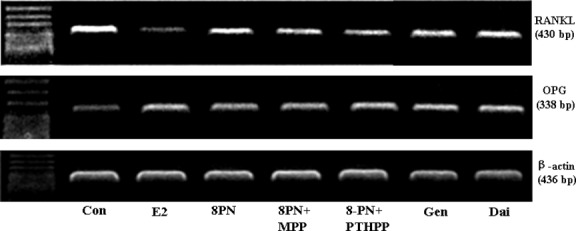
Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the expression of receptor activator for nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) mRNA in MC3T3-E1 cells. ER, estrogen receptor.
Figure 9.
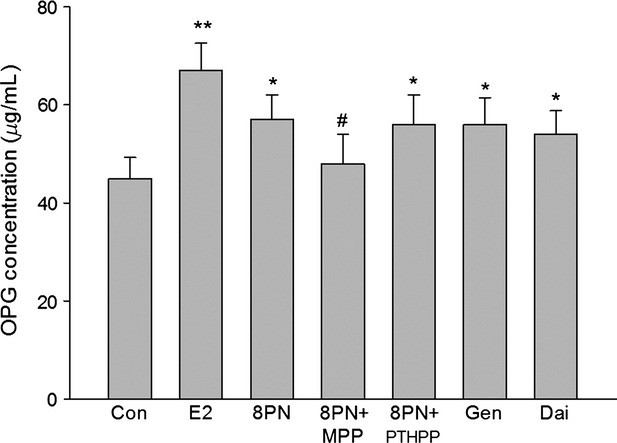
Effects of 17β-estradiol (E2, 10−8 mol/L), genistein (Gen, 10−5 mol/L), daidzein (Dai, 10−5 mol/L), and 8-prenylnaringenin (8PN, 10−5 mol/L) alone or supplemented with ERα antagonist methyl-piperidino-pyrazole (MPP, 10−7 mol/L) or ERβ antagonist 4-[2-phenyl-5,7-bis (tri-fluoro-methyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PTHPP, 1.5 × 10−7 mol/L) on the secretion of osteoprotegerin (OPG) protein in MC3T3-E1 cells. *P < 0.05 and **P < 0.01 versus control group (Con), #P < 0.05 versus 8PN group. ER, estrogen receptor.
Discussion
Effects of 8-PN on bone metabolism have been reported. Epidemiological surveys have shown that the quantitative ultrasound values of phalangeal bone were higher in the women with long-term beer consumption than the women with non-beer consumption (Pedrera-Zamorano et al. 2009). Ovariectomized rats treated with 8-PN for 12 weeks showed obviously improved bone biomechanical characteristics and bone mineral density (Sehmisch et al. 2008). 8-PN also enhanced ALP activity of human osteoblasts hFOB/ERα9 and increased ALP gene transcription level of human osteosarcoma cells U-2 OS/ER α and U-2 OS/ERβ (Effenberger et al. 2005). In addition, 8-PN promoted bone marrow stromal cells differentiation into osteoblasts and mineralization, boosted osteoclasts apoptosis and inhibited osteoclasts differentiation (Ming et al. 2012, 2013).
In the present study, 8-PN was shown enhancing ALP activity, increasing OCN protein levels, and promoting calcification nodule formation of osteoblast cell line MC3T3-E1. On the other hand, 8-PN reduced the osteoclastic differentiation from multinuclear macrophage RAW264.7 and inhibited bone resorption shown as decreased lacunae area in bone slices. Additionally, osteoclast differentiation and maturation could not be separated from the bone microenvironment in where osteoblasts, osteocyte, bone marrow stromal cells, fibroblasts, and other cells could regulate osteoclast differentiation and function by secreting cytokines such as RANKL, OPG, and M-CSF, and so on. Higher RANKL/OPG ratio contributed to promote the differentiation of osteoclasts and bone resorption, and vice versa (Yasuda 2006). In this study, RANKL/OPG ratio in MC3T3-E1 culturing medium was less in 8-PN group than in control group since 8-PN induced secretion of OPG and inhibited RANKL secretion, which suggested that 8-PN may regulate osteoclasts indirectly via osteoblasts MC3T3-E1 besides directly regulate the differentiation and function of osteoclasts. The effects of 8-PN on osteoblast MC3T3-E1 and osteoclast-like RAW264.7 mentioned above were abolished mainly by ERα-selective antagonist MPP but not ERβ-selective antagonist PTHPP, and the effects of 8-PN were weaker than those of estrogen, but stronger than those of genistein and daidzein.
It has been confirmed that 8-PN performed estrogen-like role via binding the ER in breast, endometrium and bone (Roelens et al. 2006). For the two subtypes of ER, ERα and ERβ, rapid yeast estrogen bioassays stably expressing human ERs have showed that 8-PN exhibited higher preference for ERα than for ERβ (Bovee et al. 2004). Our results confirmed that ERα, not ERβ, mainly mediated the effects of 8-PN on promoting osteoblastic bone formation and inhibiting osteoclastic bone resorption. Additionally, it has been reported that estrogen may inhibit bone resorption and promote bone formation mainly mediated by ERα and the role of ERβ remains unclear or just negatively modulating the effects of ERα in those processes (Barkhem et al. 1998; Windahl et al. 2001; Almeida et al. 2013). Meanwhile, the efficacy of daidzein, genistein, or other phytoestrogens has also been reported to be similar on the two subtypes or even stronger on ERβ than on ERα (Barkhem et al. 1998; Bovee et al. 2004). The estrogen-like activity of 8-PN was about an order of magnitude stronger than other phytoestrogens and 1–2 orders of magnitude weaker compared to estrogen (Barkhem et al. 1998; Milligan et al. 2002; Bovee et al. 2004). Our results were similar to these studies. So the special ER selectivity and efficacy might also explain that effects of 8-PN on bone were weaker than those of estrogen, but stronger than those of other phytoestrogens. It was found that the stimulation of 8-PN on uterine and endometrial had been 10 times lower than E2 under the corresponding dose of bone protection (Humpel et al. 2005). Additionally, 8-PN could inhibit breast cancer cells MCF-7 proliferation, regulate blood lipid, be anti-inflammatory, and protect cardiovascular (Heyerick et al. 2006; Böttner et al. 2008; Brunelli et al. 2009; Paoletti et al. 2009). Our findings as well as results of other research suggested that 8-PN could be a more valuable candidate for the prevention and the treatment of postmenopausal osteoporosis than other phytoestrogens, although it needed to be further confirmed.
In conclusion, the present study suggested that phytoestrogen 8-PN promotes osteoblastic MC3T3-E1 differentiation and maturation, directly inhibits mononuclear macrophage RAW264.7 osteoclast differentiation and bone resorption, and also indirectly controls osteoclasts by regulating the expression and secretion of OPG and RANKL. These effects of 8-PN were mediated not by ERβ but by ERα and were stronger than those of genistein and daidzein.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30872122, 30500184), and Jiangxi Province Science and Technology Foundation.
Conflict of Interest
None declared.
References
- Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Invest. 2013;123:394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S, et al. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Böttner M, Christoffel J, Wuttke W. Effects of long-term treatment with 8-prenylnaringenin and oral estradiol on the GH-IGF-1 axis and lipid metabolism in rats. J. Endocrinol. 2008;1982:395–401. doi: 10.1677/JOE-08-0127. [DOI] [PubMed] [Google Scholar]
- Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, andgreen fluorescent protein: a comparison of different compounds with both receptortypes. J. Steroid Biochem. Mol. Biol. 2004;91:99–109. doi: 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- Brunelli E, Pinton G, Chianale F, Graziani A, Appendino G, Moro L. 8-Prenylnaringenin inhibits epidermal growth factor-induced MCF-7 breast cancer cell proliferation by targeting phosphatidylinositol-3-OH kinase activity. J. Steroid Biochem. Mol. Biol. 2009;113:163–170. doi: 10.1016/j.jsbmb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Chadwick LR, Pauli GF, Farnsworth NR. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine. 2006;13:119–131. doi: 10.1016/j.phymed.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effenberger KE, Johnsen SA, Monroe DG, Spelsberg TC, Westendorf JJ. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005;96:387–399. doi: 10.1016/j.jsbmb.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54:164–175. doi: 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Hu R, Liu W, Li H, Yang L, Chen C, Xia ZY, et al. A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J. Biol. Chem. 2011;286:12328–12339. doi: 10.1074/jbc.M110.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel M, Isaksson P, Schaefer O, Kaufmann U, Ciana P, Maggi A. Tissue specificity of 8-prenylnaringenin: protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J. Steroid Biochem. Mol. Biol. 2005;97:299–305. doi: 10.1016/j.jsbmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Keiler AM, Zierau O, Kretzschmar G. Hop extracts and hop substances in treatment of menopausal complaints. Planta Med. 2013;79:576–579. doi: 10.1055/s-0032-1328330. [DOI] [PubMed] [Google Scholar]
- Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012;23:576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagari VS, Levis S. Phytoestrogens for menopausal bone loss and climacteric symptoms. J. Steroid Biochem. Mol. Biol. 2012;139:294–301. doi: 10.1016/j.jsbmb.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J. Clin Invest. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, et al. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–242. [PubMed] [Google Scholar]
- Ming LG, Ge BF, Wang MG, Chen KM. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells' osteogenic differentiation in vitro. Cell Prolif. 2012;45:508–515. doi: 10.1111/j.1365-2184.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming LG, Lv X, Ma XN, Ge BF, Zhang P, Song P, et al. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology. 2013;154:1202–1214. doi: 10.1210/en.2012-2086. [DOI] [PubMed] [Google Scholar]
- Paoletti T, Fallarini S, Gugliesi F, Minassi A, Appendino G, Lombardi G, et al. Anti-inflammatory and vascularprotective properties of 8-prenylapigenin. Eur. J. Pharmacol. 2009;620:120–130. doi: 10.1016/j.ejphar.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrera-Zamorano JD, Lavado-Garcia JM, Roncero-Martin R, Calderon-Garcia JF, Rodriguez-Dominguez T, Canal-Macias ML. Effect of beer drinking on ultrasound bone mass in women. Nutrition. 2009;25:1057–1063. doi: 10.1016/j.nut.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Roelens F, Heldring N, Dhooge W, Bengtsson M, Comhaire F, Gustafsson JA, et al. Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors alpha and beta. J. Med. Chem. 2006;49:7357–7365. doi: 10.1021/jm060692n. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer O, Hümpel M, Fritzemeier KH, Bohlmann R, Schleuning WD. 8-Prenylnaringenin is a potent ERalpha selective phytoestrogen present in hops and beer. J. Steroid Biochem. Mol. Biol. 2003;84:359–360. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Sehmisch S, Hammer F, Christoffel J, Seidlova-Wuttke D, Tezval M, Wuttke W, et al. Comparison of the phytohormones genistein, resveratrol and 8-prenylnaringenin as agents for preventing osteoporosis. Planta Med. 2008;74:794–801. doi: 10.1055/s-2008-1074550. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G, et al. Female estrogen receptor beta−/− mice are partially protected against age-related trabecular bone loss. J. Bone Miner. Res. 2001;16:1388–1398. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- Yasuda H. Bone and bone related biochemical examinations. Bone and collagen related metabolites. Receptor activator of NF-kappaB ligand (RANKL) Clin. Calcium. 2006;16:964–970. [PubMed] [Google Scholar]
- Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008;116:383–396. doi: 10.1016/j.jep.2008.01.011. [DOI] [PubMed] [Google Scholar]


