Abstract
This study was undertaken to determine the in vitro antimicrobial activities of 15 commercial essential oils and their main components in order to pre-select candidates for potential application in highly perishable food preservation. The antibacterial effects against food-borne pathogenic bacteria (Listeria monocytogenes, Salmonella Typhimurium, and enterohemorrhagic Escherichia coli O157:H7) and food spoilage bacteria (Brochothrix thermosphacta and Pseudomonas fluorescens) were tested using paper disk diffusion method, followed by determination of minimum inhibitory (MIC) and bactericidal (MBC) concentrations. Most of the tested essential oils exhibited antimicrobial activity against all tested bacteria, except galangal oil. The essential oils of cinnamon, oregano, and thyme showed strong antimicrobial activities with MIC ≥ 0.125 μL/mL and MBC ≥ 0.25 μL/mL. Among tested bacteria, P. fluorescens was the most resistant to selected essential oils with MICs and MBCs of 1 μL/mL. The results suggest that the activity of the essential oils of cinnamon, oregano, thyme, and clove can be attributed to the existence mostly of cinnamaldehyde, carvacrol, thymol, and eugenol, which appear to possess similar activities against all the tested bacteria. These materials could be served as an important natural alternative to prevent bacterial growth in food products.
Keywords: Antimicrobial activity, essential oil, food spoilage bacteria, food-borne pathogenic bacteria
Introduction
Pathogenic and food spoilage bacteria have been considered as the primary causes of food-borne diseases and food quality deterioration in both developed and developing countries. In order to assure the food safety and to extend the shelf life of food products, additions of chemical preservative agents into food products or decontamination treatments via physical, chemical or biological process or their combinations have been widely applied in food industries (Brul and Coote 1999; Gould 2000). However, critical concerns have been raised due to limitations of treatment processes and since survival of environment-adapted bacteria after treatment processes may lead to high resistance of bacteria such as pathogenic Escherichia coli O157:H7, Listeria monocytogenes, and some Salmonella serovars (Whitney et al. 2007; Hugas and Tsigarida 2008; Rajkovic et al. 2009). The different diseases such as campylobacteriosis, listeriosis, hemorrhagic colitis, and salmonellosis caused by food-related pathogenic bacteria were still reported (Newell et al. 2010; EFSA and ECDC 2011). In highly perishable foods such as meat and meat products, spoilage bacteria contribute to shorten the shelf life by causing off-odors, off-flavors, discoloration, gas production, and slime production (Ercolini et al. 2009). Need for natural alternative is due to consumers' preference for fewer chemicals and more natural foods. Regulatory approval is easy (GRAS) for being natural antimicrobials. Apparently, essential oils have been considered as potential alternatives. These secondary metabolites can be obtained from flowers, buds, seeds, leaves, bark, herbs, fruits, and roots of plants through expression, solvent extraction, steam or hydro distillation. These volatile oils containing bioactive compounds were known for biological activity, remarkably antioxidant activity (Mechergui et al. 2010; Viuda-Martos et al. 2010) and antimicrobial activity against food-borne pathogens and food spoilage bacteria (Burt 2004; Dadalioğlu and Evrendilek 2004; Oussalah et al. 2007; Sarac and Ugur 2008; Viuda-Martos et al. 2008; Ruiz-Navajas et al. 2012). Several studies on application of essential oils as antimicrobials have been conducted and shown to increase the safety and shelf life of food products besides being used as flavoring agent in foods (Burt 2004; Bajpai et al. 2012).
In the study of Oussalah et al. (2007), some commercial oils from Canadian supplier such as Corydothymus capitatus, Cinnamomum cassia, Cinnamomum verum, and Origanum heracleoticum were observed to strongly inhibit pathogenic Staphylococcus aureus, L. monocytogenes, E. coli O157:H7, and Salmonella Typhimurium on Brain Heart Infusion (BHI) agar. Out of 21 essential oils from an Indian producer, Cinnamomum zeylanicum, Eugenia caryophyllata, and Citrus aurantium oils were the most effective in inhibiting some tested bacterial strains of Bacillus subtilis, S. aureus, E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Pseudomonas vulgaris on Mueller-Hinton (MHA) agar (Prabuseenivasan et al. 2006). Syzygium aromaticum oil was the strongest among the 10 commercial oils tested in inhibiting the growth of four strains of E. coli O157:H7 in BHI broth (Moreira et al. 2005). No antimicrobial activity determination of their main components was included in these works. Some studies claim that oxygenated monoterpenes present in herbs and spices essential oils might also play a major role in their antimicrobial activity. Twenty-one constituents showed variation in antimicrobial activities against 25 bacterial strains including E. coli, Salmonella Pullorum, P. aeruginosa, and Brochothrix thermosphacta when assayed by agar well diffusion method using Iso-Sensitest agar (Dorman and Deans 2000). Carvacrol, cinnamic acid, eugenol, and thymol were also tested against E. coli and S. Typhimurium using Bioscreen for MIC determination in Luria broth (LB) (Olasupo et al. 2003). Kotan et al. (2007) reported that only linalool, nerol, terpinen-4-ol, α-terpineol, and fenchol, out of 21 oxygenated monoterpenes were mostly active against 63 bacterial strains including Salmonella Enteritidis, S. Typhimurium and P. aeruginosa on nutrient agar (NA) using paper disk diffusion method. However, the outputs reported from these different studies are difficult to compare directly by different test methods, diverse bacterial strains, culture media, and antimicrobial sample sources.
Nowadays the essential oils or extracts from daily-used-culinary herbs and spices are commercially acquired with Europe Union as the world's biggest importer (UNIDO and FAO 2005). In spite of all the information available on several essential oils, the investigation dealing with this kind of commercial products, which are generally the ones used by mostly flavor and fragrance industries, especially food and beverage industries, have been inadequate. Therefore, the aim of this study was to determine the in vitro antimicrobial activity of commercial essential oils and standard constituents against food-borne pathogens and food spoilage bacteria in order to preselect potential candidates for application in food preservation.
Materials and Methods
Essential oils
In this study, the essential oils provided by Pranarom International (Ghislenghien, Belgium) and Lionel Hitchen Limited (Hampshire, United Kingdom) were screened for antimicrobial activity. The list of essential oils and their properties are given in Table 1. Some individual constituents (carvacrol, trans-cinnamaldehyde, eugenol, linalool, and thymol) commonly found in these essential oils were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). These oils were stored at 4°C before use.
Table 1.
List of essential oils and their properties
| Botanical species | Common name | Family | Part | Main composition (%)1 | Manufacturers |
|---|---|---|---|---|---|
| Cinnamomum cassia | Chinese cinnamon | Lauraceae | Leaf-branch | E-cinnamaldehyde (77.90), trans-o-methoxy-cinnamaldehyde (10.50) | Pranarom |
| Cinnamomum verum | Ceylon cinnamon | Lauraceae | Bark | E-cinnamaldehyde (63.56), cinnamyl acetate (8.33) | Pranarom |
| Coriandrum sativum | Coriander | Apiaceae | Fruit | Linalool (70.07), camphor (5.52), α-pinene (4.86) | Pranarom |
| Cymbopogon flexuosus | Indian lemongrass | Gramineae | Herb grass | NA | Lionel Hitchen |
| Cymbopogon nardus | Ceylon citronella | Gramineae | Herb grass | Geraniol (24.08), camphene (9.01), geranyl acetate (8.81) | Pranarom |
| Eugenia caryophyllus | Clove | Myrtaceae | Bud | Eugenol (84.75), eugenyl acetate (7.12), β-caryophyllene (4.60) | Pranarom |
| Kaempferia galanga | Aromatic ginger | Zingiberaceae | Rhizome | NA | Lionel Hitchen |
| Origanum compactum | Oregano | Lamiaceae | Flowering plant | Carvacrol (46.37), thymol (13.70), p-cymene (13.33) | Pranarom |
| Origanum heracleoticum | Greek oregano | Lamiaceae | Flowering plant | Carvacrol (68.14), thymol (7.47), γ-terpinene (6.06) | Pranarom |
| Origanum majorana | Sweet marjoram | Lamiaceae | Flowering plant | Terpinene-4-ol (24.21), α-terpinene (8.44), sabinene (7.12) | Pranarom |
| Salvia officinalis | Dalmatian sage | Lamiaceae | Flowering plant | NA | Lionel Hitchen |
| Salvia sclarea | Clary sage | Lamiaceae | Flowering plant | Linalyl acetate (62.38), linalool (21.47), α-terpineol (2.45) | Pranarom |
| Thymus capitatus | Oregano | Lamiaceae | Flowering plant | NA | Lionel Hitchen |
| Thymus mastichina | Spanish marjoram | Lamiaceae | Flowering plant | NA | Lionel Hitchen |
| Thymus vulgaris thymoliferum | Common thymol thyme | Lamiaceae | Flowering plant | Thymol (39.74), p-cymene (18.74), γ-terpinene (11.12) | Pranarom |
NA, not available.
Based on the data of the gas-chromatography analysis of essential oils provided by manufacturers.
Bacterial strains
To assess the antibacterial properties of the test samples, six strains of pathogenic bacteria were used in the study: L. monocytogenes NCTC 11994, L. monocytogenes S0580 (isolated from raw pork meat), S. Typhimurium ATCC 14028, S. Typhimurium S0584 (isolated from pig carcass), Escherichia coli O157:H7 ATCC 35150 and E. coli O157:H7 S0575 (isolated from minced beef). The S0580, S0584, and S0575 strains have been isolated by internal laboratory for microbiological analysis. Other two strains of spoilage bacteria B. thermosphacta ATCC 11509 and Pseudomonas fluorescens ATCC 13525 were also included. Bacterial strains were grown in BHI broth and incubated at 37°C for 24 h, except for P. fluorescens ATCC 13525 (30°C) and B. thermosphacta ATCC 11509 (22°C).
Antibacterial activity assays
As a preliminary step, the antibacterial activities of the essential oils were determined by using paper disk diffusion method to screen the efficacy of essential oils among all samples. The essential oils were diluted with analytical grade ethanol at the following concentration 1, 1/1, 1/10, 1/20, and 1/40 (v/v). A volume of 20 μL of each concentration was, respectively, impregnated into the paper disk with 6 mm diameter (Biomérieux, Marcy-l'Etoile, France), and then placed onto Mueller-Hinton agar (MHA) plates (Oxoid, Badhoevedorp, Netherlands), which were previously inoculated on the surface agar with 200 μL of 106 cfu/mL suspension for each tested bacterium. Ethanol was used as a control. Some individual components (carvacrol, cinnamaldehyde, eugenol, linalool, and thymol), frequently present as major component in essential oils, were also tested. Three standard reference antibiotics, ampicillin (10 μg/disk), chloramphenicol (30 μg/disk), and streptomycin (10 μg/disk), were used as reference controls for the tested bacteria. The plates were then incubated at 37°C for 24 h for L. monocytogenes, S. Typhimurium and E. coli O157:H7, and at 30°C for 24 h for P. fluorescens, and at 22°C for 48 h for B. thermosphacta. The antibacterial activity was evaluated by measuring the diameter of inhibitory zones in millimeters using digital calliper Top Craft (Globaltronics GmbH & Co. KG, Hamburg, Germany) and the means were expressed as the results of five determinations.
Determination of minimum inhibitory and bactericidal concentrations
The essential oils, which exhibited the best antimicrobial activity in the paper disk diffusion assay, and some individual constituents, were selected for determining the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) using broth dilution method. One colony of each bacterial strain was sampled with a loop, then inoculated in 25 mL BHI broth and incubated for 18–24 h at 37°C in order to get a bacterial suspension of 109 cfu/mL. Only P. fluorescens and B. thermosphacta were incubated at, respectively, 30 and 22°C. Each stock solution was diluted with buffered peptone water (Oxoid) to obtain 105 cfu/mL bacterial suspensions. Serial dilutions of essential oils (0.125–5 μL/mL) were prepared with BHI broth medium in test tube and mixed with bacterial suspensions to give a volume of 4 mL and a final concentration of bacteria of approximately 5 × 104 cfu/mL. Final solutions were incubated at the temperature mentioned earlier. The MIC was considered as the lowest concentration that prevented the visible growth. The MBC was determined by subculturing 100 μL from each negative test tube onto plate count agar (PCA) plates. MBC was defined as the lowest concentration resulting in a negative subculture or giving presence of only one colony after incubation. The experiments were carried out in four replicates.
Statistical analysis
The mean values ± standard deviations were calculated. Analysis of variance was performed on the basis of mean values to determine the significant difference between essential oils at P ≤ 0.05. Statistical analysis was undertaken using the SAS version 9.1 (Cary, NC).
Results and Discussion
Antimicrobial activity of essential oils
The antibacterial activities of essential oils against eight bacterial strains are summarized in Tables 2–5. The results represent the diameter of inhibition zone including diameter of paper disk (6 mm). A broad variation in antimicrobial properties of the analyzed oils was observed in the study. The essential oils of C. cassia, C. verum, Origanum compactum, O. heracleoticum, Thymus capitatus, and Thymus vulgaris thymoliferum showed consistently strong antimicrobial activity against tested bacteria at different diluted concentrations (1, 1/2, 1/10, 1/20, and 1/40), whereas Cymbopogon flexuosus essential oil showed only strong activity against Gram-positive bacteria. Essential oil of Eugenia caryophyllus showed consistently moderate activity against all tested bacteria. On the other hand, Cymbopogon nardus and Salvia sclarea oils were weak or failed to inhibit the growth of Gram-negative bacteria, while Kaempferia galanga oil showed no antimicrobial activity against any of the tested bacterial strains. Interestingly, oil of Origanum majorana was more active against Gram-negative bacteria than Gram-positive bacteria. Overall, L. monocytogenes NCTC 11994, L. monocytogenes S0580, and B. thermosphacta ATCC 11509 were inhibited by 14 oils, followed by S. Typhimurium S0584 (13 oils), S. Typhimurium ATCC 14028 and E. coli O157:H7 S0575 (12 oils), E. coli O157:H7 ATCC 35150 (11 oils) and P. fluorescens ATCC 13525 (10 oils). Obviously, P. fluorescens showed least susceptibility to the tested essential oils. Generally, the Gram-positive bacteria were more sensitive to essential oils or antibacterial compounds than Gram-negative bacteria, which is in a good agreement with previous reports (Russell 1995; Smith-Palmer et al. 1998; Dorman and Deans 2000; Burt 2004; Shan et al. 2007). This resistance could be ascribed to the structure of the cellular walls of Gram-negative bacteria, mainly with regard to the presence of lipoproteins and lipopolysaccharides that form a barrier to restrict entry of hydrophobic compounds (Russell 1995; Cox and Markham 2007).
Table 2.
Antimicrobial activity of essential oils against Listeria monocytogenes NCTC 11994 and L. monocytogenes S0580 by paper disk diffusion method
| L. monocytogenes NCTC 11994 | L. monocytogenes S0580 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 1/21 | 1/101 | 1/201 | 1/401 | 1 | 1/2 | 1/10 | 1/20 | 1/40 | |
| Essential oils | Diameter of inhibition zone (mm)2 | |||||||||
| Cinnamomum cassia | 33.8 ± 0.2a | 30.1 ± 0.5a | 25.4 ± 0.1a | 14.6 ± 0.8a | 8.9 ± 0.3a | 34.1 ± 0.3a | 28.4 ± 0.8a | 23.1 ± 0.4a | 16.6 ± 0.7a | 8.5 ± 0.4a |
| Cymbopogon flexuosus | 34.8 ± 0.3b | 24.3 ± 0.8b | 9.1 ± 0.1b | 8.2 ± 0.2b | 7.1 ± 0.1b | 34.0 ± 0.3a | 26.0 ± 0.4b | 8.3 ± 0.2b | 7.9 ± 0.1b | – |
| Cymbopogon nardus | 11.8 ± 0.6c | 10.5 ± 0.3c | – | – | – | 10.8 ± 0.3b | 8.9 ± 0.3c | – | – | – |
| Coriandrum sativum | 11.7 ± 0.6c | 8.0 ± 0.5d | – | – | – | 10.6 ± 0.4b | 7.3 ± 0.5d | – | – | – |
| Cinnamomum verum | 34.0 ± 0.4a | 28.5 ± 0.2e | 21.9 ± 0.6c | 8.7 ± 1.0c | – | 34.0 ± 0.5a | 27.9 ± 1.0e | 17.6 ± 0.8c | 8.7 ± 0.2c | – |
| Eugenia caryophyllus | 14.9 ± 0.5d | 11.3 ± 0.8f | 8.8 ± 0.5b | 7.0 ± 0.9d | – | 14.4 ± 0.1c | 12.7 ± 0.2f | 7.0 ± 0.1d | – | – |
| Kaempferia galanga | – | – | – | – | – | – | – | – | – | – |
| Origanum compactum | 26.8 ± 0.6e | 22.6 ± 0.3 g | 11.1 ± 0.3d | 8.7 ± 0.1c | 7.5 ± 0.3b | 26.8 ± 0.5d | 24.3 ± 0.7 g | 11.4 ± 0.4e | 8.7 ± 0.2c | 7.8 ± 0.1b |
| Origanum heracleoticum | 31.5 ± 0.3f | 25.9 ± 0.3 h | 18.1 ± 0.2e | 12.7 ± 0.2e | 10.0 ± 0.2c | 31.1 ± 0.5e | 27.4 ± 0.5e | 17.6 ± 0.3c | 12.8 ± 0.1d | 9.9 ± 0.1c |
| Origanum majorana | 10.9 ± 0.2 g | – | – | – | – | 11.0 ± 0.2b | 7.0 ± 0.1d | – | – | – |
| Salvia officinalis | 10.3 ± 0.2 g | 8.4 ± 0.4d | – | – | – | 8.8 ± 0.2f | 7.2 ± 0.3d | – | – | – |
| Salvia sclarea | 10.4 ± 0.9 g | 7.2 ± 0.3i | – | – | – | 9.8 ± 0.3 g | 7.0 ± 0.1d | – | – | – |
| Thymus capitatus | 30.2 ± 0.7 h | 23.6 ± 0.3j | 15.6 ± 0.3f | 11.8 ± 0.2f | 9.9 ± 0.2c | 29.3 ± 0.8 h | 24.6 ± 0.6 g | 16.9 ± 0.4f | 12.5 ± 0.2d | 10.2 ± 0.2c |
| Thymus mastichina | 9.5 ± 0.4i | – | – | – | – | 10.8 ± 1.1b | 7.0 ± 0.1d | – | – | – |
| Thymus vulgaris thymoliferum | 30.3 ± 0.6 h | 27.5 ± 0.7k | 13.0 ± 0.4 g | 9.8 ± 0.4 g | 8.0 ± 0.1d | 32.6 ± 0.4i | 29.0 ± 0.4 h | 13.6 ± 0.5 g | 9.9 ± 0.2e | 8.3 ± 0.1a |
| Ampicillin3 | 33.7 ± 1.2 | ND | ND | ND | ND | 33.4 ± 0.4 | ND | ND | ND | ND |
| Chloramphenicol3 | 27.8 ± 1.0 | ND | ND | ND | ND | 26.7 ± 0.6 | ND | ND | ND | ND |
| Streptomycin3 | 18.9 ± 0.6 | ND | ND | ND | ND | 21.6 ± 0.4 | ND | ND | ND | ND |
| Blank control (ethanol) | – | – | – | – | – | – | – | – | – | – |
Diameter of inhibitory zone <7 mm considered as no antimicrobial activity. ND, not determined.
Concentrations (1, 1/2, 1/10, 1/20, 1/40) used were v/v.
Values are mean diameter of inhibitory zone (mm) ±SD of five replicates, followed by different letters in column are significantly different (P < 0.05). The diameter of paper disk (6 mm) is included.
Ampicillin (10 μg), chloramphenicol (30 μg), and streptomycin (10 μg) used as positive control.
Table 5.
Antimicrobial activity of essential oils against Brochothrix thermosphacta ATCC 11509 and Pseudomonas fluorescens ATCC 13525 using paper disk diffusion method
| B. thermosphacta ATCC 11509 | P. fluorescens ATCC 13525 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 1/21 | 1/101 | 1/201 | 1/401 | 1 | 1/2 | 1/10 | 1/20 | 1/40 | |
| Essential oils | Diameter of inhibition zone (mm)2 | |||||||||
| Cinnamomum cassia | 24.9 ± 0.4a | 22.7 ± 0.3a | 19.3 ± 0.2a | 13.3 ± 0.3a | 7.3 ± 0.5a | 23.1 ± 0.6a | 21.1 ± 1.6a | 16.1 ± 0.4a | 11.2 ± 0.7a | 8.5 ± 0.3ac |
| Cymbopogon flexuosus | 18.9 ± 0.3b | 15.8 ± 0.1b | 9.7 ± 0.4b | 8.7 ± 0.2b | 7.7 ± 0.1a | 9.4 ± 0.0b | 7.5 ± 0.1b | 7.2 ± 0.0b | – | – |
| Cymbopogon nardus | 15.4 ± 0.3c | – | – | – | – | – | – | – | – | – |
| Coriandrum sativum | 16.3 ± 0.3d | 13.5 ± 0.5c | – | – | – | 12.8 ± 0.6c | 10.5 ± 0.9c | 7.3 ± 0.2b | – | – |
| Cinnamomum verum | 24.2 ± 0.7e | 23.1 ± 0.1a | 17.2 ± 0.4c | 12.0 ± 0.3c | – | 23.6 ± 0.3d | 21.4 ± 0.3a | 12.5 ± 0.4c | 10.4 ± 0.1b | 8.5 ± 0.2ac |
| Eugenia caryophyllus | 15.6 ± 0.2c | 14.7 ± 0.1d | 12.5 ± 0.2d | 7.8 ± 0.1d | – | 9.9 ± 0.3e | 9.3 ± 0.2d | 8.5 ± 0.1d | 7.0 ± 0.1c | – |
| Kaempferia galanga | – | – | – | – | – | – | – | – | – | – |
| Origanum compactum | 27.3 ± 0.5f | 25.3 ± 0.4e | 20.8 ± 0.7e | 14.2 ± 0.9e | 8.4 ± 0.2b | 9.5 ± 0.4b | 9.2 ± 0.0d | 8.3 ± 0.2df | 7.4 ± 0.2d | 7.1 ± 0.2b |
| Origanum heracleoticum | 30.3 ± 0.8 g | 29.0 ± 0.4f | 25.8 ± 0.2f | 22.3 ± 0.8f | 11.7 ± 0.9c | 12.3 ± 0.5f | 11.8 ± 0.3e | 10.5 ± 0.2e | 9.4 ± 0.1e | 8.7 ± 0.1a |
| Origanum majorana | 16.7 ± 0.2d | 12.0 ± 0.2g | – | – | – | 15.9 ± 0.6g | 12.9 ± 0.2f | 8.1 ± 0.4f | 7.7 ± 0.2d | – |
| Salvia officinalis | 14.0 ± 0.2h | 12.0 ± 0.4g | 7.2 ± 0.1g | – | – | – | – | – | – | – |
| Salvia sclarea | 10.5 ± 0.2i | 9.4 ± 0.2h | – | – | – | – | – | – | – | – |
| Thymus capitatus | 23.5 ± 0.6j | 21.5 ± 0.5i | 18.7 ± 0.4h | 16.4 ± 0.2g | 12.8 ± 0.1d | 11.7 ± 0.3h | 11.0 ± 0.1g | 9.5 ± 0.1g | 9.2 ± 0.2e | 8.3 ± 0.1c |
| Thymus mastichina | 11.9 ± 0.4k | 10.4 ± 0.3j | 7.1 ± 0.1g | – | – | – | – | – | – | – |
| Thymus vulgaris thymoliferum | 29.6 ± 0.8 l | 27.3 ± 0.5k | 24.7 ± 0.4i | 18.2 ± 1.2h | 8.9 ± 0.2b | 11.0 ± 0.3i | 10.2 ± 0.2c | 8.4 ± 0.0df | 7.6 ± 0.2d | 7.1 ± 0.3b |
| Ampicillin3 | 31.0 ± 1.1 | ND | ND | ND | ND | – | ND | ND | ND | ND |
| Chloramphenicol3 | 29.8 ± 0.4 | ND | ND | ND | ND | – | ND | ND | ND | ND |
| Streptomycin3 | – | ND | ND | ND | ND | 12.4 ± 0.4 | ND | ND | ND | ND |
| Blank control (ethanol) | – | – | – | – | – | – | – | – | – | – |
(–)Diameter of inhibitory zone <7 mm considered as no antimicrobial activity. ND, not determined.
Concentrations (1, 1/2, 1/10, 1/20, 1/40) used were v/v.
Values are mean diameter of inhibitory zone (mm) ±SD of five replicates, followed by different letters in column are significantly different (P < 0.05). The diameter of paper disk (6 mm) is included.
Ampicillin (10 μg), chloramphenicol (30 μg), and streptomycin (10 μg) used as positive control.
Table 3.
Antimicrobial activity of essential oils against Salmonella Typhimurium ATCC 14028 and S. Typhimurium S0584 using paper disk diffusion method
| S. Typhimurium ATCC 14028 | S. Typhimurium S0584 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 1/21 | 1/101 | 1/201 | 1/401 | 1 | 1/2 | 1/10 | 1/20 | 1/40 | |
| Essential oils | Diameter of inhibition zone (mm)2 | |||||||||
| Cinnamomum cassia | 27.3 ± 0.5a | 23.0 ± 0.7a | 18.6 ± 0.6a | 12.0 ± 0.6a | 8.8 ± 0.3a | 28.2 ± 1.2a | 22.2 ± 0.8a | 19.3 ± 0.8a | 15.5 ± 0.8a | 9.1 ± 0.1a |
| Cymbopogon flexuosus | 9.8 ± 0.2b | 8.3 ± 0.2bj | – | – | – | 10.3 ± 0.4b | 9.4 ± 0.1b | – | – | – |
| Cymbopogon nardus | – | – | – | – | – | 7.4 ± 0.3c | 7.0 ± 0.1c | – | – | – |
| Coriandrum sativum | 10.1 ± 0.8b | 8.6 ± 0.4b | – | – | – | 13.9 ± 0.4d | 12.4 ± 0.3d | 7.4 ± 0.3b | – | – |
| Cinnamomum verum | 27.7 ± 0.4c | 23.7 ± 0.4c | 16.6 ± 0.4b | 12.6 ± 0.3b | 8.1 ± 0.2b | 28.5 ± 0.7a | 23.5 ± 0.8e | 19.8 ± 0.9af | 13.4 ± 0.2b | 9.1 ± 0.2a |
| Eugenia caryophyllus | 15.1 ± 0.2d | 13.3 ± 0.3d | 10.8 ± 0.2c | 7.9 ± 0.1c | 7.2 ± 0.2c | 16.7 ± 0.4e | 14.6 ± 0.4f | 13.5 ± 0.5c | 9.1 ± 0.1c | 7.8 ± 0.1b |
| Kaempferia galanga | – | – | – | – | – | – | – | – | – | – |
| Origanum compactum | 16.6 ± 0.3e | 15.1 ± 0.5e | 11.9 ± 0.6e | 10.7 ± 0.5d | 8.9 ± 0.3a | 23.7 ± 0.5f | 21.2 ± 0.6g | 16.6 ± 0.4d | 11.2 ± 0.2d | 9.4 ± 0.2a |
| Origanum heracleoticum | 20.7 ± 0.4f | 18.2 ± 0.7f | 15.8 ± 0.4f | 13.1 ± 0.2e | 12.3 ± 0.3d | 27.4 ± 0.9g | 24.5 ± 0.3h | 21.7 ± 0.6e | 19.6 ± 0.5e | 12.3 ± 0.2c |
| Origanum majorana | 14.1 ± 0.8g | 9.7 ± 0.4g | 7.4 ± 0.1g | – | – | 23.3 ± 0.8f | 19.0 ± 1.4i | 7.6 ± 0.2b | 7.1 ± 0.1f | – |
| Salvia officinalis | 7.3 ± 0.3h | – | – | – | – | 7.3 ± 0.5c | – | – | – | – |
| Salvia sclarea | – | – | – | – | – | – | – | – | – | – |
| Thymus capitatus | 21.0 ± 1.0f | 18.8 ± 0.3h | 14.9 ± 0.2h | 12.0 ± 0.3a | 11.1 ± 0.2e | 26.1 ± 0.3 h | 23.8 ± 0.5e | 20.1 ± 0.5f | 15.0 ± 0.4a | 11.9 ± 0.2c |
| Thymus mastichina | 9.0 ± 0.1i | 8.1 ± 0.1i | – | – | – | 9.7 ± 0.6i | 8.2 ± 0.1j | – | – | – |
| Thymus vulgaris thymoliferum | 19.3 ± 0.2j | 16.2 ± 0.3j | 12.3 ± 0.2i | 11.0 ± 0.1d | 9.4 ± 0.2f | 27.5 ± 0.4 g | 23.6 ± 0.5e | 15.3 ± 0.7g | 11.4 ± 0.4d | 10.3 ± 0.4d |
| Ampicillin3 | 28.8 ± 0.3 | ND | ND | ND | ND | 29.2 ± 0.5 | ND | ND | ND | ND |
| Chloramphenicol3 | 29.0 ± 0.6 | ND | ND | ND | ND | 28.9 ± 0.8 | ND | ND | ND | ND |
| Streptomycin3 | 16.2 ± 0.4 | ND | ND | ND | ND | 18.4 ± 0.4 | ND | ND | ND | ND |
| Blank control (ethanol) | – | – | – | – | – | – | – | – | – | – |
(–)Diameter of inhibitory zone <7 mm considered as no antimicrobial activity. ND, not determined.
Concentrations (1, 1/2, 1/10, 1/20, 1/40) used were v/v.
Values are mean diameter of inhibitory zone (mm) ±SD of five replicates, followed by different letters in column are significantly different (P < 0.05). The diameter of paper disk (6 mm) is included.
Ampicillin (10 μg), chloramphenicol (30 μg), and streptomycin (10 μg) used as positive control.
Table 4.
Antimicrobial activity of essential oils against Escherichia coli O157:H7 ATCC 35150 and E. coli O157:H7 S0575 using paper disk diffusion method
| E. coli O157:H7 ATCC 35150 | E. coli O157:H7 S0575 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 1/21 | 1/101 | 1/201 | 1/401 | 1 | 1/2 | 1/10 | 1/20 | 1/40 | |
| Essential oils | Diameter of inhibition zone (mm)2 | |||||||||
| Cinnamomum cassia | 28.1 ± 0.7a | 18.4 ± 0.6a | 16.8 ± 0.3a | 14.2 ± 0.5a | 9.3 ± 0.5a | 27.8 ± 0.9a | 20.2 ± 1.3a | 19.1 ± 1.2a | 15.1 ± 0.5a | 8.5 ± 0.3a |
| Cymbopogon flexuosus | 9.4 ± 0.1b | 9.1 ± 0.1b | – | – | – | 9.8 ± 0.2b | 7.7 ± 0.1b | – | – | – |
| Cymbopogon nardus | – | – | – | – | – | – | – | – | – | – |
| Coriandrum sativum | 9.5 ± 0.2b | 9.1 ± 0.4b | – | – | – | 11.1 ± 0.3c | 10.8 ± 0.4c | – | – | – |
| Cinnamomum verum | 28.1 ± 0.8a | 24.7 ± 0.2c | 21.6 ± 0.6b | 15.6 ± 0.8b | 8.5 ± 0.4b | 27.6 ± 0.7a | 23.5 ± 0.9d | 21.0 ± 1.1b | 14.9 ± 0.5a | 7.9 ± 0.7b |
| Eugenia caryophyllus | 14.6 ± 0.6c | 13.1 ± 0.6d | 11.5 ± 0.2c | 8.7 ± 0.1c | 7.5 ± 0.1c | 14.4 ± 0.4d | 13.2 ± 0.4e | 11.5 ± 0.2c | 9.3 ± 0.4b | 7.1 ± 0.1c |
| Kaempferia galanga | – | – | – | – | – | – | – | – | – | – |
| Origanum compactum | 15.4 ± 0.2d | 14.9 ± 0.5e | 11.3 ± 0.3c | 10.1 ± 0.4d | 8.6 ± 0.0b | 17.5 ± 0.3e | 16.3 ± 0.5f | 12.1 ± 0.8d | 10.7 ± 0.5c | 9.2 ± 0.2d |
| Origanum heracleoticum | 20.2 ± 0.4e | 19.4 ± 0.5f | 16.0 ± 0.1d | 13.7 ± 0.3e | 11.1 ± 0.2d | 22.1 ± 0.8f | 19.7 ± 0.4g | 16.7 ± 0.6e | 14.1 ± 0.2d | 11.2 ± 0.3e |
| Origanum majorana | 15.6 ± 0.3d | 14.0 ± 0.5g | 7.2 ± 0.2e | – | – | 18.0 ± 0.4e | 13.9 ± 0.6 h | 7.4 ± 0.2f | – | – |
| Salvia officinalis | – | – | – | – | – | 7.3 ± 0.2g | – | – | – | – |
| Salvia sclarea | – | – | – | – | – | – | – | – | – | – |
| Thymus capitatus | 20.9 ± 0.7f | 18.3 ± 0.5a | 14.5 ± 0.5f | 12.5 ± 0.3f | 10.5 ± 0.2e | 20.7 ± 0.8h | 18.4 ± 0.4i | 14.5 ± 0.2g | 12.6 ± 0.4e | 10.4 ± 0.1f |
| Thymus mastichina | 8.5 ± 0.7g | 7.1 ± 0.1h | – | – | – | 8.8 ± 0.4i | – | – | – | – |
| Thymus vulgaris thymoliferum | 17.6 ± 1.0h | 17.0 ± 0.6i | 12.3 ± 0.0g | 11.5 ± 0.2g | 10.1 ± 0.2e | 19.2 ± 0.7j | 17.3 ± 0.5j | 12.6 ± 0.2d | 11.4 ± 0.2f | 10.0 ± 0.3f |
| Ampicillin3 | 20.0 ± 0.1 | ND | ND | ND | ND | 21.6 ± 0.4 | ND | ND | ND | ND |
| Chloramphenicol3 | 22.3 ± 0.5 | ND | ND | ND | ND | 22.3 ± 0.7 | ND | ND | ND | ND |
| Streptomycin3 | 23.1 ± 0.3 | ND | ND | ND | ND | 22.4 ± 0.2 | ND | ND | ND | ND |
| Blank control (ethanol) | – | – | – | – | – | – | – | – | – | – |
(–)Diameter of inhibitory zone <7 mm considered as no antimicrobial activity. ND, not determined.
Concentrations (1, 1/2, 1/10, 1/20, 1/40) used were v/v.
Values are mean diameter of inhibitory zone (mm) ±SD of five replicates, followed by different letters in column are significantly different (P < 0.05). The diameter of paper disk (6 mm) is included.
Ampicillin (10 μg), chloramphenicol (30 μg), and streptomycin (10 μg) used as positive control.
Antimicrobial activity of essential oils components
Some standard components such as carvacrol, cinnamaldehyde, eugenol, linalool, and thymol were tested under identical conditions (Table 6). As the main constituents in some essential oils, these components have been proven to be particularly effective against some species of Gram-positive and Gram-negative bacteria (Cosentino et al. 1999; Dorman and Deans 2000; Bagamboula et al. 2004; Kotan et al. 2007; Shan et al. 2007; Hussain et al. 2008; Castilho et al. 2012). The oxygenated components, trans-cinnamaldehyde, carvacrol, and thymol were shown in this study to possess stronger antibacterial activity in comparison with eugenol and linalool, which could explain the high antimicrobial activity of cinnamon, oregano, and thyme oils (Aligiannis et al. 2001; Baydar et al. 2004; Shan et al. 2007; Castilho et al. 2012). Cinnamaldehyde exhibited high levels of antimicrobial activity against all tested strains, whereas carvacrol and thymol, with the only exception against P. flurorescens, showed a lower activity. Figure 1 shows typical inhibition halos obtained for O. heracleoticum, C. verum, E. caryophyllus, carvacrol, cinnamaldehyde, and eugenol against S. Typhimurium and P. fluorescens.
Table 6.
Inhibitory diameters and minimum inhibitory and bactericidal concentrations of essential oil constituents against food-borne and food spoilage bacteria
| Tested bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | L. monocytogenes NCTC 11994 | L. monocytogenes S0580 | S. Typhimurium ATCC 14028 | S. Typhimurium S0584 | E. coli O157:H7 ATCC 35150 | E. coli O157:H7 S0575 | B. thermosphacta ATCC 11509 | P. fluorescens ATCC 13525 |
| Paper disk diffusion method (inhibition diameter, mm)1 | ||||||||
| trans-Cinnamaldehyde | 31.7 ± 0.5a | 35.5 ± 0.3a | 28.9 ± 0.5a | 30.9 ± 0.1a | 29.6 ± 0.5a | 30.1 ± 0.2a | 30.7 ± 0.5a | 28.9 ± 0.7a |
| Carvacrol | 27.3 ± 0.3b | 30.0 ± 0.8b | 21.7 ± 0.2b | 30.4 ± 0.9a | 22.4 ± 0.3b | 23.9 ± 0.4b | 32.9 ± 0.4b | 15.1 ± 0.7b |
| Eugenol | 14.4 ± 0.2c | 15.2 ± 0.3c | 15.6 ± 0.2b | 18.2 ± 0.7b | 15.9 ± 0.4c | 16.1 ± 0.4c | 18.8 ± 0.3c | 14.8 ± 0.4b |
| Linalool | 11.4 ± 0.1d | 12.8 ± 0.3d | 10.5 ± 0.2d | 16.1 ± 0.3c | 12.4 ± 0.2d | 13.7 ± 0.5d | 15.5 ± 0.2d | 8.7 ± 0.3c |
| Thymol | 31.0 ± 0.2a | 35.6 ± 0.4a | 23.4 ± 0.5e | 33.3 ± 0.6d | 23.0 ± 0.4b | 25.5 ± 0.6e | 39.7 ± 0.4e | 19.0 ± 0.5d |
| Minimum inhibitory and bacterial concentrations (MIC/MBC, μL/mL)2 | ||||||||
| trans-Cinnamaldehyde | 0.125/0.5 | 0.25/0.5 | 0.25/0.25 | 0.125/0.25 | 0.125/0.25 | 0.125/0.25 | 0.125/1 | 0.25/0.5 |
| Carvacrol | 0.125/0.25 | 0.125/0.25 | 0.125/0.375 | 0.188/0.25 | 0.125/0.25 | 0.25/0.375 | 0.5/1 | 1/>1.5 |
| Eugenol | 0.5/1 | 0.5/1 | 1/1 | 0.5/1 | 0.5/>1.5 | 0.5/>1.5 | 1/>1.5 | 1/>1.5 |
| Linalool | 1/>1.5 | 0.75/>1.5 | >1.5/>1.5 | 1/>1.5 | 1/1.5 | 1/1 | 1/>1.5 | >1.5/>1.5 |
| Thymol | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | 0.313/0.375 | 0.25/0.25 | 0.25/0.313 | 0.5/1 | 1/>1.5 |
Values are mean diameter of inhibitory zone (mm) ±standard deviation of five replicates of each component, followed by different letters in a column are significantly different (P < 0.05). The diameter of paper disk (mm) is included.
Values are results of four replicates.
Figure 1.
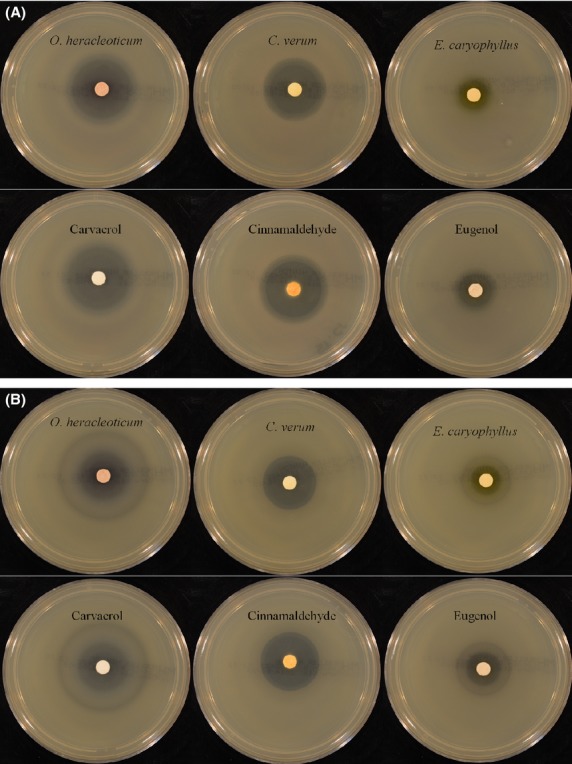
Inhibition diameter zones obtained by paper disk diffusion method for Origanum heracleoticum, Cinnamomum verum, Eugenia caryophyllus, carvacrol, cinnamaldehyde, and eugenol against (A) Salmonella Typhimurium ATCC 14028 and (B) Pseudomonas fluorescens ATCC 13525.
Determination of MIC and MBC
The results reported above revealed the potential of some essential oils such as cinnamon, clove, oregano, and thyme as natural preservatives to control food pathogenic and spoilage bacteria. To achieve precisely the antimicrobial properties of essential oils for potential application in food preservation, determination of MICs and MBCs were necessarily performed on seven selected essential oils and five standard components. The results showed variable effects of essential oils and their components on the tested bacterial strains (Tables 6, 7). Oils of C. cassia, C. verum, and T. vulgaris thymoliferum showed again strong antimicrobial activities in inhibiting the growth of pathogenic and spoilage bacteria at MICs ≤ 1 μL/mL. The bacterial growth was also inhibited by oils of O. compactum at MICs ≤ 0.5 μL/mL and E. caryophyllus at MICs ≤ 1 μL/mL except for P. fluorescens. The essential oils of C. cassia, C. verum, O. compactum, O. heracleoticum, T. capitatus, and T. vulgaris thymoliferum showed bactericidal effects at concentrations ≤1.5 μL/mL. Among tested microorganisms, as previously observed with the paper disk diffusion method, P. fluorescens was the least sensitive as higher concentrations of essential oils were needed with MICs and MBCs ranging from 1 to 1.5 μL/mL. By comparison to previously published studies, our findings presented discrepancy of antimicrobial activity of selected essential oils against food-borne and spoilage bacteria. It may be explained by the different composition and percentage content of active constituents in essential oils, which have been found to have an important role in slowing down or stopping the bacterial growth or killing the bacteria (Ouattara et al. 1997; Bozin et al. 2006). Some factors influencing this variation in composition can be species, subspecies or variety of plants (Sarac and Ugur 2008), geographical locations (Sarac and Ugur 2008; Mechergui et al. 2010), harvesting seasons (Hussain et al. 2008), drying methods (Di Cesare et al. 2003), and also extraction methods (Burt 2004; Karakaya et al. 2011). Moreover, the methods used to assess the antimicrobial activity could also affect the generated outputs (Hammer et al. 1999; Burt and Reinders 2003; Burt 2004). Other factors such as the choice of bacterial strains and their sensitivity, volume of inoculum, incubation time, and temperature should also be related to the variation in the experimental results (Smith-Palmer et al. 1998; Burt 2004; Bozin et al. 2006).
Table 7.
Minimum inhibitory (MIC) and minimum bactericidal (MBC) concentrations of selected essential oils against food-borne and food spoilage bacteria
| Test bacteria (MIC/MBC (μL/mL))1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Essential oils | L. monocytogenes NCTC 11994 | L. monocytogenes S0580 | S. Typhimurium ATCC 14028 | S. Typhimurium S0584 | E. coli O157:H7 ATCC 35150 | E. coli O157:H7 S0575 | B. thermosphacta ATCC 11509 | P. fluorescens ATCC 13525 |
| Cinnamomum cassia | 0.5/0.5 | 0.25/0.25 | 0.25/1 | 0.25/1 | 0.5/1 | 0.25/0.25 | 0.5/0.5 | 1/1 |
| Cinnamomum verum | 0.5/0.5 | 0.25/0.5 | 0.5/0.5 | 0.5/1 | 0.5/0.5 | 0.25/0.5 | 0.5/1 | 1/1.5 |
| Eugenia caryophyllus | 1/>1.5 | 1/>1.5 | 1/1.5 | 1/1.5 | 1/1 | 1/1 | 0.5/0.5 | 1.5/1.5 |
| Origanum compactum | 0.5/0.5 | 0.25/0.25 | 0.5/0.5 | 0.25/0.5 | 0.25/0.5 | 0.5/0.5 | 0.5/0.5 | 1.5/1.5 |
| Origanum heracleoticum | 0.25/0.25 | 0.25/0.25 | 0.125/0.125 | 0.25/0.25 | 0.25/0.25 | 0.25/0.25 | 0.5/0.5 | 1/1 |
| Thymus capitatus | 0.5/0.5 | 0.5/1 | 1/1 | 0.5/1.5 | 0.5/1 | 0.25/0.25 | 0.13/0.25 | 1/1 |
| Thymus vulgaris thymoliferum | 0.5/0.5 | 0.25/0.25 | 0.25/0.5 | 0.25/0.5 | 0.25/0.25 | 0.25/0.5 | 0.25/0.5 | 1.5/1.5 |
Values are results of four replicates.
Against the pathogenic L. monocytogenes, S. Typhimurium and E. coli O157:H7, the essential oil of oregano (O. heracleoticum and O. compactum), thyme (T. vulgaris thymoliferum), and cinnamon (C. cassia and C. verum) were all strongly active. Our findings indicated comparable or even better results by comparison to the outputs of Oussalah et al. (2007). As evidence, essential oils of O. heracleoticum, O. compactum, T. vulgaris thymoliferum, and E. caryophyllus showed their effectiveness against L. monocytogenes with MICs of at least two times lower that of Oussalah et al. (2007). This could be due to the higher content of main and active component in the essential oils, for instant, higher carvacrol 68% in O. heracleoticum oil to 54% in previous study of Oussalah et al. (2007), which could result in a better antilisterial activity. The results obtained with L. monocytogenes are very helpful and relevant as this microorganism can grow at refrigeration temperature, over a wide range of pH values above 4.4 and in the presence of high salt content surviving mild preservation treatment (Hazzit et al. 2006), features that make it difficult to eliminate this microorganism from foods. However, the results above were generated from two different methods, even inoculum concentrations. Therefore, bacterial sensibility to essential oil could be different (Hammer et al. 1999; Burt 2004). Consequently, these findings could be considered as extra confirmatory information in this study. In this study, antimicrobial effect against S. Typhimurium was ∼2- to 10-fold for oregano oil, 20-fold for clove oil and even 80-fold for thyme oil by comparison to findings of Hammer et al. (1999). Cinnamon oil also inactivated effectively the growth of pathogenic S. Typhimurium as similarly reported by Unlu et al. (2010). The oregano oils were much more effective than clove oil against E. coli O157:H7, which is similar to the findings of Oussalah et al. (2007), but completely opposed to the result of Moreira et al. (2005). This could be explained by the use of different bacterial strains of E. coli O157:H7, different methods for MIC and MBC determination and also different subspecies of oregano. Other authors also revealed the antimicrobial effects of these essential oils against different strains of L. monocytogenes (Lis-Balchin and Deans 1997; Faleiro et al. 2005), Salmonella (Özkan et al. 2003; Rota et al. 2008) and E. coli O157:H7 (Sağdıç et al. 2002; Özkan et al. 2003; Rota et al. 2008; Karakaya et al. 2011). Overall, the selective essential oils and their components exhibited a wide range of efficacy in inhibiting the pathogenic bacterial growths. Association of L. monocytogenes, S. Typhimurium, and enterohemorrhagic E. coli O157:H7 with food-borne outbreaks is well documented (Newell et al. 2010; EFSA and ECDC 2011). According to the broad spectrum against these food-borne pathogens, the use of these effective natural alternatives into foods could help food producers to shift away from artificial preservative and to reduce or even eliminate these food poisoning bacteria and control their contaminations in foods.
Brochothrix thermosphacta and P. fluorescens are commonly responsible for food spoilage causing off-odors, off-flavors, and slime production, especially in highly perishable products like meats and meat products. In this study, most of selected essential oils exhibited a remarkable activity against B. thermosphacta. These oils could potentially be good candidates in inhibiting the growth of B. thermosphacta. Apparently, Spanish oregano oil (T. capitatus) and thyme oil showed slightly better activity among tested oils. Only a few studies have been reported on the antimicrobial activity of such essential oils against B. thermosphacta (Ouattara et al. 1997; Baratta et al. 1998; Dorman and Deans 2000). Therefore, this study brings some interestingly complementary findings to the previously published work. Dorman and Deans (2000) have found qualitatively similar result to our finding. In contrast, Ouattara et al. (1997) demonstrated that cinnamon and clove oils were the most active, while oregano and thyme oil failed to inhibit bacterial growth. This discrepancy could be explained by a relationship between the inhibitory effect of essential oils and the presence of their active volatile constituents and sensitivity of different bacterial strains. On the other hand, Gram-negative P. fluorescens was observed as the least sensitive to majority of essential oils among the tested bacterial strains. This is in agreement with many studies having studied different strains of Pseudomonas other than P. fluorescens such as Pseudomonas putida (Oussalah et al. 2006) and P. aeruginosa (Ouattara et al. 1997; Cosentino et al. 1999; Hammer et al. 1999; Dorman and Deans 2000; Özkan et al. 2003; Prabuseenivasan et al. 2006; Bouhdid et al. 2008; Sarac and Ugur 2008; Unlu et al. 2010; Castilho et al. 2012). Only a few studies reported antimicrobial activities of essential oils, especially oregano and thyme oils from different species, against P. fluorescens and the resistance of this food spoilage bacterium is well-known (Baratta et al. 1998; Özkan et al. 2003; Sarac and Ugur 2008; Ruiz-Navajas et al. 2012). Our results proved that low antibacterial activity of carvacrol and thymol against P. fluorescens can explain the low activity of oregano and thyme oils comparing to other bacteria. The lower sensibility of this bacterium has been attributed to an active efflux mechanism and the barrier function of the outer membrane lipopolysaccharide, which can screen out and restricts entry of some antimicrobial agents or compounds (Cox and Markham 2007). However, essential oils from C. cassia, C. verum and their main constituent cinnamaldehyde clearly worked well against this food spoilage bacterium (Ouattara et al. 1997; Oussalah et al. 2006; Di Pasqua et al. 2007; Unlu et al. 2010). Thus, these substances could be potentially important to be used as antimicrobial agent in food preservation.
The essential oils and standard components were demonstrated to inhibit the growth of both food pathogenic and food spoilage bacteria. Thus, the data obtained in this study can be evidently served as a well confirmatory and complementary data to the previously published works.
Conclusion
The commercial essential oils from cinnamon, oregano, and thyme exhibit promising antimicrobial effects against selected food-borne and food spoilage bacteria, which can be attributed to the presence of the principle bioactive constituents, especially cinnamaldehyde, carvacrol, and thymol. These investigated essential oils and their main active components could be potential candidates to be used as natural alternatives for further application in food preservation to retard or inhibit the bacterial growth and for safety and to extend the shelf life of the food products. However, the confirmation of antimicrobial efficiency and organoleptic impact of these essential oils in foodstuffs need to be evaluated.
Acknowledgments
The authors acknowledge the ARES-CCD (Académie de Recherche et d'Enseignement Supérieur - Commission de la Coopération au Développement, Belgium) program for providing scholarship and also Lionel Hitchen Ltd. for providing essential oils. The authors thank Prof. Frédéric Farnir and Dr. Luc Do Duc (Unit of Biostatistics, Bioinformatics, Economics and Animal Selection, Department of Animal Production, University of Liège) for statistical analysis.
Conflict of Interest
None declared.
References
- Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001;49:4168–4170. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21:33–42. [Google Scholar]
- Bajpai VK, Baek K-H, Kang SC. Control of Salmonella in foods by using essential oils: a review. Food Res. Int. 2012;45:722–734. [Google Scholar]
- Baratta MT, Dorman HJD, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998;13:235–244. [Google Scholar]
- Baydar H, Sağdıç O, Özkan G, Karagoğan T. Antibacterial activity and composition of essential oils from Origanum Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004;15:169–172. [Google Scholar]
- Bouhdid S, Skali SN, Idaomar M, Zhiri A, Baudoux D, Amensour M, et al. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008;7:1563–1570. [Google Scholar]
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Brul S, Coote P. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999;50:1–17. doi: 10.1016/s0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Castilho PC, Savluchinske-Feio S, Weinhold TS, Gouveia SC. Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira Island, Portugal. Food Control. 2012;23:552–558. [Google Scholar]
- Cosentino S, Tuberoso CIG, Pisano B, Satta M, Mascia V, Arzedi E, et al. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999;29:130–135. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Cox SD, Markham JL. Susceptibility and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J. Appl. Microbiol. 2007;103:930–936. doi: 10.1111/j.1365-2672.2007.03353.x. [DOI] [PubMed] [Google Scholar]
- Dadalioğlu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004;52:8255–8260. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- Di Cesare LF, Forni E, Viscardi D, Nani RC. Changes in the chemical composition of basil caused by different drying procedures. J. Agric. Food Chem. 2003;51:3575–3581. doi: 10.1021/jf021080o. [DOI] [PubMed] [Google Scholar]
- Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007;55:4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- EFSA and ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009. EFSA J. 2011;9:1–158. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini D, Russo F, Nasi A, Ferranti P, Villani F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009;75:1990–2001. doi: 10.1128/AEM.02762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faleiro L, Miguel G, Gomes S, Costa L, Venâncio F, Teixeira A, et al. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 2005;53:8162–8168. doi: 10.1021/jf0510079. [DOI] [PubMed] [Google Scholar]
- Gould GW. Preservation: past, present and future. Br. Med. Bull. 2000;56:84–96. doi: 10.1258/0007142001902996. [DOI] [PubMed] [Google Scholar]
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Hazzit M, Baaliouamer A, Faleiro ML, Miguel G. Composition of the essential oils of Thymus and Orignaum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006;54:6314–6321. doi: 10.1021/jf0606104. [DOI] [PubMed] [Google Scholar]
- Hugas M, Tsigarida E. Pros and cons of carcass decontamination: the role of the European Food Safety Authority. Meat Sci. 2008;78:43–52. doi: 10.1016/j.meatsci.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hussain AI, Anwar F, Sherazi STH, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Karakaya S, El SN, Karagözlü N, Şahin S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare spp. hirtum) by using different extraction methods. J. Med. Food. 2011;14:645–652. doi: 10.1089/jmf.2010.0098. [DOI] [PubMed] [Google Scholar]
- Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. C. 2007;62:507–513. doi: 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- Lis-Balchin M, Deans SG. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997;82:759–762. doi: 10.1046/j.1365-2672.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Mechergui K, Coelho JA, Serra MC, Lamine SB, Boukhchina S, Khouja ML. Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: chemical composition and antioxidant activity. J. Sci. Food Agric. 2010;90:1745–1749. doi: 10.1002/jsfa.4011. [DOI] [PubMed] [Google Scholar]
- Moreira MR, Ponce AG, del Valle CE, Roura SI. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT. 2005;38:565–570. [Google Scholar]
- Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, et al. Food-borne diseases – the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010;139:S3–S15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olasupo NA, Fitzgerald DJ, Gasson MJ, Narbad A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl. Microbiol. 2003;36:448–451. doi: 10.1046/j.1472-765x.2003.01427.x. [DOI] [PubMed] [Google Scholar]
- Ouattara B, Simard RE, Holley RA, Piette GJP, Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997;37:155–162. doi: 10.1016/s0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Oussalah M, Caillet S, Saucier L, Lacroix M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006;73:236–244. doi: 10.1016/j.meatsci.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Oussalah M, Caillet S, Saucier L, Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. 2007;18:414–420. [Google Scholar]
- Özkan G, Sağdıç O, Özcan M. Note: inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci. Technol. Int. 2003;9:85–88. [Google Scholar]
- Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Smigic N, Uyttendale M, Medi H, De Zutter L, Devlieghere F. Resistance of Listeria monocytogenes Escherichia coli O157:H7 and Campylobacter jejuni after exposure to repetitive cycles of mild bactericidal treatments. Food Microbiol. 2009;26:889–895. doi: 10.1016/j.fm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Rota MC, Herrera A, Martínez RM, Sotomayor JA, Jordán MJ. Antimicrobial activity and chemical composition of Thymus vulgaris Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19:681–687. [Google Scholar]
- Ruiz-Navajas Y, Viuda-Martos M, Sendra E, Perez-Alvarez JA, Fernández-López J. Chemical characterization and antibacterial activity of Thymus moroderi and Thymus piperella essential oils, two Thymus endemic species from Southeast of Spain. Food Control. 2012;27:294–299. [Google Scholar]
- Russell AD. Mechanisms of bacterial resistance to biocides. Int. Biodeter. Biodegr. 1995:247–265. [Google Scholar]
- Sağdıç O, Kuşçu A, Özcan M, Özçelik S. Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli O157:H7. Food Microbiol. 2002;19:473–480. [Google Scholar]
- Sarac N, Ugur A. Antimicrobial activities of the essential oils of Origanum onites L., Origanum vulgare L. subspecies hirtum (Link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J. Med. Food. 2008;11:568–573. doi: 10.1089/jmf.2007.0520. [DOI] [PubMed] [Google Scholar]
- Shan B, Cai Y-Z, Brooks JD, Corke H, H Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007;55:5484–5490. doi: 10.1021/jf070424d. [DOI] [PubMed] [Google Scholar]
- Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765x.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- UNIDO and FAO. UNIDO, Vienna: 2005. Herbs, spices and essential oils: post-harvest operations in developing countries. [Google Scholar]
- Unlu M, Ergene E, Unlu GV, Zeytinoglu HS, Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food Chem. Toxicol. 2010;48:3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet. Int. J. Food Sci. Technol. 2008;43:526–531. [Google Scholar]
- Viuda-Martos M, Ruiz Navajas Y, Zapata ES, Fernández-López J, Pérez-Álvarez JA. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010;25:13–19. [Google Scholar]
- Whitney BM, Williams RC, Eifert J, Marcy J. High-pressure resistance variation of Escherichia coli O157:H7 strains and Salmonella serovars in tryptic soy broth, distilled water, and fruit juice. J. Food Protect. 2007;70:2078–2083. doi: 10.4315/0362-028x-70.9.2078. [DOI] [PubMed] [Google Scholar]


