Abstract
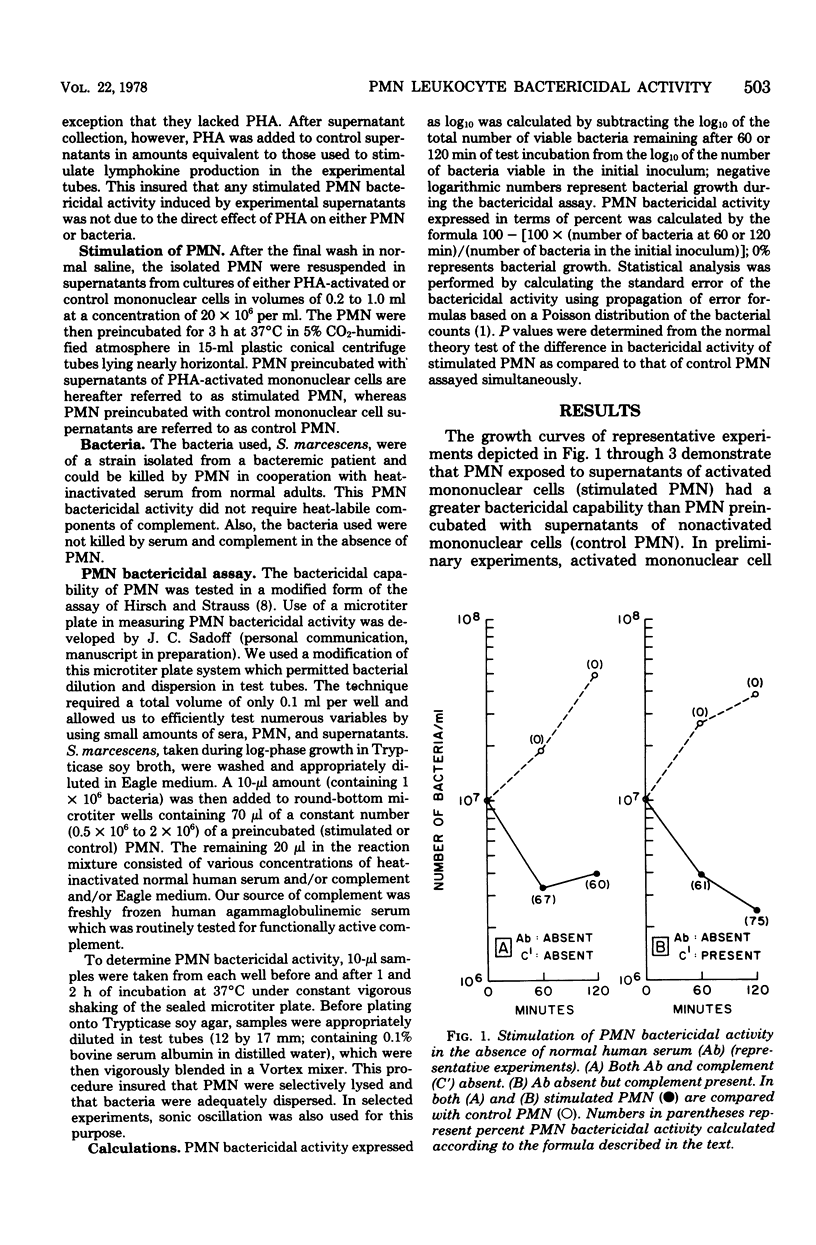
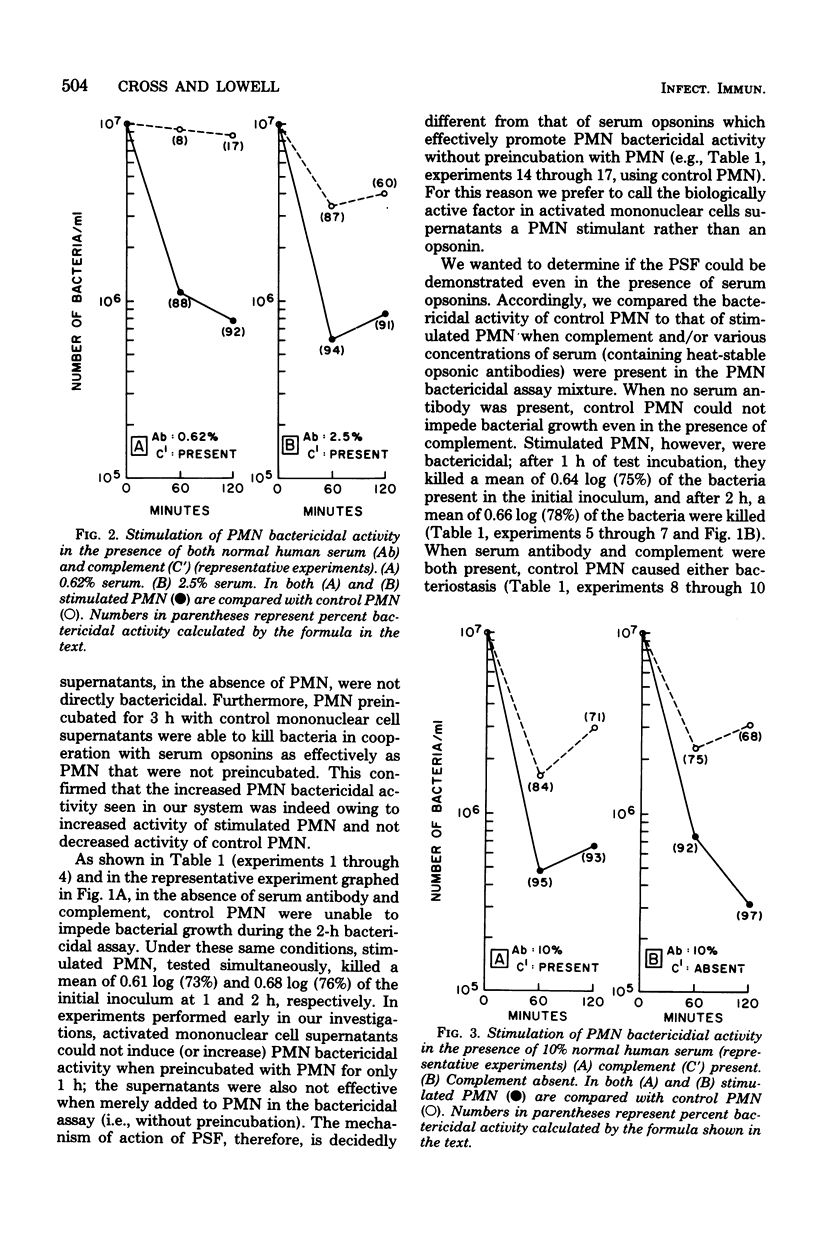
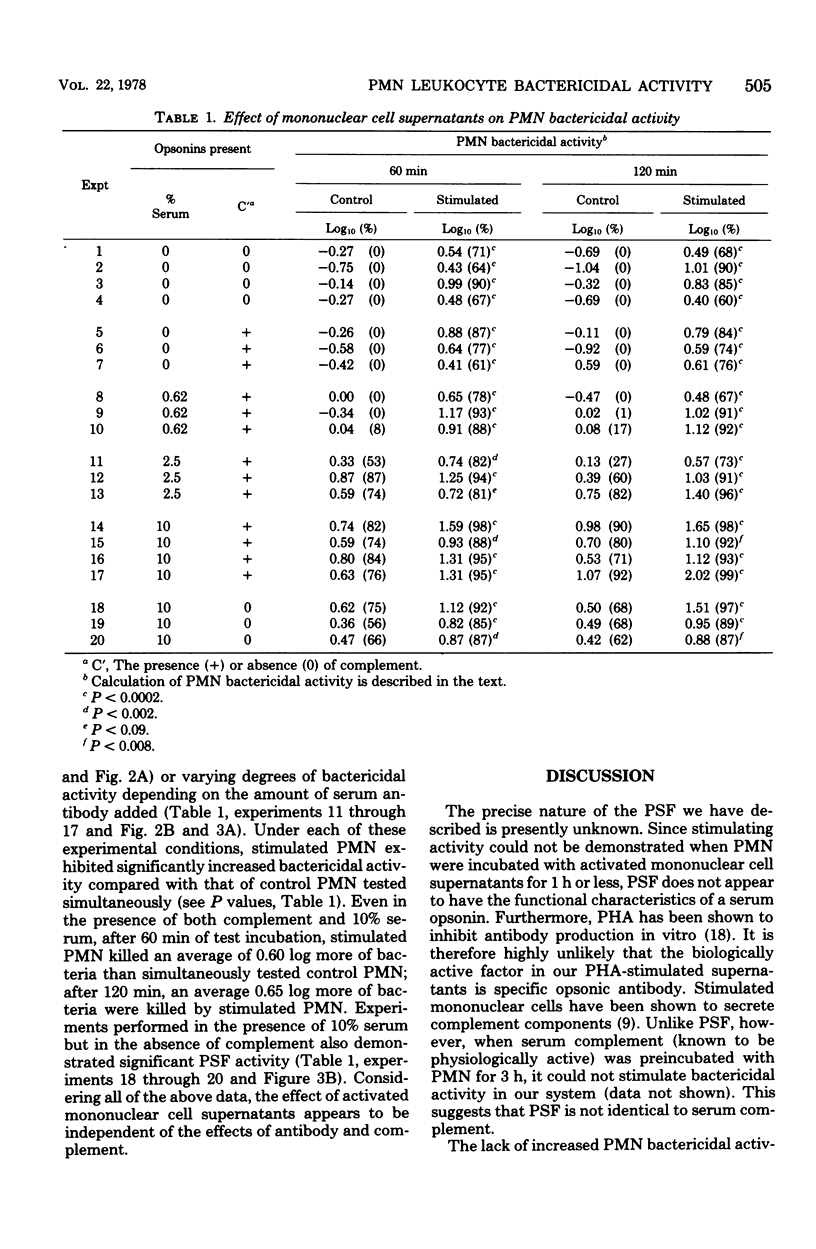
Supernatants of phytohemagglutinin-activated human mononuclear cells stimulated polymorphonuclear leukocyte (PMN) activity against the gram-negative organism Serratia marcescens. In the absence of serum opsonins, when control PMN could not impede bacterial growth, stimulated PMN averaged more than 0.6-log kill of the original bacterial inoculum. In the presence of optimal amounts of serum opsonins, when control PMN were significantly bactericidal, stimulated PMN killed, on the average, at least 0.6 log more of bacteria. Stimulation was not found when PMN were preincubated with supernatants for 1 h or less. The data strongly suggested that the action of the PMN stimulating factor was independent of and different from classically described serum opsonins. PMN stimulating activity may be an additional lymphokine-mediated immune defense mechanism enabling hosts to kill invading microorganisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974 Jun;112(6):2055–2062. [PubMed] [Google Scholar]
- Campbell P. A. Immunocompetent cells in resistance to bacterial infections. Bacteriol Rev. 1976 Jun;40(2):284–313. doi: 10.1128/br.40.2.284-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A., Smith J. W. Tuftsin deficiency: a new syndrome with defective phagocytosis. J Pediatr. 1972 Apr;80(4):564–572. doi: 10.1016/s0022-3476(72)80051-9. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Littman B. H., Ruddy S. Production of the second component of complement by human monocytes: stimulation by antigen-activated lymphocytes or lymphokines. J Exp Med. 1977 May 1;145(5):1344–1352. doi: 10.1084/jem.145.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnitzer R., Glover A., Rabson A. R. The effect of PHA-activated MN-cell supernatants on polymorphonuclear leucocyte function. Clin Exp Immunol. 1977 Sep;29(3):501–508. [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Becker E. L. The role of an activatable esterase in immune-dependent phagocytosis by human neutrophils. J Immunol. 1977 Apr;118(4):1354–1365. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E. Products of activated lymphocytes: leukocyte inhibitory factor (LIF) distinct from migration inhibitory factor (MIF). J Immunol. 1974 Apr;112(4):1461–1466. [PubMed] [Google Scholar]
- Rocklin R. E., Winston C. T., David J. R. Activation of human blood monocytes by products of sensitized lymphocytes. J Clin Invest. 1974 Feb;53(2):559–564. doi: 10.1172/JCI107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B. H., Namba Y. On soluble mediators of immunologic regulation. Cell Immunol. 1976 Jan;21(1):161–176. doi: 10.1016/0008-8749(76)90337-3. [DOI] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Young L. S., Martin W. J., Meyer R. D., Weinstein R. J., Anderson E. T. Gram-negative rod bacteremia: microbiologic, immunologic, and therapeutic considerations. Ann Intern Med. 1977 Apr;86(4):456–471. doi: 10.7326/0003-4819-86-4-456. [DOI] [PubMed] [Google Scholar]



