Abstract
OBJECTIVE
The catechol-O-methyltransferase enzyme catalyzes the methylation of the catechol estrogens, 2- or 4-hydroxyestrogen, to 2- or 4-methoxyestrogen. Both the hydroxy estrogens and methoxy estrogens were shown to modulate the effects of estrogen. Because catechol-O-methyltransferase activity controls levels of these metabolites, it may help regulate the cellular estrogenic milieu. In this study, we examined the regulation of catechol-O-methyltransferase expression in human myometrial cells.
METHODS
Catechol-O-methyltransferase expression was assessed by reverse transcription–polymerase chain reaction, Western blot, and luciferase assays in human myometrial cells after treatment with estrogen or progesterone. Catechol-O-methyltransferase expression was measured in cells after treatment with tumor necrosis factor alpha (TNFα) alone or with lactacystin, a protea-some inhibitor. Luciferase assays were also conducted using human myometrial cells containing an estrogen response element–luciferase reporter gene to measure levels of estrogen-mediated transactivation after treatment with estrogen and increasing concentrations of 2-hydroxestrogen.
RESULTS
Catechol-O-methyltransferase expression was down-regulated by progesterone or estrogen. Tumor necrosis factor alpha upregulated catechol-O-methyltransferase expression, whereas cotreatment with lactacystin attenuated this response, suggesting that TNFα activated nuclear factor kappa B to induce catechol-O-methyltransferase expression. Increased concentrations of 2-hydroxyestrogen attenuated estrogen-mediated transcription in the myometrial cells.
CONCLUSION
Catechol-O-methyltransferase expression may be regulated in the myometrium to control the local action of estrogen. Low levels of catechol-O-methyltransferase in the myometrium would result in an accumulation of 2-hydroxyestrogen and may antagonize the local effect of estrogen. High levels of catechol-O-methyltransferase in the myometrium would result in lower levels of 2-hydroxyestrogen and may increase sensitivity to estrogen.
Catechol-O-methyltransferase catalyzes the transfer of a methyl group from S-adenosyl-methio-nine to one of the phenolic hydroxyl groups in a variety of catechols including catechol estrogens and catecholamine neurotransmitters.1,2 During estrogen metabolism, catechol-O-methyltransferase converts the catechol estrogens, 2- or 4-hydroxyestrogen, to 2-or 4-methoxyestrogen. These estrogen metabolites have been shown to modulate the local effects of estrogen.3–9 Because catechol-O-methyltransferase activity ultimately controls levels of these metabolites, it appears to be a key factor in regulating the cellular estrogenic milieu.
Catechol-O-methyltransferase is present in many types of tissues, including those involved in reproduction (Salih SM, Salama SA, Jamaluddin M, Nagamani M, Al-Hendy A. Cyclic Variations of catechol-omethyltransferase [COMT] expression in human endometrium and hormonal regulation of COMT in human stromal endometrial cell line [abstract]. J Soc Gynecol Investig 2006;13:265A).5,10–12 Distinct variations in catechol-O-methyltransferase expression were detected in endometrium samples taken from cycling women, in which catechol-O-methyltransferase expression levels were higher in proliferative endometrium compared with secretory endometrium (Salih et al).13 Changes in catechol-O-methyltransferase activity in reproductive tissues were also detected during pregnancy. Catechol-O-methyltrans ferase activity was higher in decidua vera tissues taken from term pregnant women than in tissues taken from women in early pregnancy.12 Placental tissues taken from pregnant women at term also contained high levels of catechol-O-methyltransferase activity.10,11 Hormones were shown to influence catechol-O-methyltransferase expression in a tissue-specific manner.13–16 Results from computer-based bioinformatic analysis of the catechol-O-methyltransferase promoter revealed the presence of multiple half-sites of the estrogen-response element, a half-site for the glucocorticoid-response element, and multiple sites for nuclear factor κB.
In this study, we examined the regulation of catechol-O-methyltransferase expression in human myometrial cells. Catechol-O-methyltransferase expression was assessed by reverse transcription–polymerase chain reaction or Western blot analyses in primary human myometrium cells after treatment with progesterone, estrogen, or tumor necrosis factor α (TNFα), a cytokine associated with parturition. Luciferase assays were conducted using immortalized human myometrial cells containing a lucif-erase reporter controlled by the catechol-O-methyltransferase promoter, which were treated with estrogen or progesterone to confirm results obtained from reverse transcription–polymerase chain reaction and Western blot analyses. In addition, immortalized human myome-trial cells containing an estrogen response element–lucif-erase reporter gene were treated with estrogen and 2-hydroxyestrogen to measure the impact of this metabolite on estrogen-mediated transactivation.
MATERIALS AND METHODS
Primary and immortalized human myometrial cells were used in the study. Catechol-O-methyltransferase expression was assessed by reverse transcription– polymerase chain reaction and Western blot analyses in the primary cells after treatment with progesterone, estrogen, or TNFα. The immortalized myometrial cells were used in luciferase assays. Primary human myometrial cell cultures were established from hysterectomy specimens as described previously.17 All human tissues were collected, and informed consent was obtained according to a protocol (#0111) approved by the Institutional Review Board at the University of Texas Medical Branch, Galveston, Texas. Cultures consisted of a homogeneous population of smooth muscle cells based on the distribution of smooth muscle markers (eg, α-actin, desmin, and vimentin) within each culture.18 The cells used in the study were at low passage, and the presence of receptors for both estrogen and progesterone was confirmed by Western blot (data not shown). Luciferase assays were conducted using HM9 cells, which are a telomerase-immortalized human myometrial cell line that was established from a myometrial sample obtained from a pregnant woman at term (kindly provided by Dr. Melvyn Soloff, University of Texas Medical Branch, Galveston, Texas).19 These cells are well-characterized and express progesterone receptors (PR-A and PR-B, data not shown) but lack endogenous estrogen receptor-α expression. We reconstituted estrogen receptor-α expression by transfecting the HM9 cells with an adenovirus encoding the cDNA for estrogen receptor-α, as previously described.20 These adenovirus estrogen receptor-α HM9 cells expressed normal estrogen receptor-α protein in Western blots (data not shown) and responded appropriately to estrogen in proliferation (data not shown) and estrogen-response element–luciferase transactivation assays.5
A pilot study was conducted in which multiple primary human myometrial cells (each established from a patient sample) were treated with a single dose of estrogen or progesterone. After treatment, catechol-O-methyltransferase expression was assessed by Western blot, and catechol-O-methyltransferase protein levels in treated cells were compared with those in the untreated counterpart. Results from this study indicated that both estrogen and progesterone down-regulated catechol-O-methyltransferase expression. Therefore, a representative primary cell line (established from a single patient) was used in the subsequent treatment assays described below.
The primary human myometrial cells were grown to subconfluence (70–80%) and then incubated in media containing 10% charcoal-stripped fetal bovine serum (Hyclone Laboratories, Logan, UT) for 24 hours. The cells were treated with estrogen or progesterone (Sigma Aldrich, St. Louis, MO) at molar concentrations ranging from 10−6 to 10−10 mol/L or with TNFα (PreproTech Inc, Rocky Hill, NJ) at concentrations ranging from 5 to 40 ng/mL. Cells incubated in the same media used to prepare the solutions of hormones or TNFα served as vehicle control cells. Cells were incubated for 8 hours with TNFα and for 36 hours with progesterone or estrogen. After the desired incubation, cells were harvested for reverse transcription–polymerase chain reaction or Western blot analyses.
A second experiment was conducted involving treatment with TNFα, in which primary human myometrial cells were pretreated with the 26S proteasome inhibitor lactacystin (BioMol Research Labs Inc, Plymouth Meeting, PA) at 5, 10, or 20 μmol/L concentrations for 2 hours, followed by an addition of TNFα at a concentration of 5 ng/mL. Cells incubated in media alone or treated with either TNFα or lactacystin alone served as controls. Cells were subsequently incubated for 8 hours and then harvested for reverse transcription–polymerase chain reaction analyses.
Total RNA or whole-cell lysates were prepared from the treated human myometrial cells and used in reverse transcription–polymerase chain reaction or Western blot analyses, respectively. The acid guanidinium thiocyanate-phenol-chloroform method22 was used to extract the total RNA. All RNA samples were treated with a recombinant Dnase I (Ambion, Austin, TX) to remove contaminating DNA. The amount of RNA was calculated using the A260 value. Whole-cell lysates were prepared by incubating the treated cells at 4°C for 30 minutes in protein extraction buffer (50 mM sodium chloride [NaCl], 50 mM Tris, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulphate [SDS]) followed by centrifugation (4°C, 10,000 × g, 10 minutes). The concentration of total protein in each sample was determined using the bicinchoninic acid protein assay (Pierce, Rockford, Ill) following the instructions provided by the manufacturer.
Two-step reverse transcription–polymerase chain reaction assays were conducted to measure catechol-O-methyltransferase mRNA levels using reagents and procedures recommended by Applied Biosystems (ABI, Foster City, CA). Each sample used for reverse transcription included 200 ng of total RNA and random hexamers. The cDNA was synthesized in the reverse transcription samples at 48°C for 30 minutes. Equivalent amounts of the cDNA were used in the polymerase chain reaction amplification of either catechol-O-methyltransferase or glyceraldehyde 3-phosphate dehydrogenase (GAPD) using primers and probes for human catechol-O-methyltransferase or GAPD (Applied Biosystems). Amplification was detected by the ABI Prism 7700 Sequence Detection Analyzer under the following thermal cycle conditions: 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 10 seconds, and a 60°C annealing/extension for 60 seconds. Negative controls included two samples: one had water substituted for RNA to check for contamination, and the other control lacked the reverse transcriptase enzyme to confirm that amplification was not due to genomic DNA.
The threshold cycle values for catechol-O-methyltransferase and GAPD were used to calculate the relative mRNA levels. For each sample, the threshold cycle value for GAPD was used to normalize the threshold cycle value for catechol-O-methyltransferase. The levels of catechol-O-methyltransferase mRNA from treated human myometrial cells were expressed relative to those in the vehicle control cells using the delta/delta cycle threshold method described in User Bulletin #2 (Applied Biosystems).
Catechol-O-methyltransferase protein levels were detected by Western blot using standard techniques.23 Samples, each containing 10 mcg of whole cell lysate, were subjected to electrophoresis in a SDS–10% polyacrylamide gel. All reagents for the Western blot were prepared in a buffer containing 25 mmol/L Tris-Cl, 150 mM NaCl, and 0.1% Tween-20 in the presence or absence of 5% or 3% nonfat milk. The primary antibody used to detect catechol-O-methyltransferase, which binds to both the soluble catechol-O-methyltransferase and membrane catechol-O-methyltransferase forms of the protein, was used at a 1:10,000 dilution (Chemicon, Temecula, CA). The primary antibody used to detect β-actin was used at a 1:10,000 dilution (Sigma Aldrich). The proteins were visualized by chemiluminescence (Amersham Biosciences, Piscataway, NJ) and quantified by densitometry (Alpha Innotech Co, San Leando, CA). The values from the β-actin in each sample were used to normalize levels of catechol-O-methyltransferase protein.
Luciferase assays were conducted using HM9 adenovirus estrogen receptor-α, the immortalized human myometrial cells expressing estrogen receptor-α, to further confirm the regulation of catechol-O-methyltransferase expression. Two reporter constructs, catechol-O-methyltransferase P1-Luc and TK-RLuc, were transfected into the adenovirus estrogen receptor-α HM9 cells using reagents and instructions provided in the Basic Nucleofector kit (Amaxa, Gaithersburg, MD). The catechol-O-methyltransferase P1-Luc reporter encodes the firefly luciferase controlled by the catechol-O-methyltransferase promoter and measured catechol-O-methyltransferase transcription, while the TK-RLuc reporter encoded the renilla luciferase controlled by the thymidine kinase promoter and served as an internal control. After transfection, cells were incubated in media containing 10% charcoal-stripped fetal bovine serum and treated with progesterone or estrogen (from 10−6 to 10−12 mol/L), as previously described. After treatment, the cells were harvested for luciferase assays using reagents and procedures included in the Dual Reporter Luciferase Assay kit (Promega, Madison, WI). Lucif-erase values reflecting catechol-O-methyltransferase transcription (catechol-O-methyltransferase P1-Luc) were normalized using luciferase values from the internal control (Tk-RLuc).
Luciferase assays were also conducted to measure the effect of 2-hydroxyestrogen on estrogen-mediated transactivation. For these assays, we incubated the adenovirus estrogen receptor-α HM9 cells with an adenovirus containing an estrogen-response element-tk-luciferase reporter (kindly provided by Dr. Eun J. Lee, Northwestern University, Chicago, IL) at 10 plaque-forming units/cell for 5 hours at 37°C as previously described.5 The medium was removed and replaced with fresh medium containing 10−8 mol/L estrogen and 2-hydroxyestrogen (Sigma Chemicals) at different concentrations ranging from 100 nmol/L to 100 μmol/L. Untreated cells and cells treated with estrogen alone served as controls. After 24 hours the treated cells were harvested to measure luciferase activity using reagents and procedures included in the Luciferase Assay System (Promega). The luciferase activity was normalized against the number of cells per well calculated by measuring fluorescence of Hoechst 33258 dye.5
Two independent treatment assays were conducted in which each concentration of estrogen, progesterone, or TNFα was tested in duplicate samples. Two to three independent reverse transcription–polymerase chain reaction assays were conducted in which each sample was tested in duplicate. Western blot was conducted using samples for each concentration of hormone from the two treatment assays. Data from the reverse transcription–polymerase chain reaction assays were analyzed by one-way analysis of variance followed by Tukey multiple pairwise comparisons tests, and P<.05 was considered significant.
RESULTS
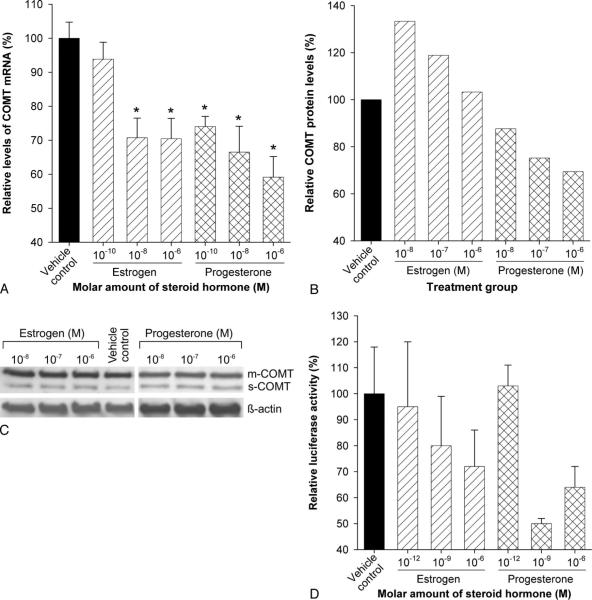
Treatment of human myometrial cells with progesterone consistently caused a downregulation in catechol-O-methyltransferase expression (Fig. 1A–D). Catechol-O-methyltransferase mRNA levels in primary human myometrial cells treated with 10−10, 10−8, and 10−6 mol/L concentrations of progesterone were 74%, 66%, and 59% compared with vehicle control cells (Fig. 1A). These differences were statistically significant (P<.05) at all three molar concentrations. Total catechol-O-methyltransferase protein (ie, both soluble and membrane-bound forms of catechol-O-methyltransferase) levels were 88%, 75%, and 69% compared with vehicle control in cells treated with 10−8, 10−7, and 10−6 mol/L of progesterone (Fig. 1B and C). Treatment of adenovirus estrogen receptor-α HM9 cells, containing the catechol-O-methyltransferase promoter–luciferase reporter gene, with 10−12, 10−9, and 10−6 mol/L of progesterone resulted in 103%, 50%, and 64% luciferase activities when compared with vehicle control (Fig. 1D).
Fig. 1.
Progesterone and estrogen downregulate catechol-O-methyltransferase (COMT) expression in human myometrial cells. Values in treated groups are expressed relative to vehicle control. An asterisk indicates a statistically significant difference (P<.05) in mRNA levels. A. Bars represent average COMT mRNA levels from three independent reverse transcriptase polymerase chain reaction assays in which samples were tested in duplicate. B. Bars represent COMT protein levels quantified from the autoradiogram shown in panel C. C. An autoradiogram showing results of Western blot analysis used to detect COMT protein. D. Bars represent average luciferase activity measured in samples from treated adenovirus estrogen receptor-α HM9 cells tested in duplicate. Error bars represent standard error of the mean. mCOMT, membrane-bound COMT; sCOMT, soluble COMT.
Wentz. Catechol-O-Methyl Transferase Regulation. Obstet Gynecol 2006.
Treatment of cells with estrogen also caused a decrease in catechol-O-methyltransferase expression (Fig. 1A-D), although higher concentrations of estrogen were required to consistently downregulate catechol-O-methyltransferase expression. Catechol-O-methyltransferase mRNA levels in primary human myometrial cells treated with 10−10, 10−8, and 10−6 mol/L concentrations of estrogen were 94%, 71%, and 70%, respectively, compared with vehicle control cells (Fig. 1A). Differences in catechol-O-methyltransferase mRNA in cells treated with 10−8 and 10−6 mol/L concentrations of estrogen were statistically significant (P<.05). In cells treated with 10−8, 10−7, and 10−6 mol/L of estrogen, total catechol-O-methyltransferase protein levels were 133%, 119%, and 103% compared with vehicle control (Fig. 1B and C). Treatment of adenovirus estrogen receptor-α HM9 cells, containing the catechol-O-methyltransferase promoter–luciferase reporter gene, with 10−12, 10−9, and 10−6 mol/L of estrogen resulted in 95%, 80%, and 72% luciferase activities when compared with vehicle control (Fig. 1D). It should be noted that the relative levels of catechol-O-methyltransferase expression obtained from Western blot were higher than those obtained from reverse transcription–polymerase chain reaction and luciferase assays. This could be attributed to the fact that Western blot may not be as sensitive as reverse transcription–polymerase chain reaction and luciferase assays.
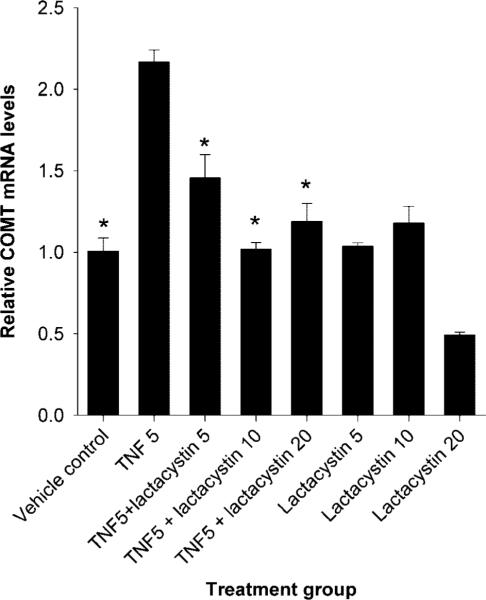
Treatment of human myometrial cells with TNFα at 5 or 10 ng/mL concentrations caused an increase in catechol-O-methyltransferase mRNA levels, whereas higher concentrations of TNFα had no effect on catechol-O-methyltransferase transcription (Fig. 2). When expressed relative to vehicle control, levels of catechol-O-methyltransferase mRNA were 4.5 times higher in human myometrial cells treated with 5 ng/mL of TNFα and two times higher in cells treated with 10 ng/mL of TNFα (Fig. 2). These differences were statistically significant (P<.05). Catechol-O-methyltransferase mRNA levels in cells cotreated with both TNFα and lactacystin (a proteasome inhibitor) at 20, 10, or 5 μmol/L concentrations were 118%, 102%, and 145% compared with vehicle control (Fig. 3). The reduction in catechol-O-methyltransferase mRNA levels induced by lactacystin at all concentrations were statistically significant (P<.05) when compared with catechol-O-methyltransferase levels in cells treated with TNFα alone. The catechol-O-methyltransferase mRNA levels in human myome-trial cells treated with 20, 10, or 5 μmol/L concentrations of lactacystin alone were 49%, 117%, and 104% compared with vehicle control (Fig. 3). Because lactacystin is a proteasome inhibitor and, hence, inhibits nuclear factor κB activity, our data suggest that TNFα induces catechol-O-methyltransferase expression via nuclear factor κB signaling.
Fig. 2.
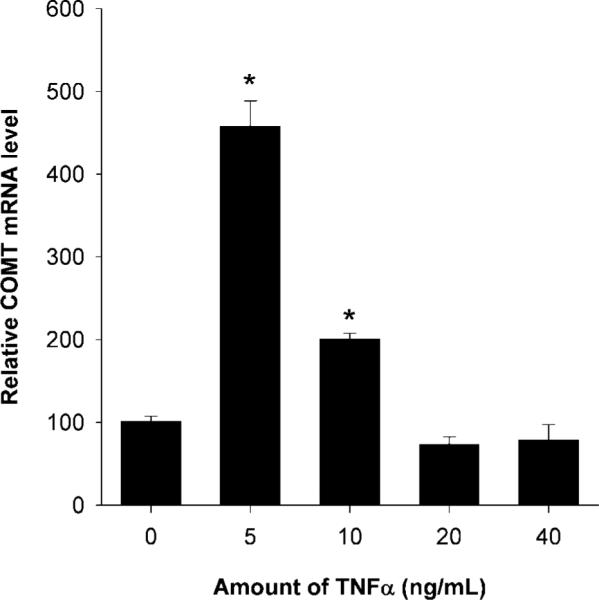
Tumor necrosis factor alpha (TNFα) activates catechol-O-methyltransferase (COMT) transcription in human myometrial cells. Values in treated groups are expressed relative to vehicle control. An asterisk indicates a statistically significant difference (P<.05) in mRNA levels. Bars represent average COMT (with standard error of the mean) mRNA levels from three independent reverse transcriptase polymerase chain reaction assays in which samples were tested in duplicate.
Wentz. Catechol-O-Methyl Transferase Regulation. Obstet Gynecol 2006.
Fig. 3.
Activation of catechol-O-methyltransferase (COMT) mRNA expression by tumor necrosis factor alpha (TNFα) involves the 26 proteasome pathway. Values are expressed relative to vehicle control. Bars represent average COMT (with standard error of the mean) mRNA levels from three independent reverse transcriptase polymerase chain reaction assays in which samples were tested in duplicate. The asterisk indicates statistically significant differences (P<.05) in mRNA when compared with the value from cells treated with TNFα alone. TNF5, TNFα at a concentration of 5 ng/mL; Lactacystin 5, 10, and 20 indicates lactacystin concentrations of 5, 10, and 20 μmol/L.
Wentz. Catechol-O-Methyl Transferase Regulation. Obstet Gynecol 2006.
Treatment of adenovirus estrogen receptor-α HM9 cells, containing the estrogen-response element-Luc reporter, with 10−8 mol/L concentrations of estrogen induced high luciferase activity (Fig. 4). Starting at a concentration of 5 μmol/L, 2-hydroxyestrogen caused a reduction in estrogen-stimulated estrogen-response element transcriptional activity (Fig. 4). This difference was statistically significant (P=.002) at 5 μmol/L and all higher doses of 2-hydroxyestrogen (Fig. 4).
Fig. 4.
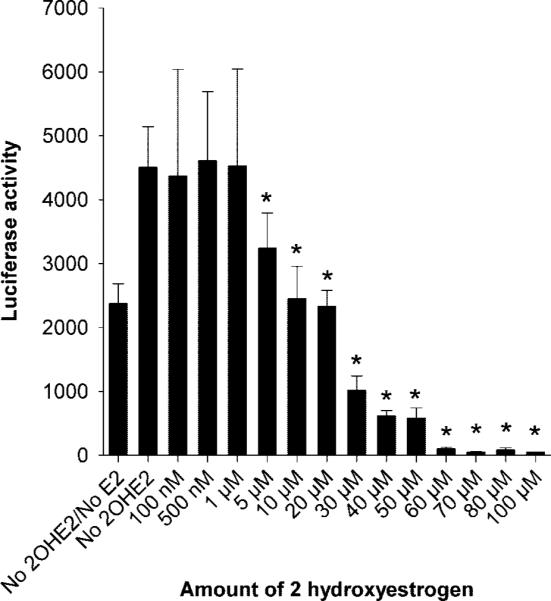
The estrogen metabolite 2-hydroxyestrogen (2-OHE2) reduces estrogen-mediated transactivation in human myometrial cells. Bars represent average luciferase activities (with the standard error of the mean) from two independent assays in which samples were tested in triplicate. The inhibitory effect of 2-OHE2 was statistically significant (P=.002) at doses of 5 μmol/L and higher as indicated by an asterisks.
Wentz. Catechol-O-Methyl Transferase Regulation. Obstet Gynecol 2006.
DISCUSSION
In this study, we demonstrated that catechol-O-methyltransferase expression was downregulated by progesterone and high levels of estrogen (Fig. 1) but upregulated by TNFα (Fig. 2) in human myometrial cells. We and other researchers have shown that the catechol-O-methyltransferase substrate, 2-hydroxyestrogen, can exhibit an antiestrogenic effect in multiple biologic assays,3–5 while the catechol-O-methyltransferase product, 2-methoxyestrogen, works as an estrogen agonist.6–9 The biologic activities of these estrogen metabolites depend on their concentrations.3,4,6–8 Low levels of catechol-O-methyltransferase in the myometrium would result in a higher 2-hydroxyestrogen to 2-methoxyestrogen ratio and could block the effect of estrogen on the myometrium (Fig. 4). Elevated catechol-O-methyltransferase levels in the myometrium would reduce the 2-hydroxyestrogen to 2-methoxyestrogen ratio, which could alleviate the antagonist effects of the 2-hydroxyestrogen, resulting in an increased sensitivity of the myometrium to estrogen.
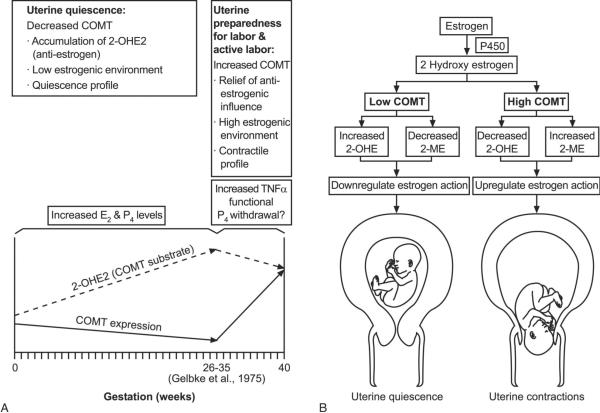
Because catechol-O-methyltransferase activity ultimately controls levels of these metabolites, we propose that catechol-O-methyltransferase expression is regulated in the myometrium to help control the local action of estrogen (Fig. 5). We propose that expression levels of catechol-O-methyltransferase may influence myometrial function. Low levels of catechol-O-methyltransferase would antagonize estrogen function and support myometrial quiescence, while high levels of catechol-O-methyltransferase may help mediate local estrogen function and facilitate myometrial contractility (Fig. 5). During the estrous cycle, elevated levels of catechol-O-methyltransferase could facilitate myometrial contractility and possibly aid in fertilization and menses. During early and midpregnancy, downregulation of catechol-O-methyltransferase by progesterone and estrogen in the myometrium may help maintain uterine quiescence. Late in pregnancy, upregulation of catechol-O-methyltransferase, possibly mediated by nuclear factor kappa B induced by TNFα, could invoke a functional estrogen activation in the myometrium in preparation for effective uterine contractions leading to a successful labor and delivery (Fig. 5).
Fig. 5.
A putative role for catechol-O-methyltransferase (COMT) in modulating the response to estrogen in the myometrium during pregnancy. Decreasing levels of COMT in the myometrium in early and midpregnancy (under influence of increasing estrogen and progesterone) result in a high accumulation of 2-hydroxyestrogen (2-OHE2) (A), which acts as an antiestrogen and may promote uterine quiescence (B). Near term, levels of COMT increase in the myometrium under the influence of proinflammatory cytokines like tumor necrosis factor alpha (TNFα) and others; this increase leads to a reduction in the levels of antiestrogen 2-OHE2 (A), which induces cellular functional estrogen activation and mediates uterine contraction (B).
Wentz. Catechol-O-Methyl Transferase Regulation. Obstet Gynecol 2006.
Caution should be exercised when extrapolating these in vitro results from cultured human myometrial cells to the intricate clinical situation during human pregnancy. Further evidence for our proposed model involving a putative function for catechol-O-methyltransferase in the myometrium during pregnancy would require repeated sampling of the human myometrium at different stages of the pregnancy. This procedure cannot be done due to ethical constraints (potential for harm to baby and mother). As an indirect assessment of catechol-O-methyltransferase activity, one can use the levels of the substrate 2-hydroxyestrogen as a surrogate. We and others have measured various estrogen metabolites, including 2-hydroxyestrone, in daily morning urine from women with normal pregnancy (Wentz MJ, Al-Hendy A. Hormonal regulation of catechol-O-methyltransferase expression in human myometrial cells [abstract]. J Soc Gynecol 2006;13:240A).23–25 Results indicated that, from early pregnancy, levels of urinary 2-hydroxyestrone gradually increased and peaked in the third trimester and then steadily decreased throughout parturition (Wentz et al) (Fig. 5A). It was reported previously that levels of urinary 2-methoxyestrone, a product of catechol-O-methyltransferase activity, were low during early and midpreg-nancy and then increased near term.25 Values for the urinary 2-hydroxyestrone23 or 2-methoxyestrone25 did not correlate with total urinary estrogens present in the samples, indicating that levels for either catechol-O-methyltransferase substrate or product did not merely reflect circulating levels of estrogen.
The functional genetic polymorphism in the catechol-O-methyltransferase gene has been linked to several estrogen-related medical disorders in women.28 The catechol-O-methyltransferase genotypes dictate the relative levels of enzymatic activity: catechol-O-methyltransferase Val/Val=high, catechol-O-methyltransferase Val/Met=intermediate, and catechol-O-methyltransferase Met/Met=low.27 The catechol-O-methyltransferase Val/Val genotype is more prevalent in African-American women (47%) than in white (19%) or Hispanic women (30%) (P=.003).5 We recently reported that the catechol-O-methyltransferase Val/Val genotype was significantly more common in women with uterine leiomyomas than in ethnically matched controls.5 Cultured human myometrial cells with the catechol-O-methyltransferase Val/Val (high) genotype exhibited higher levels of cell proliferation, estrogen-response element– mediated transactivation, and estrogen-responsive proteins when compared with human myometrial cells with the catechol-O-methyltransferase Met/Met (low) genotype.5 Cotreatment with a selective catechol-O-methyltransferase inhibitor reduced the estrogen-mediated cell proliferation and transactivation.5 These results suggest that the catechol-O-methyltransferase Val/Val high activity genotype, which is more common in African-American women, could create a hyperestrogenic cellular milieu in the myometrium and may be linked to the higher incidence of uterine leiomyomas5 and preterm labor in these women.28
Acknowledgments
This work was supported by a National Institutes of Health grant to Dr. Al-Hendy (R01 HD46228).
REFERENCES
- 1.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–5. [PubMed] [Google Scholar]
- 2.Creveling CR. The role of catechol-O-methyltransferase in the inactivation of catecholestrogen. Cell Mol Neurobiol. 2003;23:289–91. doi: 10.1023/A:1023680302975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrinol. 1989;61:239–46. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 4.Bradlow LH, Telang NT, Sepkovic DW. Osborne 2-Hydroxyesterone: the “good” estrogen. J Endocrinol. 1996;150:S259–65. [PubMed] [Google Scholar]
- 5.Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006;13:136–44. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee SN, Sengupta K, Banerjee S, Saxena NK, Banerjee SK. 2-Methoxyestradiol exhibits a biphasic effect on VEGF-A in tumor cells and upregulation is mediated through ER-alpha: a possible signaling pathway associated with the impact of 2-ME2 on proliferative cells. Neoplasia. 2003;5:417–26. doi: 10.1016/s1476-5586(03)80044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippert C, Seeger H, Mueck AO. The effect of endogenous estradiol metabolites on the proliferation of human breast cancer cells. Life Sci. 2003;72:877–83. doi: 10.1016/s0024-3205(02)02305-6. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZJ, Zhu BT. Concentration-dependent mitogenic and antiproliferative actions of 2-methoxyestradiol in estrogen receptor-positive human breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:265–75. doi: 10.1016/j.jsbmb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland TE, Schuliga M, Harris T, Eckhardt BL, Anderson RL, Quan L, et al. 2-methoxyestradiol is an estrogen receptor agonist that supports tumor growth in murine xenograft models of breast cancer. Clin Cancer Res. 2005;11:1722–32. doi: 10.1158/1078-0432.CCR-04-1789. [DOI] [PubMed] [Google Scholar]
- 10.Castren O, Saarikoski S. The simultaneous function of catechol-O-methyltransferase and monoamine oxidase in human placenta. Acta Obstet Gynecol Scand. 1974;53:41–7. doi: 10.3109/00016347409156887. [DOI] [PubMed] [Google Scholar]
- 11.Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-o-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5:121–7. doi: 10.1055/s-2007-999669. [DOI] [PubMed] [Google Scholar]
- 12.Casey ML, MacDonald PC. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am J Obstet Gynecol. 1983;145:453–7. doi: 10.1016/0002-9378(83)90316-2. [DOI] [PubMed] [Google Scholar]
- 13.Briggs MH, Briggs M. Hormonal influences on erythrocyte catechol-O-methyl transferase activity in humans. Experientia. 1973;29:278–80. doi: 10.1007/BF01926474. [DOI] [PubMed] [Google Scholar]
- 14.Parvez H, Parvez S, Raza-Bukhari A. Decreased phenylethanolamine-N-methyltransferase and catechol-O-methyltransferase activity in rabbit adrenal glands during pregnancy. Br J Pharmacol. 1976;57:413–6. doi: 10.1111/j.1476-5381.1976.tb07682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue K, Creveling CR. Induction of catechol-O-methyltransferase in the luminal epithelium of rat uterus by progesterone. J Histochem Cytochem. 1991;39:823–8. doi: 10.1177/39.6.2033240. [DOI] [PubMed] [Google Scholar]
- 16.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–8. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Rauk PN, Surti U, Roberts JM, Michalopoulos G. Mitogenic effect of basic fibroblast growth factor and estradiol on cultured human myometrial and leiomyoma cells. Am J Obstet Gynecol. 1995;173:571–7. doi: 10.1016/0002-9378(95)90284-8. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–31. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Soloff MS, Jeng YJ, Ilies M, Soloff SL, Izban MG, Wood TG, et al. Immortalization and characterization of human myome-trial cells from term-pregnant patients using a telomerase expression vector. Mol Hum Reprod. 2004;10:685–95. doi: 10.1093/molehr/gah086. [DOI] [PubMed] [Google Scholar]
- 20.Lee EJ, Duan WR, Jakacka M, Gehm BD, Jameson JL. Dominant negative ER induces apoptosis in GH(4) pituitary lactotrope cells and inhibits tumor growth in nude mice. Endocrinology. 2001;142:3756–63. doi: 10.1210/endo.142.9.8372. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Springs Harbor Laboratory Press; New York (NY): 1989. [Google Scholar]
- 23.Gelbke HP, Bottger M, Knuppen R. Excretion of 2-hydroxyestrone in urine throughout human pregnancies. J Clin Endocrinol Metab. 1975;41:744–50. doi: 10.1210/jcem-41-4-744. [DOI] [PubMed] [Google Scholar]
- 24.Hobkirk R, Nilsen M. Observations on the occurrence of six estrogen fractions in human pregnancy urine. I. Normal pregnancy. J Clin Endocrinol Metab. 1962;22:134–41. doi: 10.1210/jcem-22-2-134. [DOI] [PubMed] [Google Scholar]
- 25.Hobkirk R, Nilsen M. 2-methoxyestrone as an estrogen metabolite in the human subject. J Clin Endocrinol Metab. 1963;23:274–8. doi: 10.1210/jcem-23-3-274. [DOI] [PubMed] [Google Scholar]
- 26.Tempfer CB, Schneeberger C, Huber JC. Applications of polymorphisms and pharmacogenomics in obstetrics and gynecology. Pharmacogenomics. 2004;5:57–65. doi: 10.1517/phgs.5.1.57.25687. [DOI] [PubMed] [Google Scholar]
- 27.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981–98. Paediatr Perinat Epidemiol. 2002;16:8–15. doi: 10.1046/j.1365-3016.2002.00394.x. [DOI] [PubMed] [Google Scholar]