Abstract
Sensitive probing of temperature variations on nanometer scales represents an outstanding challenge in many areas of modern science and technology1. In particular, a thermometer capable of sub-degree temperature resolution over a large range of temperatures as well as integration within a living system could provide a powerful new tool for many areas of biological, physical and chemical research; possibilities range from the temperature-induced control of gene expression2–5 and tumor metabolism6 to the cell-selective treatment of disease7,8 and the study of heat dissipation in integrated circuits1. By combining local light-induced heat sources with sensitive nanoscale thermometry, it may also be possible to engineer biological processes at the sub-cellular level2–5. Here, we demonstrate a new approach to nanoscale thermometry that utilizes coherent manipulation of the electronic spin associated with nitrogen-vacancy (NV) color centers in diamond. We show the ability to detect temperature variations down to 1.8 mK (sensitivity of ) in an ultra-pure bulk diamond sample. Using NV centers in diamond nanocrystals (nanodiamonds, NDs), we directly measure the local thermal environment at length scales down to 200 nm. Finally, by introducing both nanodiamonds and gold nanoparticles into a single human embryonic fibroblast, we demonstrate temperature-gradient control and mapping at the sub-cellular level, enabling unique potential applications in life sciences.
Many promising approaches to local temperature sensing1 are currently being explored. These include scanning probe microscopy1, 9, Raman spectroscopy10, and fluorescence-based measurements using nanoparticles11, 12 and organic dyes13, 14. Fluorescent polymers13 and green fluorescent proteins (GFPs)14 have recently been used for temperature mapping within a living cell. However, many of these existing methods are limited by drawbacks such as low sensitivity and systematic errors owing to fluctuations in the fluorescence rate11, 12, the local chemical environment13 and the optical properties of the surrounding medium14. Moreover, while promising, GFP-based methods rely on cellular transfection14 that proves to be difficult to achieve in certain primary cell types15. Our new approach to nanoscale thermometry utilizes the quantum mechanical spin associated with nitrogen vacancy (NV) color centers in diamond. As illustrated in Fig. 1, in its electronic ground state, the NV center constitutes a spin-1 system. These spin states can be coherently manipulated using microwave pulses and efficiently initialized and detected via laser illumination (see SI). In the absence of an external magnetic field, the precise value of the transition frequency (Δ) between the |ms = 0〉 and |ms = ±1〉 states exhibits a temperature dependence (dΔ/dT =−(2π)77 kHz/K) due to thermally induced lattice strains16–18.
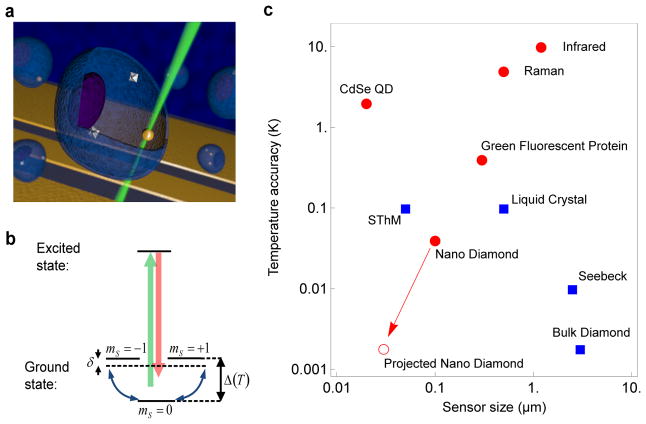
Figure 1. NV-based nanoscale thermometry.
a, Schematic image depicting nanodiamonds and gold nanoparticles (Au NPs) within a living cell. The controlled application of local heat is achieved via laser illumination of the Au NP, while nanoscale thermometry is achieved via precision spectroscopy of the NV spins in nanodiamonds. b, Simplified NV level diagram showing a ground state spin triplet and an excited state. At zero magnetic field, the | ± 1〉 sub-levels are split from the |0〉 state by a temperature-dependent zero field splitting Δ(T). Pulsed microwave radiation is applied (detuning δ) to perform Ramsey-type spectroscopy. c, Comparison between the NV quantum thermometer and other reported techniques as a function of sensor size and temperature accuracy. Red circles indicate methods that are biologically compatible. The red open circle indicates the ultimate expected accuracy for our measurement technique in solution (see Methods).
The operational principle of NV-based thermometry relies upon the accurate measurement of this transition frequency, which can be optically detected with high spatial resolution (Fig. 1). For a sensor containing N color centers, the temperature sensitivity is given by
| (1) |
where Tcoh is the NV-spin coherence time and t is the integration time. Here, we also introduce a factor C to account for imperfections in readout and initialization19. Assuming Tcoh is on the order of a few milliseconds and C ≈ 0.0319, a single NV can potentially exhibit a sensitivity better than . Beyond high sensitivity, NV-based thermometry also offers several distinct advantages over existing methods in biological and chemical temperature sensing. First, owing to diamond’s chemical inertness, it is generally robust to changes in the local chemical environment. Second, it can be applied over a wide range of temperatures, 200–600 K17, 18, which is of particular interest in the study of nanoscale chemical reactions20.
As a first benchmark experiment, we demonstrate the high temperature sensitivity of NV-based thermometry in a bulk diamond sample. While the NV’s magnetic sensitivity has rendered it a competitive magnetometer21, 22, to accurately determine the temperature, it is necessary to decouple the NV electronic spin from fluctuating external magnetic fields. This is achieved via a modified spin-echo sequence that makes use of the spin-1 nature of the NV defect23, allowing us to eliminate the effects of an external, slowly varying, magnetic field. Specifically, we apply a microwave pulse at frequency ω (Fig. 1b) to create a coherent superposition , where . After half the total evolution time τ we apply a 2π echo-pulse that swaps the population of the | + 1〉 and | − 1〉 states (Fig. 2a). Following another period of free evolution for time τ, quasi-static, magnetic-field-induced shifts of these |±1〉 levels are eliminated, allowing for accurate temperature sensing. In the experiment, we use a CVD-grown, isotopically pure diamond (99.99 % spinless 12C isotope) sample24 to further reduce magnetic-field fluctuations originating from the intrinsic 13C nuclear spin bath. As shown in Fig. 2a, this allows us to observe coherence fringes approaching 0.5 ms. Interestingly, for all NVs tested, we observe a characteristic low-frequency beating of the fluorescence signal that varies from NV to NV, which is most likely due to locally fluctuating charge traps25. Despite this beating, for a fixed evolution time 2τ, the NV spin depends sensitively on the sample temperature (Fig. 2b). We observe a temperature sensitivity of for 2τ = 250 μs. With 30 seconds of integration, we achieve a measurement accuracy δT = 1.8 ±0.3 mK (see Methods). While the measurement sequence for a single value of 2τ allows us to determine the temperature only up to a multiple of (2dΔ/dT2τ)−1, absolute temperature variations can be determined by repeating the measurement for 2τ′< 2τ as shown in Fig. 2b.
Figure 2. Sensitivity of single NV Thermometer.

a, Measured fluorescence as a function of echo evolution time 2τ (red points); the black solid line indicates a fit corresponding to a damped cosine function with two distinct frequencies. The characteristic beating can be explained by fluctuating proximal charge traps located at distances of about 50 nm. The inset depicts the microwave 2π-echo-pulse sequence used to cancel unwanted external magnetic field fluctuations23. b, Measured fluorescence (red points), corresponding error bars (one standard deviation) and best fit line as function of temperature for an echo time of 2τ = 250 μs (bottom) and 2τ′= 50 μs (top). The fixed evolution times of 2τ and 2τ ′ are indicated in (a) by red arrows. The temperature is controlled by a Peltier element at the sample mount, while the (local) x-axis temperature is determined via a thermistor located immediately next to the sample. The fluorescence is converted to population by normalizing to two reference measurements where the spin is prepared in ms = 0 (ms =−1).
We now demonstrate the high spatial resolution of NV-based thermometry. This is achieved by using nanodiamonds. In most commercially available nanodiamonds, the NV coherence time is limited to approximately 1 μs due to additional paramagnetic impurities. While this shortened coherence time reduces the intrinsic temperature sensitivity for a single defect, this decrease can be offset by using an ensemble of NVs to enhance the signal to noise ratio by a factor of . Note that unlike NV-based magnetometry, where the proximity to the source (often limited by nanodiamond size) is critical to the maximum sensitivity, NV thermometry is not subject to such a constraint; in fact, the excellent thermal conductivity of diamond ensures that all NV centers within a nanocrystal are in thermal equilibrium with the local heat environment. To maximize the number of NV centers and to minimize the lattice strain, our measurements are performed on single-crystalline nanodiamonds containing approximately 500 NV centers (Adamas Nanotechnologies). The zero-field splitting Δ of the NV ensemble, and thus the temperature, is determined by recording a continuous-wave electron spin resonance (ESR) spectrum. Specifically, we measure changes to the zero-field splitting by recording the fluorescence at four different frequencies centered around Δ = 2.87 GHz (Fig. 3a). This procedure eliminates unwanted effects from fluctuations in the total fluorescence rate, ESR contrast, Rabi frequency and magnetic field, yielding a robust thermometer (see Methods).
Figure 3. Sub-micron thermometry using nanodiamonds.
a, Frequency scan of a single nanodiamond containing approximately 500 NV centers. The four red points indicate the measurement frequencies used to extract the temperature as detailed in Methods. b, Two-dimensional confocal scan of nanodiamonds (circles) and Au NPs (cross) spin-coated onto a glass coverslip. The color bar represents fluorescence given in counts per second (cps). c, Temperature of a single nanodiamond as a function of laser power for two different laser-focus locations. The red data points depict the dramatic heating of a nanodiamond as a result of laser illumination on a nearby Au NP. The blue data points depict the same measurement with the laser focus displaced by 0.8 μm from the Au NP location; this results in the negligible heating of the nanodiamond as a function of laser power. The inset shows the measured temperature change of a nanodiamond, when the surrounding temperature is controlled by a Peltier element. d, Temperature changes measured (red points) at the six nanodiamond locations in (b) as a function of distance from the illuminated Au NP (cross). The blue curve represents the theoretical temperature profile based upon a steady-state solution of the heat equation. All data in this figure are obtained on a glass coverslip, and all error bars correspond to one standard deviation.
Combining our nanodiamond thermometer with the laser heating of a gold nanoparticle (Au NP) allows us to both control and monitor temperature at nanometer length scales (Fig. 3). Both nanodiamonds and Au NPs (nominal diameter 100 nm) are initially spin-coated on a microscope coverslip. Using a confocal microscope with two independent scanning beams, we co-localize Au NPs and nanodiamonds with ~ 100 nm resolution (see SI). While locally heating the Au NP via continuous illumination with a variable-power green laser (focused to a diffraction limited spot), we simultaneously measure the temperature at the nanodiamond location using ESR spectroscopy.
The ability to measure temperature with NDs is verified by heating the substrate temperature over a range of 2.5 K and simultaneously monitoring the zero-field splitting (see Fig. 3c, inset). To demonstrate nano-scale temperature control Fig. 3c depicts the temperature change recorded by the ND as a function of the green laser power applied to the Au NP at a distance of 0.8±0.1 μm. To further verify that the temperature change originates from local heating, we repeat the measurement with the excitation laser displaced from the ND by 0.8 μm in the opposite direction. In this case, the temperature measured by the nanodiamond remained constant as a function of laser power (blue points), thereby confirming the locality of the heat source. From a linear fit to the data we estimate the accuracy of our ND sensor to be δT = (44±10) mK. The measured temperature change agrees with the theoretically expected temperature profile based upon a steady-state solution of the heat equation, , where Q̇ is the heat dissipation, κ is the thermal conductivity of glass and r is the distance between the nanodiamond and the Au NP. As shown in Fig. 3b, by recording the temperature of six nanodiamonds at different distances from the laser-heated Au NP we find that the measured temperature profile (Fig. 3d) is in excellent agreement with the theoretical steady-state prediction (solid line). This allows us to directly estimate the temperature change at the location of the Au NP to be 72 ± 6 K.
To demonstrate that nanodiamond thermometry is compatible with living cells, we introduce nanodiamonds and Au NPs into human embryonic fibroblast WS1 cells via nanowire-assisted delivery15. Just as in the control experiments described above, we probe the temperature at two different locations (NV1 and NV2) within a single cell while locally heating an individual Au NP (Fig. 4a). As shown in Fig. 4b, NV1, which is significantly closer to the heat source, exhibits a stronger temperature dependence as a function of laser power than NV2. Varying the incident power allows us to generate controlled temperature gradients of up to 5 K over distances of approximately 7 μm. To ensure that this temperature gradient is created by the controlled illumination of the NP and does not result from heating of the cellular cytoplasm, we displace the laser spot from the Au NP; this then results in a negligible temperature change at the location of NV1 with ΔT = (−20 ± 50) mK (green square, Fig. 4b). The increased measurement uncertainty for larger laser powers is the result of heating fluctuations from drift of the Au NP.
Figure 4. Nanoscale thermometry in cells.

a, Confocal scan of a single cell under 532 nm excitation with collection above 638 nm. The cross corresponds to the position of the Au NP used for heating, while circles represent the location of the nanodiamonds (NV1 and NV2) used for thermometry. The dotted line provides a guide to the eye and outlines the cell membrane. Color bars indicate the fluorescence in cps. b, Measured change in temperature at the position of NV1 and NV2 relative to the incident laser power applied to the Au NP. Dashed lines are linear fits to the data. Each point consists of an average of 5 measurements with each individual measurement taking 4 seconds. The error bars (one standard deviation) are set by fluctuations in the laser heating of the Au NP. c, Fluorescence scan of stained cells (live/dead assay) with excitation at 494/528 nm and emission at 515 nm (green - cell alive) and 617 nm (red - cell dead). The bar plot depicts the temperature of a single nanodiamond (circle) with local heat applied at two different locations (cross). d, Confocal fluorescence scans of an individual cell under varying illumination power. Excitation occurs at 532 nm and collection is above 630 nm. Cell death is indicated by the penetration of ethidium homodimer-1 through the cell membrane, staining the nucleus. At low laser powers, the cell remains alive, while cell-death occurs as laser-induced heating is increased.
The experiments shown in Fig. 4b clearly demonstrate the sub-micron measurement of an intra-cellular heat gradient. However, the substantial heating induced by constant illumination for an extended period of time ultimately leads to the death of the cell, which is confirmed using a standard live/dead assay (Calcein AM/Ethidium Homodimer-1). To demonstrate that our technique can be employed within living cells, we increase the concentration of Au NPs to allow for heat generation at different locations by simply moving the laser focus. Then, we measured the temperature variation at a single nanodiamond (bar plot in Fig. 4c) while introducing a slight heating of Au NPs in two differing locations (crosses). After our measurement, the viability of the cell is confirmed (Fig. 4c).
Finally, we demonstrate that our method can be used to control cell viability. To start, we heat the cell with 12 μW of laser power and measure a temperature change of 0.5 ± 0.2 K at the nanodiamond location; this corresponds to a change of approximately 10K at the Au NP spot. At this point, the cell is still alive, as confirmed by the absence of ethidium homodimer-1 fluorescence inside the membrane (Fig. 4d). By increasing the laser power to 120μW, we induce a temperature change of 3.9 ± 0.1K at the nanodiamond location (approximately 80K at the location of the laser focus); in this case, the cell is flooded with fluorescence from the ethidium homodimer, thus signaling cell death. This proof-of-principle experiment indicates that nanodiamond thermometry may enable the optimization of NP-based photothermal therapies8.
Our experiments demonstrate that NV centers in diamond can be used as robust temperature sensors that combine the virtues of sub-micron spatial resolution, sub-degree thermal sensitivity and bio-compatibility. The sensitivity of our current measurement can be enhanced by improving the relevant coherence time and by increasing the number of NV centers within the nanocrystal. Optimizing these factors should allow us to reach sensitivities of (see Methods), yielding the ability to sense sub-kelvin temperature variations with milli-second time resolution. In solution, the ultimate accuracy of our method will likely be limited by residual heating during the measurement process. As discussed in the Methods, this limit is in the range of 50 μK to 5 mK, depending on experimental conditions. While the present work focuses on monitoring temperature variations, the use of diamond samples with low strain or, alternatively, ensembles of NDs, should allow for the realization of an absolute thermometer (see Methods). The spatial resolution of our method can be further improved by using far-field sub-diffraction techniques26.
The present observations open up a number of intriguing possibilities. For instance, the simultaneous measurement and control of a sub-cellular thermal gradient could enable the accurate control of gene expression27. Further improvements in sensitivity may allow for real-time observations of non-equilibrium sub-cellular processes in biological and medical applications11. The large dynamic range of our quantum thermometer and it’s intrinsic robustness may also allow for the direct microscopic monitoring and control of chemical reactions20. Moreover, combining our technique with two-photon microscopy28, 29 may enable in vivo identification of local tumor activity by mapping atypical thermogenesis at the single-cell level30. Finally the combination of thermoablative therapy with our temperature sensor constitutes a potent tool for the selective identification and killing of malignant cells without damaging surrounding tissue 7, 8.
Methods
Experimental apparatus, sensitivity and accuracy
Our experimental apparatus consists of a confocal microscope with two independent excitation/collection paths allowing measurement and heating at two independent locations simultaneously. The experiments use either a Nikon Plan Fluor 100x oil immersion, NA = 1.3, (nanodiamonds) or a Nikon Plan Apo 100x air, NA = 0.95, objective (bulk sample), resulting in C ≈ 0.03, which can be further improved by employing a solid immersion lens or diamond nano patterning. Microwaves are delivered via a lithographically defined coplanar waveguide on top of a glass coverslip. For experiments with nanodiamonds we use neutral density filters in the collection path to avoid saturation of the APD. The temperature accuracy δT for bulk diamond is estimated from the measurement shown in Fig. 2b. Using the standard deviation σ (shown error bars) we evaluate the accuracy as where c is the oscillation amplitude and 2τ is the free evolution time. We find that for integration times t < 30 s (limited by temperature stability) the temperature accuracy improves as , giving a sensitivity . A linear dependence of the dissipated heat as a function of laser power (Fig. 3b) is used to determine the measurement accuracy for NDs. A linear function, with slope m, is fitted to the q data (red dashed line) and the measurement accuracy is given by , with Ti the measured temperature and Pi the corresponding laser power. The error bars are evaluated as , where Γ(·) indicates the Gamma distribution.
Ultimate sensitivity
The ultimate sensitivity of our method is limited by the NV coherence time and the number of defect centers. In our current experiment, we have demonstrated a sensitivity of (with a free evolution time of 250 μs). Two natural extensions enable longer NV coherences: 1) decreasing the 13C concentration to suppress the nuclear spin bath and 2) further dynamical decoupling. These methods can, in principle, allow us to extend the evolution time up to T1/2 ~ 3 ms. In combination with a nanocrystal that contains ~ 1000 NV centers, this could yield an ultimate sensitivity limit of . Further improvement may be possible by employing spin squeezed states. Finally, the determination of absolute temperature is limited by variations of the zero-field splitting due to spatially varying strain. For low strain diamond samples, we find that the variation in the zero-field splitting is less than 60 kHz, allowing for sub-K absolute temperature determination. In addition, the use of an ensemble of NV centers in different NDs with uncorrelated strain values allows for a further increase in absolute sensitivity by a factor , where n is the number of NDs.
Ultimate accuracy in solution
In cases where our method is used to probe a system that is in solution (e.g. cells, chemical reactions), the primary accuracy limit is set by heat dissipation during the measurement process. In particular, the microwave spectroscopy used to detect changes in the NV zero-field splitting also induces heating of the solution. In the present experiment, we utilize a lithographically fabricated microwave loop (diameter 200 μm) to generate an ac-magnetic field, B ≈ 10 mGauss, for spin manipulations. Estimating the effective dipole field created by the microwave loop shows that the solution (water) absorbs 10−6 W of power yielding a temperature increase of 5 mK in the steady state. By using a smaller microwave loop (20 μm) and reducing the duty cycle, it could be possible to decrease the heating of the solution to approximately 50 μK.
Injection of nanodiamonds into cells
Nanodiamonds and Au NPs were introduced into WS1 cells via silicon nanowire-mediated delivery15. Silicon nanowires were treated with 3-amino-propyltrimethoxysilane to present NH2 functionality on the surface, and nanodiamonds / Au NPs were subsequently attached via electrostatic binding. Afterwards, human embryonic fibroblast WS1 cells were plated on the silicon nanowire substrates and cultured overnight. The cells were removed by trypsin treatment and re-plated on a glass slide with lithographically defined strip lines for ESR measurements. The samples were stained with calcein-AM and ethidium homodimer-1 for the live/dead assay.
Supplementary Material
Acknowledgments
We thank R. Walsworth, V. Denic, C. Latta, L. Jiang, A. Gorshkov, P. Cappellaro, A. Sushkov and I. Lovchinsky for helpful discussions and experimental help. This work was supported by NSF, the Center for Ultracold Atoms, the Defense Advanced Research Projects Agency (QUASAR programs), Army Research Office (MURI program), NIH Pioneer Awards (5DP1OD003893-03) and NHGRI (1P50HG006193-01), the Swiss National Science Foundation (PBSKP2_143918) (P.C.M.).
Footnotes
Author Contributions
P.C.M., H.J.N., H.P. and M.D.L. conceived the study. G.K., P.C.M. and N.Y.Y. designed and conducted the experiments and analyzed the data. M.K., P.K.L. and H.P. prepared the biological samples. All authors participated in discussions and writing of the manuscript.
Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
The authors declare no competing financial interests.
References
- 1.Yue Y, Wang X. Nanoscale thermal probing. Nano reviews. 2012;3 doi: 10.3402/nano.v3i0.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucchetta E, Lee J, Fu L, Patel N, Ismagilov R. Dynamics of drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434:1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SV, Wigge PA. H2a. z-containing nucleosomes mediate the thermosensory response in arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Lauschke VM, Tsiairis CD, François P, Aulehla A. Scaling of embryonic patterning based on phase-gradient encoding. Nature. 2012;493:101–105. doi: 10.1038/nature11804. [DOI] [PubMed] [Google Scholar]
- 5.Kamei Y, et al. Infrared laser–mediated gene induction in targeted single cells in vivo. Nature methods. 2008;6:79–81. doi: 10.1038/nmeth.1278. [DOI] [PubMed] [Google Scholar]
- 6.Vreugdenburg T, Willis C, Mundy L, Hiller J. A systematic review of elastography, electrical impedance scanning, and digital infrared thermography for breast cancer screening and diagnosis. Breast Cancer Research and Treatment. 2013;137:665–676. doi: 10.1007/s10549-012-2393-x. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder A, et al. Treating metastatic cancer with nanotechnology. Nature Reviews Cancer. 2011;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 8.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer letters. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar A. Scanning thermal microscopy. Annual review of materials science. 1999;29:505–585. [Google Scholar]
- 10.Kim SH, et al. Micro-raman thermometry for measuring the temperature distribution inside the microchannel of a polymerase chain reaction chip. Journal of Micromechanics and Microengineering. 2006;16:526. [Google Scholar]
- 11.Yang J, Yang H, Lin L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS nano. 2011;5:5067–5071. doi: 10.1021/nn201142f. [DOI] [PubMed] [Google Scholar]
- 12.Vetrone F, et al. Temperature sensing using fluorescent nanothermometers. ACS nano. 2010;4:3254–3258. doi: 10.1021/nn100244a. [DOI] [PubMed] [Google Scholar]
- 13.Okabe K, et al. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nature communications. 2012;3:705. doi: 10.1038/ncomms1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donner J, Thompson S, Kreuzer M, Baffou G, Quidant R. Mapping intracellular temperature using green fluorescent protein. Nano letters. 2012;12:2107–2111. doi: 10.1021/nl300389y. [DOI] [PubMed] [Google Scholar]
- 15.Shalek AK, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proceedings of the National Academy of Sciences. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acosta V, et al. Temperature dependence of the nitrogen-vacancy magnetic resonance in diamond. Physical Review Letters. 2010;104:70801. doi: 10.1103/PhysRevLett.104.070801. [DOI] [PubMed] [Google Scholar]
- 17.Toyli D, et al. Measurement and control of single nitrogen-vacancy center spins above 600 k. Physical Review X. 2012;2:031001. [Google Scholar]
- 18.Chen XD, et al. Temperature dependent energy level shifts of nitrogen-vacancy centers in diamond. Applied Physics Letters. 2011;99:161903–161903. [Google Scholar]
- 19.Taylor J, et al. High-sensitivity diamond magnetometer with nanoscale resolution. Nature Physics. 2008;4:810–816. [Google Scholar]
- 20.Jin C, Li Z, Williams R, Lee K, Park I. Localized temperature and chemical reaction control in nanoscale space by nanowire array. Nano letters. 2011;11:4818–4825. doi: 10.1021/nl2026585. [DOI] [PubMed] [Google Scholar]
- 21.Maze J, et al. Nanoscale magnetic sensing with an individual electronic spin in diamond. Nature. 2008;455:644–647. doi: 10.1038/nature07279. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian G, et al. Nanoscale imaging magnetometry with diamond spins under ambient conditions. Nature. 2008;455:648–651. doi: 10.1038/nature07278. [DOI] [PubMed] [Google Scholar]
- 23.Hodges J, Englund D. Time-keeping with electron spin states in diamond. 2011. arXiv preprint arXiv:1109.3241. [Google Scholar]
- 24.Balasubramanian G, et al. Ultralong spin coherence time in isotopically engineered diamond. Nature materials. 2009;8:383–387. doi: 10.1038/nmat2420. [DOI] [PubMed] [Google Scholar]
- 25.Dolde F, et al. Electric-field sensing using single diamond spins. Nature Physics. 2011;7:459–463. [Google Scholar]
- 26.Maurer P, et al. Far-field optical imaging and manipulation of individual spins with nanoscale resolution. Nature Physics. 2010;6:912–918. [Google Scholar]
- 27.Xu G, et al. Identification of proteins sensitive to thermal stress in human neuroblastoma and glioma cell lines. PloS one. 2012;7:e49021. doi: 10.1371/journal.pone.0049021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nature methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 29.Wee TL, et al. Two-photon excited fluorescence of nitrogen-vacancy centers in proton-irradiated type ib diamond. The Journal of Physical Chemistry A. 2007;111:9379–9386. doi: 10.1021/jp073938o. [DOI] [PubMed] [Google Scholar]
- 30.Tsoli M, et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Research. 2012;72:4372–4382. doi: 10.1158/0008-5472.CAN-11-3536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.