Summary
Radiometals play an important role in diagnostic and therapeutic radiopharmaceuticals. This field of radiochemistry is multidisciplinary, involving radiometal production, separation of the radiometal from its target, chelate design for complexing the radiometal in a biologically stable environment, specific targeting of the radiometal to its in vivo site, and nuclear imaging and/or radiotherapy applications of the resultant radiopharmaceutical. The critical importance of inorganic chemistry in the design and application of radiometal-containing imaging and therapy agents is described from a historical perspective to future directions.
Keywords: Radiometals, Bifunctional chelates, Specific targeting, Nuclear imaging, Radiotherapy
Introduction
The application of radioisotopes of inorganic elements to nuclear medicine, both diagnostic and therapeutic, has been of interest almost since the discovery of radioactivity. The middle to late 1930s saw the development of radionuclides with potential medical applications with 32P, 131I, and 89Sr and the first human studies for leukemia, thyroid and bone therapy, respectively, were initiated [1]. Since the early studies, many diagnostic and some therapeutic radiopharmaceuticals have been developed. Diagnostic nuclear imaging requires the use of penetrating radiations from radionuclides that emit either gamma rays or annihilation photons from positron emission. Radiotherapy requires particulate emission from alpha or beta decay (although there is some interest in Auger electrons) so that the energy of decay is deposited over a relatively short range (e.g., in the cancer cells). The development of the 99Mo/99mTc generator in the late 1950s led to the predominance of 99mTc in diagnostic nuclear medicine and was the entry into the field of radiometal based imaging and therapeutic radiopharmaceuticals [2, 3]. Recent shortages of 99Mo have brought to light the precarious position of the field of nuclear medicine because of its dependence on aging reactors for production of 99Mo [4].
Many excellent reviews (and the references therein) covering the production of radionuclides for medical applications[5–10] and the state of diagnostic and therapeutic radiopharmaceuticals[9, 11–25] have been published over the last 20 years. Here we describe the current status and trends in inorganic radiopharmaceutical chemistry and future directions. We have elected to not include those radiometals whose use is strictly as the aqua ions (e.g., 82Rb+, 201Tl+. 89Sr2+) and to focus on those with reasonable half-lives for shipping or that are available in generator form.
Radiometals in radiopharmaceuticals
Radiometals have become an integral component of many radiopharmaceuticals because their nuclear properties (Table 1)[26] are more suitable for diagnostic and therapeutic nuclear medicine applications than those of their Main Group non-metal radionuclides. Not only are the nuclear emissions (α, β−, β+, γ) and their energies and half-lives important, but their availability and cost are more often the determining factors in their application for routine medical use. Additionally, there is a requirement for high specific activity (activity per unit mass) for many medical applications, particularly those involving receptor targeted radiopharmaceuticals. An excellent example illustrating these concepts is the advent of the 99Mo/99mTc generator, which made the 6 h, high specific activity Tc-99m widely available at a reasonable cost.
Table 1.
Radionuclide properties (National Nuclear Data Center, http://www.nndc.bnl.gov)
| Radionuclide | Decay path and maximum particle emission (%) |
Gamma emission (%) | Half-life |
|---|---|---|---|
| Cu-62 | 2.926 MeV β+ (97.4%) EC decay (2.6%) |
511 keV (194.9) | 9.74 min |
| Cu-64 | 0.653 MeV β+ (17.6%) EC decay (43.9%) 0.579 MeV β− (38.5%) |
511 keV (35.2) | 12.7 h |
| Cu-67 | 0.562 MeV β− | 184.6 keV (48.7) 93.3 keV (16.1) 91.3 keV (7) |
2.58 d |
| Ga-67 | EC decay | 393.5 keV (4.6) 300 keV (16.6) 184.6 keV (21.4) 93.3 keV (38.8) |
3.2617 d |
| Ga-68 | 0.836 MeV β+ (90%) EC decay (10%) |
1077 keV (3 %) 511 keV (178.3%) |
67.71 min |
| Zr-89 | 0.902 MeV β+ (22.74%) EC Decay (77.26%) |
511 keV (45.5) 909 keV (99) |
3.27 d |
| Y-90 | 2.28 MeV β− | 64.053 h | |
| Tc-99m | IT | 140 keV (89) | 6.0067 h |
| Rh-105 | 0.5672 MeV β− | 318.9 keV (19.1) | 35.36 h |
| In-111 | EC decay | 245 keV (94.1) 171 keV (90.7) |
2.8047 d |
| Pm-149 | 1.072 MeV β− (95.9%) | 286 keV (3.1) | 53.08 h |
| Sm-153 | 0.8076 MeV β− | 103 keV (29.25) | 46.50 h |
| Tb-161 | 0.593 MeV β− | 74.6 keV (10.2) | 6.89 d |
| Dy-166 | 0.4868 MeV β− | 82.5 keV (13) | 81.6 h |
| Ho-166 | 1.8547 MeV β− | 80.57 keV (6.56) | 26.824 h |
| Lu-177 | 0.498 MeV β− | 208.4 keV (10.36) 112.95 keV (6.17) |
6.647 d |
| Re-186 | 1.07 MeV β− | 137.2 keV (9.47) | 3.7186 d |
| Re-188 | 2.12 MeV β− | 155 keV (15.6) | 17.003 h |
| Au-198 | 1.372 MeV β− | 411.8 keV (95.62) | 2.695 d |
| Au-199 | 0.452 MeV β− | 208.2 keV (8.72) 158.4 keV (40) |
3.139 d |
| Bi-212 | 2.252 MeV β− (64.06%) 6.09 MeV α (35.94%) 8.78 MeV α (Po-212) |
727.3 keV (6.67) | 60.55 min |
| Bi-213 | 1.423 MeV β− (97.8%) 5.87 MeV α (2.1%) 8.375 MeV α (Po-213) |
440.5 keV (25.94) | 45.59 min |
| Ac-225 | 5.83 MeV α 6.34 MeV α (Fr-221) 7.07 MeV α (At-217) 1.423 MeV β− (Bi-213) |
99 keV (5.8) | 10 d |
Radiometals offer an advantage over radiolabeling organic molecules (e.g., with 18F, 11C, etc.) in that lyophilized “kit” formulations, which allow rapid radiolabeling, are often available. The radiopharmaceutical “kits” contain all ingredients except for the radiometal. To formulate the final radiopharmaceutical, the radiometal is added to the “kit” and the instructions (e.g., let stand at room temperature for 30 minutes, heat in a boiling water bath for 30 minutes, etc.) are followed, quality control is performed typically resulting in product yields greater than 90%, which are suitable for patient administration. An example of a “kit” formulation is that for Cardiolite® (Figure 1), which is clinically approved for myocardial imaging and requires the addition of 99mTc pertechnetate and heating.
Figure 1.
Structure of Cardiolite.
The radiometals listed in Table 1 include some of the more widely used and potentially useful radionuclides for either diagnostic imaging or radiotherapy. There is no ideal diagnostic radiometal and no ideal therapeutic radiometal; each has its own unique benefits and associated problems. Technetium-99m was considered to be the ideal diagnostic radionuclide for many years because of its widespread availability, low cost, and relatively easy product formulations. The recent shortages of 99mTc have made the nuclear medicine field consider alternatives as it is not clear that the issues that led to the shortage will be solved. Not only are the aging reactors that make fission 99Mo an issue, but so are concerns of non-proliferation shifting the field to consider conversion to low enriched 235U or moving toward 98Mo(n,γ) production of 99Mo. Low enriched 235U fuel will require irradiation of higher masses of uranium fuel to obtain the same activity (quantity) of 99Mo, which will require modifications to the separation method and likely higher radioactive waste generation. The 98Mo(n,γ) production of 99Mo will result in lower specific activity 99Mo (i.e., more mass because 98Mo would not be separated from 99Mo) and thus a larger or different generator system would be required.
The various radiometals listed in Table 1 have excellent nuclear properties for nuclear medicine applications. Their chemical properties (substitution rates (both on and off), redox chemistry, hydrolysis rates, etc.) on the radiotracer level, where the radiometal is often nM in concentration or lower, pose challenges. The availability of a matched pair of radionuclides, one for diagnosis and one for therapy, would be of value but is rarely available. True matched pairs would include different radioisotopes of the same element as with the diagnostic radionuclide 64Cu and the therapeutic radionuclide 67Cu. Pseudo-matched pairs would involve radioisotopes of similar but different elements as with the diagnostic radionuclide 99m Tc and the therapeutic radionuclides 188/186Re. Indium-111 analogues have been used as an “ersatz” diagnostic matched pair for the radiotherapeutic 90Y (which has no imageable γ emission), however the inorganic chemistry, in vivo chemistry, and in vivo pharmacokinetics of the 111In product may be different from that of the analogous 90Y product depending on the chelate and their relative kinetic stabilities. Thus, particular caution must be applied with “matched pairs” if the radiometals are not the same element.
Targeting strategies for radiometal conjugates
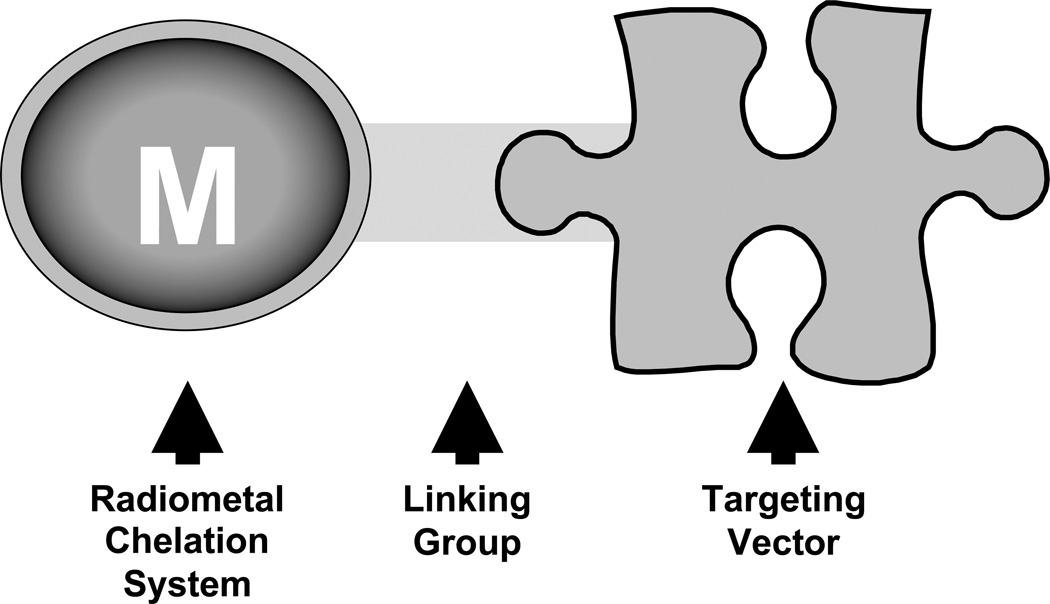
The current driving force behind the clinical application of radiopharmaceuticals in nuclear medicine is the ability to selectively direct, or target, radiolabeled molecules to active sites of human disease. In order to effectively accomplish this goal, a suitable diagnostic or therapeutic radiometal must be strategically attached to a biological targeting vector. Figure 2 illustrates a general schematic of the most common approach to a targeted radiopharmaceutical using a suitable bifunctional chelating agent (BFCA) directly linked to a biological targeting vector. Although direct labeling (with Tc and Re) has been reported, the labeling is generally non-specific and used primarily for antibody labeling. This discussion will be limited to the BFCA approach only.
Figure 2.
Cartoon of a generic bifunctional chelating agent.
The BFCA serves the dual purpose of providing a means to stably complex the radiometal and also allows for selective attachment directly to the selected biological targeting vector. A widely employed strategy for linking or conjugation of a BFCA to a biological targeting vector involves coupling a free primary amine (or activated carboxylic acid) located on the structure of the BFCA with an activated carboxylic acid (or primary amine) from the biological targeting vector to generate a stable amide bond linkage. In some cases, BFCA conjugation to a biological targeting vector requires the addition of an organic linking group in order to maximize in vivo targeting, or alter non-target tissue pharmacokinetics [27]. A variety of linking groups have been successfully employed including simple multi-amino acid linkages, aliphatic carbon chain linkages, and small organic heterocyclic compounds [27]. In all cases, the goal has been to generate a combination of radiometal, BFCA, and linking technology that results in a radiopharmaceutical to selectively target a human disease process while minimizing non-disease and non-target tissue background accumulation of radioactivity.
Numerous strategies for developing targeted radiopharmaceuticals have been employed over the past several decades, including the radiolabeling of small proteins, antibodies, antibody fragments, peptides, and small organic molecules [27–32]. For the purpose of this discussion, we are limiting our scope to focus only on peptide targeting vectors, which have either recently shown clinical potential, or are currently being investigated pre-clinically for their future use as targeted radiopharmaceuticals [32–39]. Peptides, as a class in themselves, offer tremendous flexibility for modification when biological issues such as in vivo clearance kinetics and metabolic stability are taken into consideration. Further supporting the use of peptides as selective biological targeting vectors is the increasing evidence of the presence and up-regulation of various peptide receptors associated with human cancers. Table 2 provides an overview of various peptides and their respective receptor families currently under development for use as the biological targeting vector component of site directed radiopharmaceuticals. The list was selected based on current interest and use in the development of targeted radiopharmaceuticals where a significant body of literature is being assembled supporting the validity of these radiolabeled peptides including positive results upon in vitro evaluation, animal model biodistribution studies, animal model in vivo imaging, and in several cases human patient studies [32, 37]. Several of the peptide targeting vectors listed have undergone extensive evaluation as potential radiopharmaceuticals resulting in anywhere from thirty to several hundred unique peptide targeting vectors radiolabeled to obtain an optimum candidate for potential clinical evaluation [32, 37].
Table 2.
Peptides and peptide receptors currently under evaluation as biological targeting vectors in diagnostic and therapeutic radiopharmaceuticals
| Peptide | Receptor | Tumor expression |
|---|---|---|
| Somatostatin (SST) | Somatostatin receptor subtype – 2 (SST2) | Neuroendocrine including gastroenteropancretic, carcinoids, pituitary, breast, brain, small cell lung cancer |
| Bombesin (BBN) / Gastrin Releasing Peptide (GRP) | Bombesin receptor subtype – 2 (BB2) | Prostate, breast, pancreas, small cell lung, colorectal |
| α-Melanotropin (α−MSH) | Melanocortin-1 receptor (MC1R) | Melanomas |
| Neurotensin (NT) | Nuerotensin receptor (NTR1) | Colon, Ewing’s sarcoma, breast, exocrine pancreatic cancer |
| Neuropeptide Y (NPY) | Neuropeptide receptor Y1 (Y-1) | Breast cancer, ovary, adrenal, brain, kidney, Ewing’s sarcoma |
| Cholecystokinin (CCK) | Cholecystokinin-B (CCK-B) | Small cell lung cancer, medullary thyroid cancer, astrocytomas |
| Arg-Gly-Asp (RGD) | αVβ3 integrin | Tumor angiogenesis in melanoma, ovarian, lung carcinoma, neuroblastomas, glioblastomas, breast cancer |
An example of a bioconjugate under current investigation is the bombesin peptide conjugated to 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). These peptide targeting vectors have been conjugated with a variety of bifunctional chelating agents in order to complex and evaluate the spectrum of radiometals available for diagnostic and therapeutic radiopharmaceutical development [40].
Radiometal chelate considerations
Numerous chelates have been developed and evaluated for various radiometals as the basis of BFCAs that can be appended to biomolecules for targeting. It is clear that both thermodynamic and kinetic stability of the resultant radiometal complexes are important for the development of safe and efficacious radiopharmaceuticals, with kinetic stability being more important under the high dilution on injection in vivo [41, 42]. The dissociation rate (koff) will determine in vivo stability (Ks = kon/koff) since equilibrium conditions are no longer applicable once the radiopharmaceutical has distributed throughout the blood volume. Any loss of the radiometal from its chelate in vivo leads to undesirable and higher non-target irradiation. Some radiometals additionally require the BFCA to provide redox stability to oxidation and/or reduction. Technetium and rhenium complexes are examples where the radiometal can be susceptible to in vivo oxidation to pertechnetate or perrhenate, while Cu(II) and Au(III) complexes are examples where the radiometals can be susceptible to reduction to either Cu(I) or Au(0); in all cases specific targeting is lost. There is no universal chelate that will function as the best BFCA for all radiometals. Each metal, in a given oxidation state, has its own unique and specific requirements for which donor atoms will yield sufficiently kinetically inert radiometal complexes for in vivo applications. Below we discuss the radiometals that we consider potentially the most useful for site directed radiopharmaceuticals in which peptides act as the targeting moiety.
Technetium
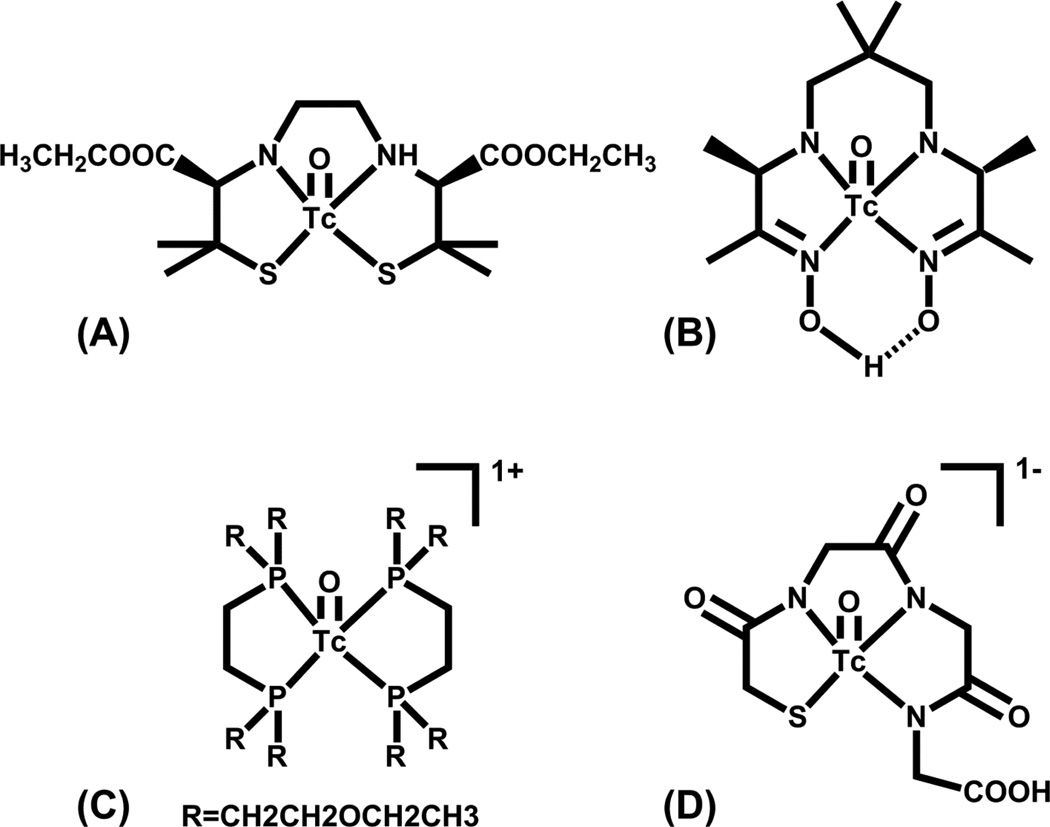
The availability of technetium (as pertechnetate, TcO41−) from a relatively inexpensive 99Mo/99mTc generator, together with its nuclear properties, which are ideal for SPECT imaging, has made 99mTc the workhorse of the nuclear imaging community. Technetium is situated in the middle of the d-block transition elements in Group 7 between manganese and rhenium, and this position gives the metals in this triad one of the wider ranges of available oxidation states. Compounds of Tc have been reported in oxidation states ranging from +7 to −1, with Tc(V), Tc(III) and Tc(I) most commonly utilized in nuclear medicine applications. Over the years, many Tc complexes in a variety of oxidations states with a multitude of chelators have been synthesized and evaluated in vivo. The seminal work of Deutsch and Davison in the late 1970s and 1980s demonstrated the importance of Tc-99 inorganic chemistry to radiopharmaceutical development [43–48]. The chemistry of technetium labeled radiopharmaceuticals has been extensively reviewed and a few examples are shown in Figure 3 [27, 49, 50].
Figure 3.
Structures of (A) Neurolite®, (B) Ceretec®, (C) Myoview®, and (D) MAG3.
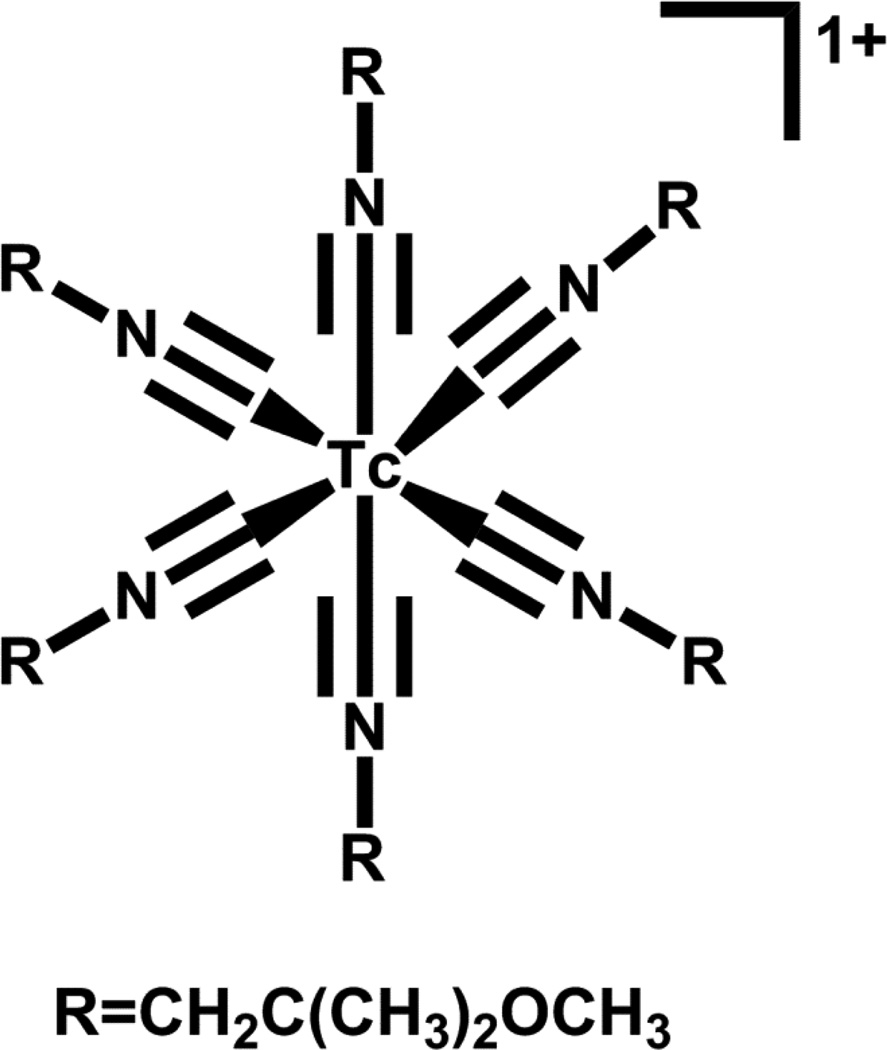
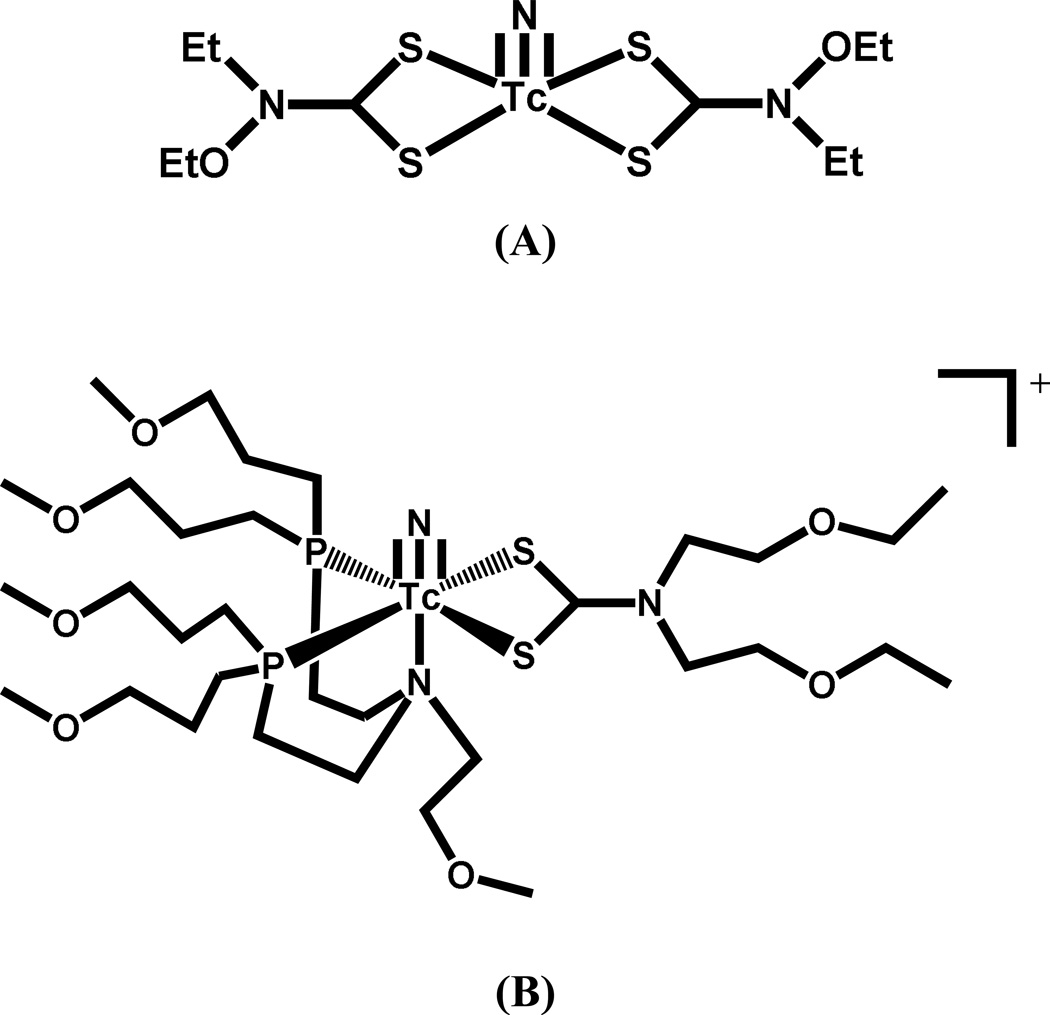
Currently there are numerous clinically approved radiopharmaceuticals based on 99mTc that are essentially small molecules and not based on the bifunctional chelate approach. Technetium(V) is the most accessible oxidation state from Tc(VII) as evidenced by the many approved Tc(V) radiopharmaceuticals such as Neurolite®, Ceretec®, Myoview® and MAG3® (Figure 3), which are approved for cerebral blood flow imaging, cerebral blood flow imaging and white blood cell labeling, myocardial perfusion, and renal filtration imaging, respectively. Current interest in small molecule 99mTc radiopharmaceutical development is in the area of potential myocardial perfusion imaging agents with faster liver clearance than the current agents (Cardiolite® and Myoview®). Duatti and co-workers developed the neutral 99mTcN-NOET (Figure 4A), which contains the Tc-nitrido core and two N-ethyl-N-ethoxydithiocarbamates bound to the Tc(V) center[51, 52]. Evaluation in humans showed faster liver clearance [51]. Duatti, Tisato, Liu and Santos have all reported +1 cationic analogs in which one of the dithiocarbamate moieties is replaced with a tridentate PNP donor set (Figure 4B); addition of various ether groups were evaluated for the effect(s) on clearance [53–58].
Figure 4.
Structures of (A) TcN-NOET and (B) TcN(S2)(PNP)+.
Pertechnetate as eluted from the 99Mo/99mTc generator behaves similarly to iodide, being taken up by the thyroid and excreted through the renal-urinary system. This rapid excretion of pertechnetate can be considered a benefit as this is the chemical species that will be generated by in vivo instability or metabolism of technetium complexes in lower oxidation states. As Tc(VII) in pertechnetate is coordinatively saturated, it is reduced during radiopharmaceutical preparations to generate stable radiometal complexes for in vivo applications.
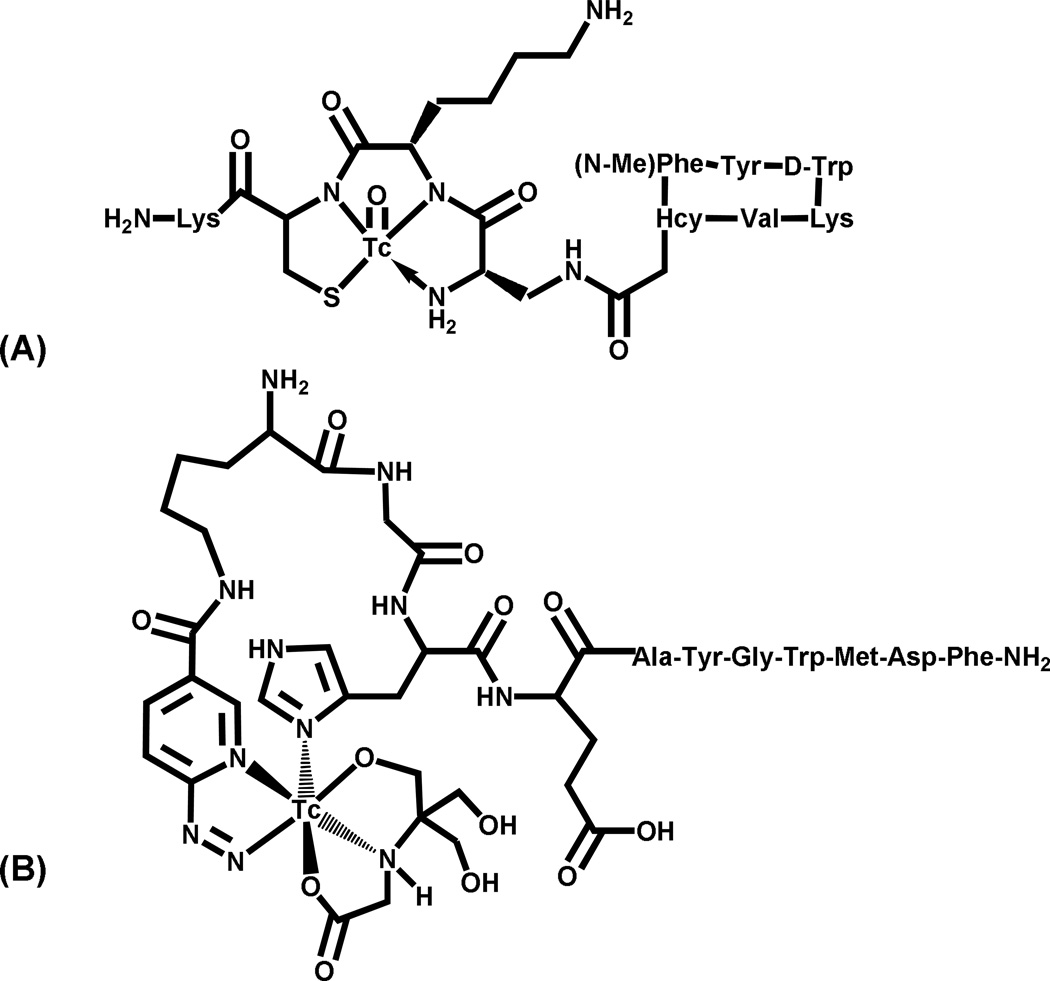
Technetium(V) has a d2 electronic configuration and is dominated by oxo chemistry in aqueous solution. These complexes tend to be either mono-oxo with square pyramidal or distorted octahedral geometries, or dioxo with octahedral geometry; examples of both are shown in Figure 3. Technetium(V) is easily accessible from pertechnetate (Tc(VII)), and radiopharmaceutical kit formulations often utilize Sn(II) as the reducing agent. Glucoheptonate or citrate are used in kit formulations when the chelate is slow to react compared to hydrolysis, which would result in the formation of colloidal TcO2 if a suitable chelate is not present. A range of donor atoms, including N, S, P and O, have been used to form Tc(V) complexes. Tetradentate chelates are generally used to make this somewhat labile system more kinetically inert, and the most commonly used BFCAs include mercaptoacetylglycylglycylglycine derivatives (N3S MAG3 derivatives), diaminedithiol or amidoaminedithiol derivatives, the linear tetraamine 1, 4,8,11-tetraazaundecane (a 2-3-2 carbon backbone), and 6-hydrazinonicotinic acid (HYNIC) analogues [27, 59, 60]. The use of the HYNIC BFCA for 99mTc is attractive because it involves a simple labeling procedure for biomolecules without difficult chelate synthesis. However, HYNIC is generally a monodentate BFCA and thus requires the use of co-ligands to satisfy the coordination sphere of the Tc(V) center. Unfortunately, the co-ligands yielding the best pharmacokinetic properties tend to form multiple products while those that form single entities (e.g., thiol, phosphine donors) tend to be quite lipophilic with poor pharmacokinetics [61–64]. Two recent examples of Tc(V) complexes for targeting peptide receptors are 99mTc-depreotide (diamido amine thiol [N3S] chelator linked to a somatostatin peptide)[65] and 99mTc-HYNIC-GRP [63], both shown in Figure 5.
Figure 5.
Structures of (A) 99mTc-depreotide and (B) 99mTc-HYNIC-GRP peptide.
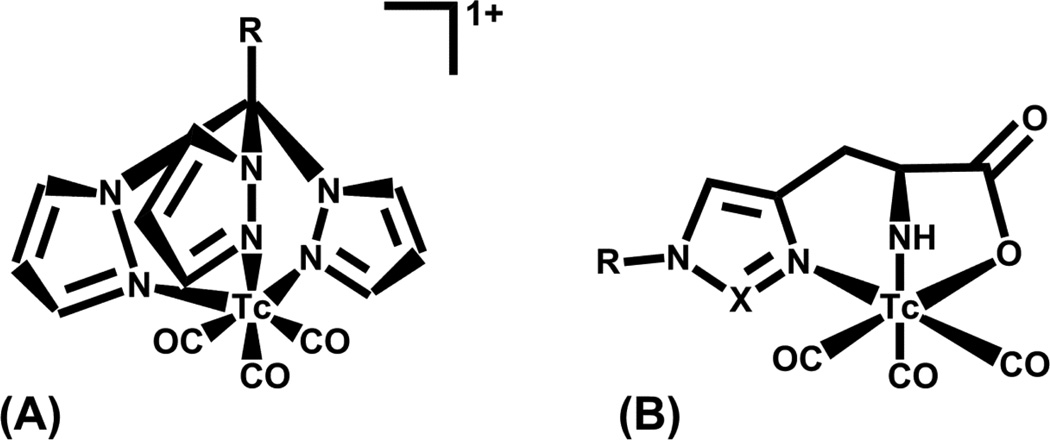
The nitrido core has been evaluated as an alternative to the oxo core typically utilized with Tc(V) complexes. Duatti and Tisato utilized the PXP donor set along with bidentate (YZ) ligands as potential bifunctional chelates; only the [TcN(PXP)(YZ)]0/+ (and no [TcN(PXP)2]2+ or [TcN(YZ)2]0/2−) formed at the 99mTc level making this a possible route to radiolabeling peptides [66].
Technetium(III) with its low-spin d4electronic configuration is more kinetically inert than Tc(V); however, Tc(III) requires additional reducing agent for access from Tc(VII) pertechnetate and often higher temperatures. Technetium(III) has been shown to coordinate to soft and hard donors (N, O, P, and S) in both octahedral (paramagnetic) and 7-coordinate (diamagnetic) ligand environments. Teboroxime [TcCl(CDO)3BCH3] is an example of a Tc(III) approved radiopharmaceutical for myocardial imaging (Figure 6). Very few Tc(III) complexes have been evaluated as bifunctional chelates, with the ‘4+1’ NS3/P system showing some promise [67, 68]. The challenges with this system include the harsher reducing conditions and controlling the higher lipophilicity associated with the sulfur and phosphorus donor ligands.
Figure 6.
Structure of Teboroxime.
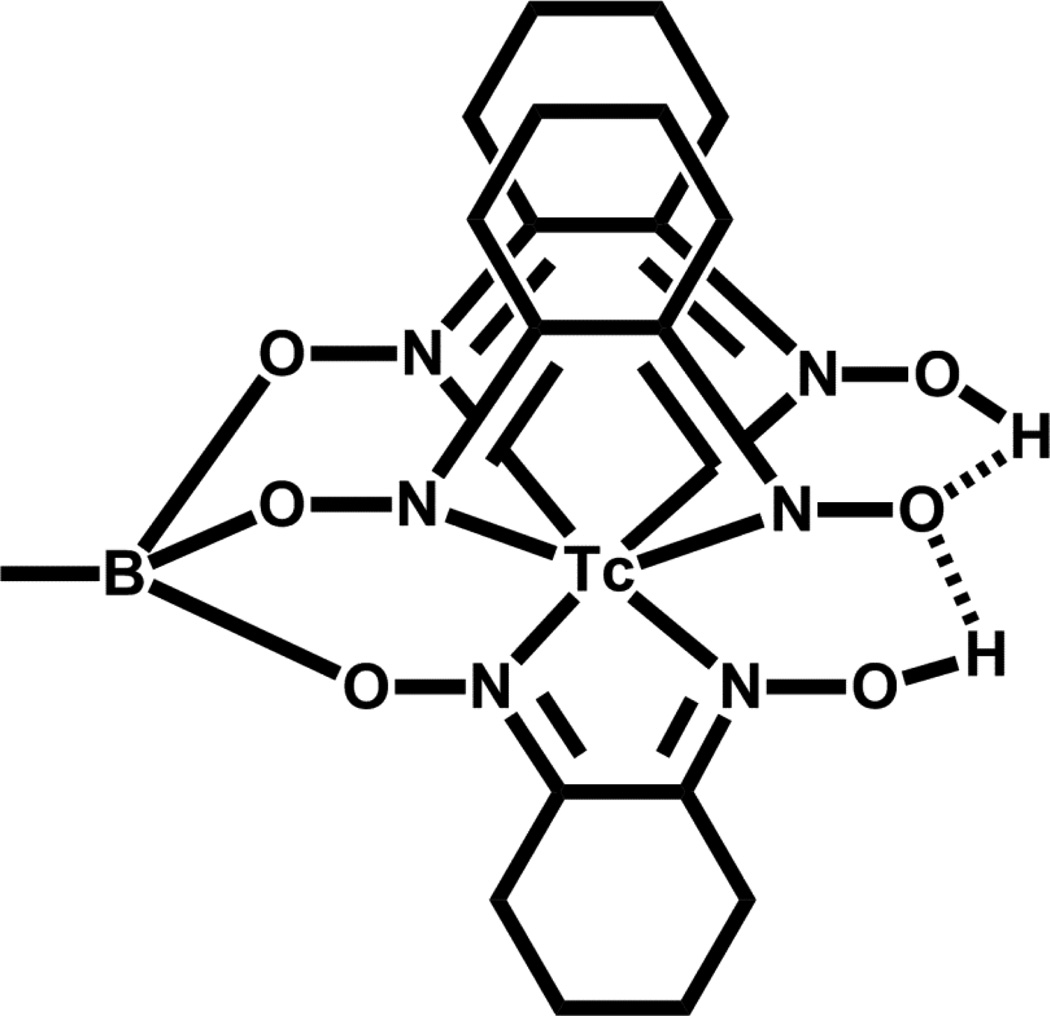
The low-spin d6 electronic configuration of Tc(I) is kinetically inert and makes this oxidation state desirable as the basis of new Tc radiopharmaceutical development. The report by Alberto and co-workers demonstrating the accessibility of [Tc(CO)3(OH2)3]+ from pertechnetate in aqueous solution accelerated interest in potential Tc(I) radiopharmaceuticals [69–72]. The three carbon monoxide ligands are inert to substitution while the three coordinated water molecules offer an easy route for ligand exchange with a suitable tridentate chelate. The current availability of the commercial IsoLink® kit from Covidien makes this a desirable starting complex for radiolabeling with 99mTc. This allows for relatively fast exchange onto the Tc(I) center with a molecular targeting tridentate BFCA. Tc(I) is considered a “soft” metal center and as such prefers “soft” donors, which tend to make the resultant complex more lipophilic; however a balance must be achieved with clearance properties from non-target tissues in vivo. Thus, amine, imine and carboxylate donors are often used with the [Tc(CO)3]+ core to reduce lipophilicity and thus facilitate clearance. Scorpionates [73], single amino acids (especially histadine analogues) [64], and other chelates were initially investigated as potential chelators for the bifunctional chelate approach for the [Tc(CO)3]+ core [74–79]. A representation of these binding moieties for the tricarbonyl core is given in Figure 7 [73, 75].
Figure 7.
99mTc(I) tricarbonyl complexes anchored by (A) tris(pyrazoyl)methane and (B) histadine (X = CH2, N) functionalized tridentate chelates.
Benny and co-workers utilized “click” chemistry to improve radiolabeling efficiency of the tricarbonyl technetium(I) core at lower temperatures than reported by Schibli et al. [75, 80] to minimize potential damage to appended biological targeting vectors [81]. Two approaches were reported that differed primarily in the order of radiolabeling the [Tc(CO)3]+ core. One approach involved coupling the BFCA containing a pendant primary alkyne with an azido moiety containing the biological targeting group and subsequently radiolabeling it. The second approach involved radiolabeling the BFCA containing the primary alkyne and then reacting it with the azido targeting moiety [81]. Both approaches yielded the same product, but neither approach gave sufficient radiolabeling yields at 25°C at less than 10−5 M BFCA [81].
Although the availability of the Isolink kit has made this core easily accessible and the Tc(I) oxidation state offers high kinetic stability, an inherent issue with the [Tc(CO)3]+ core has been the resultant increased lipophilicity of its complexes. Increasing complex lipophilicity results in increased and often slow hepatobiliary clearance, which hinders imaging the pelvic/abdominal region.
Rhenium
Rhenium has been referred to as the radiotherapeutic matched pair for the diagnostic 99mTc. Its position as the 3rd row congener of Tc in Group 7 makes its chemistry similar to that of technetium in many ways, but there are differences which manifest themselves when redox chemistry or substitution kinetics are involved. Rhenium is more difficult to reduce than Tc and is slower to substitute. This leads to re-oxidation to perrhenate in vivo if the chelate systems are not carefully selected. Fortunately, perrhenate clears from the body quickly and thus does not accumulate in non-target organs/tissues. The slower substitution rates have led to lower lability in the site trans to the oxo group in Re(V) complexes, which has resulted in different products for Re(V) compared to Tc(V) [82]. The Re(V) complexes may have a ligand in the site trans to the oxo group whereas Tc(V) does not or substitution slows down reduction resulting in a completely different complex for Re(V) [82]. This may also be the reason that a Re(V) complex of propyleneamine oxime analogues has not been reported (i.e., the Re analogue of Ceretec®, Figure 3). Because reduction is more difficult for Re, the +5 oxidation state is the most accessible from perrhenate, although the ability to utilize the low spin d6 tricarbonyl Re(I) core has received a lot of attention.
Rhenium(V) chemistry, like Tc(V), is dominated by oxo chemistry. Generally, chelators that are NxS4-x donors have been utilized to complex and stabilize the oxorhenium(V) core [83]. Diamine dithiols (DADT), monamine monoamide dithiols (MAMA), and diamide dithiols have all been shown to successfully chelate the oxorhenium (V) core and are most widely used as potential BFCAs for Re(V). The susceptibility of Re(V) complexes to oxidation to perrhenate on high dilution in vivo requires careful selection and testing of chelates for Re(V); thiolate and phosphine donors seem to be most suitable at stabilizing Re(V) to oxidation, perhaps because both are themselves reducing in nature. The N3S MAG3 type chelates do not function as well with Re(V) on the radiotracer level (especially in vivo) as they do with Tc(V) because oxidation is an issue. A second thiolate may add sufficient stability although the N2S2 chelates tend to generate more lipophilic complexes.
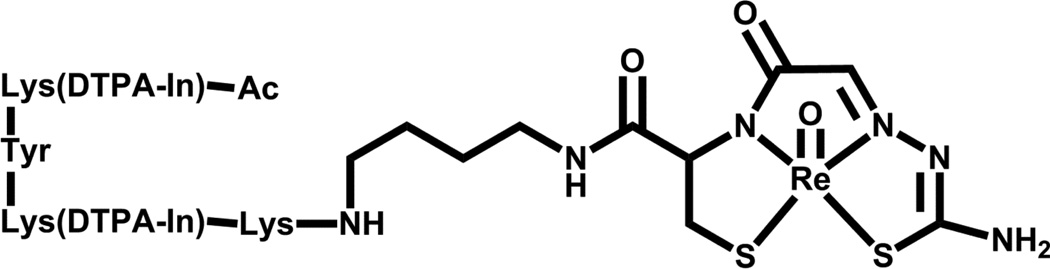
Previous clinical trials have focused on the use of rhenium radiopharmaceuticals for the palliation of metastatic bone cancer [84–87], advanced lung cancer [88], and inoperable hepatocellular carcinoma [89]. The majority of current research with Re(V) includes potential use in bifunctional chelators, for example Re-N2S2-IMP 192 (Figure 8) [83, 90].
Figure 8.
Structure of Re-N2S2-IMP 1.
Since Re is more difficult to reduce than Tc, very few lower oxidation state complexes have been evaluated on the radiotracer level. The ‘4+1’ NS3/P type system for Re(III) has been reported, however challenges exist in the reduction of 188ReO4− directly to Re(III) and a two step formulation involving 188Re(EDTA) or 188Re(thiourea) complexes as the intermediate has been investigated to minimize formation of colloidal reduced, hydrolyzed 188ReO2, which arises from the amount of reducing agent necessary and the pH conditions required for its synthesis [91].
As with Tc, the low spin d6 Re(I) oxidation state is kinetically inert and is currently being investigated predominantly as a surrogate to Tc(I) tricarbonyl complexes on the macroscopic level. Although reduction of perrhenate in aqueous solution to the +1 oxidation state is difficult, it may be useful in the future for radiotherapeutic applications similar to those imaging applications discovered for the technetium (I) tricarbonyl analogues [49, 73, 92, 93]. Valliant and co-workers have shown that the chemistry of tricarbonyl complexes with Re(I) and Tc(I) carborane complexes may be different for some carboranes due to differences in redox potentials of the two metals and perhaps to their differing substitution rates [94].
The availability of high specific activity radiorhenium (186 or 188) is essential for the development and approval of Re-based radiopharmaceuticals for radiotherapy. Although a 188W/188Re generator is available, its routine availability is of question. The availability of high specific activity 186Re would have a major impact for the field because the half-life and beta energy of 186Re are more suitable for radiotherapeutic applications than those of 188Re. Although production of 186Re from an accelerator is possible, it is currently not being exploited [95–99]. The longer half-life of 186Re would make it suitable for radiolabeling antibodies or other longer circulating biomolecules.
Copper
Development of copper radiopharmaceuticals has received much attention due the availability of multiple isotopes with radiopharmaceutical relevance [100]. The Cu isotopes of most interest include 64Cu (imaging and therapy), 62Cu (imaging) and 67Cu (therapy) (see Table 1). The Cu (II) oxidation state is preferred for radiopharmaceutical use as it is more kinetically inert than the more common, yet labile Cu(I) oxidation state. Copper(II) complexes tend to be Jahn-Teller distorted and kinetic lability remains an issue [101–105]. Thus much research in this area addresses suitable chelates for kinetically inert, “in vivo” stable Cu(II) complexes. Copper(II) is considered a borderline soft metal, favoring nitrogen and oxygen donor groups; thiolates and phosphines tend to reduce Cu(II) to the more labile Cu(I) unless care is taken. Early work in the development of a chelate system for Cu(II) focused on DOTA and 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid, TETA; however these complexes have demonstrated limited in vivo stability. Cross-bridged derivatives of DOTA and TETA, CB-DO2A and CB-TE2A, as well as the sarcophagine cage have proven more useful for chelation of Cu(II) for in vivo applications. BFCAs based on cross-bridged derivatives of DOTA and TETA and the sarcophagine cage have proven difficult to synthesize or easily modify and thus many investigators prefer to use the commercially available DOTA analogues even though they are not sufficiently stable under in vivo conditions. Brechbiel and co-workers have described a functionalized CB-TE2A analog with a reported 13% yield from cross-bridged cyclam, which was subsequently conjugated to RDG peptide analogs and labeled with 64Cu showing excellent in vitro stability [106]. Additionally, these particular analogues are quite hydrophobic and hepatobiliary clearance is often observed, leading to less than desirable pharmacokinetics. Anderson and co-workers and references therein provide an excellent and comprehensive recent review of copper radiopharmaceutical research [100].
Since last reviewed, two NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) Cu-64 conjugates[107–110] have been reported. The 64Cu- NO2A-8-AOC-BBN(7–14)NH2 conjugate[110] and the 64Cu-NOTA-RGD-BBN conjugate[108, 109] were synthesized and showed nM affinities for GRP and both αvβ3 and GRP receptors, respectively. Both showed favorable biodistribution results and PET imaging in tumor bearing mice. A subsequent head-to-head study comparing 64Cu- NO2A-8-AOC-BBN(7–14)NH2, 64Cu-DOTA-8-AOC-BBN(7–14)NH2, and 64Cu-CB-TE2A-8-AOC-BBN(7–14)NH2 was also reported; while 64Cu-CB-TE2A-8-AOC-BBN(7–14)NH2 exhibited more favorable stability in vivo; a reduced uptake and retention was observed in the tumor tissue compared to 64Cu- NO2A-8-AOC-BBN(7–14)NH2 [107].
The NOTA chelate system may prove to be more suitable for Cu(II) than the analogous DOTA or TETA systems, as the coordination number of Cu(II) is better matched. This chelate system seems to form significantly more hydrophilic complexes with Cu(II) based on the reports of Smith and Liu [107–110]. Copper-64 is available to researchers on a regular basis from a few sources; however 62Cu and especially 67Cu are not readily available and it is questionable whether 67Cu will be available in sufficient quantities for radiotherapy.
Recent studies with the small molecule 64CuATSM, which is taken up and retained in hypoxic tissue [111], have focused on understanding the mechanism of uptake and retention under reducing conditions. Fujibayashi et al. [111] reported that 64CuATSM not only is retained in hypoxic cells but also cells having mitochondrial dysfunction with higher levels of NADH and NADPH (reductases), even under normoxic conditions. Their findings indicate that 64CuATSM may be more broadly applicable than other hypoxia imaging compounds such as 18F-misonitroimidazole [111].
Gallium, indium, radiolanthanides, bismuth and actinium
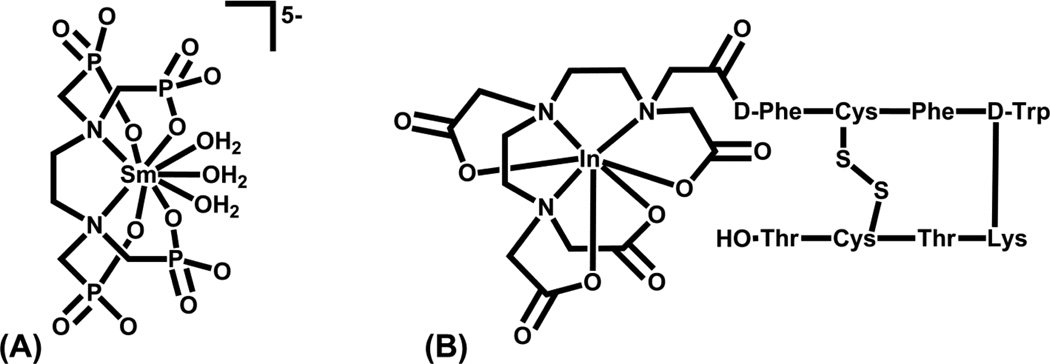
Gallium, indium, the radiolanthanides, bismuth, and actinium have been grouped together because the aminocarboxylate chelates have been utilized to stabilize these radiometals for in vivo applications. These metals all form +3 cations and thus show some similarities in their chemistries, although they differ in their coordination numbers and thus the optimal chelate for in vivo stability. Gallium(III) is generally 6-coordinate, In(III) 6- to 8-coordinate (usually 7-coordinate), Y(III) 6- to 12-coordinate (8- and 9-coordinate most common), Ln(III) 6- to 12-coordinate (9-coordinate most common, but depends on the size of the metal ion), and Bi(III) 6- to 8-coordinate. The primary criterion for in vivo stability of these metal ions is to completely surround (saturate) their coordination sphere with donor atoms from the appropriate chelator. DTPA (diethylenetriaminepentaacetic acid), DOTA, NOTA, and various other amine carboxylate donor ligands have been used to complex these metal ions. Too many or too few donor atoms within a chelate to fully saturate the coordination sphere of the particular metal ion or a poor cavity fit between the chelate and the metal ion result in an unstable complex in vivo.
Approved radiopharmaceuticals incorporating these radiometals include Quadramet® (153Sm-EDTMP) for bone pain palliation associated with metastatic disease (Figure 9A), Octreoscan® (111In-DTPA-octreotide) for imaging somatostatin receptor positive cancers (Figure 9B), 67Ga-citrate for tumor imaging, 111In(oxine)3 for platelet imaging, 111In-Prostascint® for prostate cancer imaging, 111In-Oncoscint® for colorectal and ovarian cancer imaging, and 90Y-Zevalin® for treating non-Hodgkin’s lymphoma, the last three all incorporating monoclonal antibody targeting vectors.
Figure 9.
Structures of (A) Quadramet® and (B) Octreoscan®.
Both DTPA and DOTA conjugated to antibodies or peptides have proven to be suitable chelates for In(III) for in vivo applications. Conjugation of the peptide or antibody to DTPA or DOTA through one of the carboxylate arms leaves the required 7-coordination sites available for complexation to In(III). Indium-111 is readily available in high specific activity and both DTPA and DOTA analogs are suitable for use as BFCAs [112]. The DTPA is often the chelate of choice for In3+ because of its faster complex formation kinetics compared with DOTA. Various aminecarboxylates (DTPA, DOTA, NOTA) have been evaluated along with tris(2-mercaptobenzyl)amine and tris(2-hydroxybenzyl)amine chelates for Ga(III) [21, 40, 113]. Although studies utilizing DTPA and DOTA have been carried out for Ga(III), neither is optimal for the smaller metal ion. The cavity size of DTPA and DOTA are somewhat large for Ga(III) and its strong preference for forming 6-coordinate complexes leaves dangling carboxylate arms. NOTA may have a better size and coordination number for Ga(III) [114, 115]. The stability of the resultant radiometal complexes is important for minimizing non-target tissue radiation doses and for obtaining suitable diagnostic images. Gallium and indium, if lost from their chelator, will behave similarly in vivo to Fe(III) and will be complexed by transferrin in the blood and localized to the liver. Much of the current research for gallium and indium relies on the bifunctional chelate approach where the metal is stably complexed to a chelator that is ligated to a biological molecule or biological targeting vector, for example Octreoscan® (111In- DTPA complexed to peptide) shown in Figure 9B [34].
Several radiolanthanides have nuclear properties suitable for radiotherapy applications including 153Sm, 177Lu, 149Pm, 166Dy, 166Ho and 161Tb (Table 1). The lanthanides are hard Lewis acids and form primarily ionic bonds in aqueous solution. Thus, in order to form complexes that are kinetically inert in vivo, they must be complexed with octadentate macrocyclic chelates such as DOTA although some hindered DTPA analogues (e.g., CHX-DTPA) have shown in vivo stability with radiolanthanides [116–120]. Use of DOTA as a BFCA may require either carbon backbone or aminocarboxylate arm derivatization to retain the octadenticity of the DOTA analogue, although there are many studies using one of the carboxylate arms for conjugation to the targeting agent [27, 116]. This derivatization is necessary as the radiolanthanides show significant uptake in the skeleton (as a calcium mimic) and the liver (where they are found as hydroxide colloids) in vivo if their complexes are not sufficiently stable [27, 116, 117]. The aminophosphonate chelate ethylenediaminetetramethylenephosphonic acid (EDTMP) was used to develop 153Sm-EDTMP (Quadramet®) as a bone pain palliation agent; 1,4,7,10-tetraazacyclododecane-1,4,7,10-methylenephosphonic acid (DOTMP) has been investigated more recently to form a more kinetically inert complex with 166Ho [116, 118]. Several excellent reviews on the lanthanide chemistry of radiopharmaceuticals have been published [27, 116, 117, 121].
Yttrium(III) is often considered a pseudo-lanthanide as it is situated above La(III) in Group 3. As 90Y has no gamma emissions (Table 1), researchers have often used 111In to assess its in vivo biodistribution even though this is not ideal; when 90Y is lost from its chelate it localizes primarily to bone, liver and other organs similar to the radiolanthanides [3], while 111In localizes with transferrin to the liver. Yttrium and the radiolanthanides undergo hydrolysis if they are lost from their chelate in the body resulting in colloidal M(OH)3, which will accumulate in the liver and other blood rich organs; some of the radiometal will localize in the bone if hydrolysis occurs more slowly [118]. Sterically hindered DTPA analogs (CHX-DTPA and CyDTPA) have been determined to be suitable for 90Y labeling with faster formation kinetics than DOTA [122–126]. Yttrium-90-Zevalin® (an antibody-CHX-DTPA bifunctional chelator) is an example of a 90Y radiopharmaceutical currently in clinical use [127, 128].
Bismuth(III) is of interest because two of its radioisotopes, 212Bi and 213Bi (Table 1), are alpha emitters with tremendous potential for targeted radiotherapy [129–131]. For convenience, DTPA and DOTA analogues have been evaluated as chelators for Bi(III); Brechbiel et al. recently reported on a decadentate DOTA analog, namely 3p–C-DEPA, which showed very good labeling yields with 205/206Bi at room temperature (~94% in 1 h) and very good in vivo stability in mice [132]. Clinical studies of patients with advanced myeloid leukemia showed the difficulties (loss of radiometal from chelate; short half-life) and small successes (selected destruction of leukemia cells with few side effects) of targeted alpha therapy from [213Bi]-HuM195 (a DTPA conjugated antibody) [129, 133, 134]. Because of the limited success of these initial studies, further clinical studies were extended to [225Ac]-HuM195 to yield more dose per injected radiopharmaceutical from the cumulative effects of all daughter product emissions [129, 133]. Any loss of bismuth from its chelate would result in localization and predominant increased radiation exposure to the kidneys [129]. Recently, Maecke and co-workers demonstrated the therapeutic potential of a 213Bi labeled peptide, 213Bi-DOTA-PESIN as compared directly to the analogous 177Lu labeled peptide, 177Lu-DOTA-PESIN [135]. The results from this preclinical study conducted in a mouse model of prostate cancer clearly confirmed that α therapy using 213Bi was much more efficacious than β therapy employing 177Lu in controlling tumor growth while at the same time also confirming previous findings of marked kidney damage associated with 213Bi therapy.
Actinium-225 has been suggested for use as an in vivo generator system for a decay chain that would result in a therapeutic dose from 4 alphas and 2 betas, and includes 213Bi [129]. This additional therapeutic dose would be beneficial for treatment, but there is a risk that the recoiling daughter nucleus would be lost from the chelator and result in undesirable non-target irradiation in vivo unless the decay occurs inside the targeted cancer cell [136]. The DOTA and DTPA chelates have been evaluated for 225Ac; if lost from its chelate, 225Ac generally localizes in the liver or skeletal system in vivo, but proper chelation can reduce the localization in non-target tissue [129, 136]. Several reviews have reported the benefits, usefulness and challenges of actinium as a potential nanogenerator [129–131, 133, 136–138].
The +3 radiometals have similarities in the BFCAs that are used with them; however there are issues that need to be addressed for each in order for them to become clinically useful. All of these radiometals are susceptible to hydrolysis and thus the reaction pH is critical for their formation, especially at the radiotracer level; hydrolysis becomes an issue at least two pH units earlier than at the macroscopic level and thus radiolabeling must be accomplished under acidic conditions even though protons are released on complexation. The commercially available 68Ge/68Ga generator makes 68Ga, a 68 minute positron emitter readily available to the diagnostic nuclear medicine community. Although currently available 68Ge/68Ga generator systems have 68Ge breakthrough issues, using suitable solid phase ion exchange technology it is possible to further purify the eluted 68Ga [139, 140]. Gallium-67, a SPECT radionuclide, is available in high specific activity from accelerator production on a routine basis although the gamma emission is not optimum for SPECT image acquisition. There is no ideal chelator that has been identified for 67/68Ga3+ to date. Recent reports with various nitrogen (amine and imine) and oxygen (carboxylate, phenolate, hydroxamate) donor chelates suggest that perhaps the NOTA analogs will prove to be ideal, however there has been no effort to determine the best donor atoms for Ga3+ [141, 142].
The radiolanthanides (and there are several of them with suitable nuclear properties; see Table 1) have the advantage that a BFCA that is suitable for one of them should work for all of them. Because their bonding is ionic in nature, octadentate macrocyclic DOTA analogs are the ideal BFCA [116, 118, 143]. The issue of high specific activity production of radiolanthanides must be addressed in order to promote routine use.
Bismuth radioisotopes suffer from availability, as does 225Ac. Although the alpha particles emitted by these radionuclides have high cell killing potential, their routine availability remains an issue for clinical use. Additionally, there has been very little, if any, chelate design specifically for these two radiometals. DOTA has been the BFCA of choice without consideration of other potential chelates (i.e., donors).
Zirconium
Zirconium has become of interest in the radiopharmaceutical arena because one of its radioisotopes, 89Zr (Table 1), has nuclear properties suitable for use in PET imaging. Zirconium is considered a labile, +4 metal ion with chemistry somewhat similar to that of lutetium in that it is a hard metal center preferring hard, anionic oxygen donors [144]. Zirconium forms 8-coordinate complexes; however unlike the lanthanides that favor DOTA and DTPA chelates, zirconium has been shown to be lost in vivo from these complexes [145]. In fact, the stability constant (log KML) for Zr-DTPA is ~36 [40]; once again kinetics is the dominant factor for in vivo stability. In vivo studies of 89Zr-ZrCl4 showed high liver uptake, while 89Zr(C2O4)44− showed uptake in the skeletal system [145]. 89Zr can be produced at a cyclotron and has been utilized as a PET radionuclide for labeling antibodies and peptides (some using desferrioxamine as the chelator) [133, 146–149]. A recent study showed that 89Zr-desferrioxamine B-J591 can be used to successfully diagnose prostate specific membrane antigen positive prostate tumors in vivo [145]. Although the availability of a longer-lived positron emitter (3.26 days for 89Zr) would be beneficial by allowing delayed PET imaging, the 100% abundance emission of a 909 keV gamma is not desirable; and may be a significant deterrent.
Gold
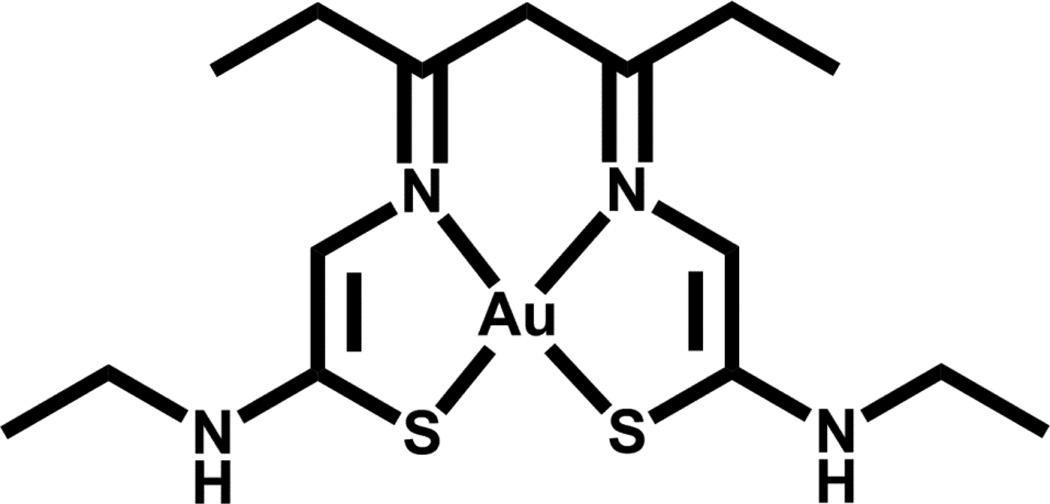
Two radioisotopes of Au are of interest for radiotherapeutic applications, namely 198Au and 199Au (Table 1). Gold-199 has more suitable beta and gamma emissions for nuclear medicine applications and is available in high specific activity from an enriched Pt target. The high abundance, higher energy gamma of 198Au (412 keV) makes it less favorable for in vivo applications but it is more readily available for development. These two radionuclides are readily available (both are reactor produced) to interested researchers. Unfortunately, the chemistry of Au(III) is not straight-forward and both hydrolysis and reduction to Au(0) are problems that need to be overcome. At the radiotracer level, hydrolysis becomes an issue for Au(III) above pH 4; extracting (t-Bu4N)[198/199AuCl4] into an organic solvent such as CHCl3 prior to reaction with the chelate addresses this issue [150].
Recently, Bottenus and co-workers investigated a series of gold(III) bis-thiosemicarbazone complexes [150]. Although one of the complexes reported, Au(3,4-HxTSE) (Figure 10), showed favorable in vitro stability, biodistribution studies in CF-1 normal mice showed >50% ID/g remaining in the bloodstream at 4 h p.i. with high lung uptake possibly resulting from complex binding to serum albumin [150]. Stabilizing Au(III) to reduction under in vivo conditions continues to be an issue that must be overcome to generate a potential 199Au radiotherapeutic agent.
Figure 10.
Structure of [Au(3,4-HxTSE)]
Gold-198 nanoparticles have received recent attention for potential radiotherapeutic applications as in vivo reduction is not an issue. Gum Arabic coated nanoparticles (GA-198AuNP) were formed by addition of an alanine based phosphine reducing agent P(CH2NHCH(CH3)COOH)3 (THPAL) to a mixture of 198AuCl4− and Gum Arabic under acidic conditions. Nanoparticle formation was accompanied by a color change from yellow to purple, and TEM images indicated gold particle diameters in the range of 12 – 18 nm [151–153]. Animal studies in PC-3 tumor bearing SCID mice where GA-198AuNPs were administered via intratumoral injection demonstrated slowed tumor growth when compared to the non-radioactive treated control group [151].
Because of the difficulties encountered with Au(III) chemistry, the availability of suitable chelates is limited. Design of a chelate to essentially encapsulate the Au(III) center so that its axial sites are not available for attack and reduction may be what is needed. The chelate itself must be stable to oxidation or Au(0) will form through internal redox chemistry.
Rhodium
Rhodium-105 is an interesting isotope for radiopharmaceutical use. Its moderate β− emission is useful for therapeutic applications, while low abundance γ emissions are available for in vivo mapping of the Rh-105 containing radiopharmaceutical as well as for dosimetry determinations (Table 1). The low spin d6 electronic configuration of Rh(III) makes its complexes kinetically inert. In fact, Rh(III) undergoes extremely slow water exchange (T1/2 ~ 30 years), second only to its 3rd row congener Ir(III) [154]. This allows for formation of metal chelate complexes with favorable in vivo stability. This high kinetic inertness is overcome in substitution reactions by using refluxing alcohol (ethanol for biological applications) to reduce Rh(III) to the more labile Rh(I) in situ to facilitate substitution; carrying out the reactions in a normoxic atmosphere results in oxidation back to Rh(III) following substitution [155].
Much of the early work towards development of a Rh-105 chelate system focused on nitrogen and oxygen donor atoms such as cyclam and cyclen derivatives, amine oximes, amine phenols, and amine porphyrin ligands [156, 157]. A variety of tetrathioether chelates (macrocyclic and acyclic) have been evaluated and were shown to give 105Rh-S4 complexes in >90% yield with high stability [157–163]. Additionally, NS3, N2S2 and N4 macrocyclic and N2S2 acyclic ligands were evaluated; although the complexes formed were very stable, their yields dropped with increasing N donor atoms [161, 164–167]. More recently, the acyclic N2P2 and S2P2 chelates were evaluated for 105Rh complexation and again stabilities were very high [168]. The macrocyclic S4 and acyclic N2S2 chelates conjugated to the bombesin peptide BBN(7–14)NH2 with various linkers showed good binding to GRP receptors, indicating promise for targeting prostate cancer [162, 164, 166]. Radiochemical yields of >90% were achieved with many of the chelate systems that have been reported. Overall, the presence of amine donor atoms increased the pH necessary for complexation and resulted in lower yields at the radiotracer level due to the competing hydrolysis reaction; the more amine N donors present, the higher the pH needed and the lower the yield [161, 164–167]. The presence of phosphine donors allowed reduced temperatures and lower concentrations of ethanol to be used at the radiotracer level (phosphine acts as both a reducing agent and chelate) but at the expense of significantly higher ligand concentrations (~1000 fold increase needed) [168]. Although higher temperatures and ethanol concentrations are required for the tetrathioether chelate systems, they may prove to be more suitable BFCAs due to ligand stability and the use of lower ligand concentrations for generating the 105Rh bioconjugate. Based on literature reports to date, either the 222-S4 or 333-S4 acyclic ligand systems will be most useful as they each only generate one isomer on complexation with Rh(III) (cis- and trans-dichloro, respectively) [159, 162].
Rhodium-105 is available in high specific activity. Its radiotracer complexes are kinetically inert in vivo and thus transchelation will not be an issue. Its slow substitution kinetics can be a hurdle in radiolabeling as higher temperatures are needed (e.g., refluxing ethanol) to generate Rh(I) in situ to allow faster substitution [160, 163, 168]. The higher temperatures required may be a problem for some biotargeting groups. As with many metals, hydrolysis is an issue above pH 6–7 and this must be considered during any radiosyntheses.
Future directions
Inorganic chemistry will continue to play an important role in diagnostic and therapeutic radiopharmaceuticals. There is no single radionuclide or BFCA that is optimal for all applications in either imaging or treatment. The recent worldwide shortages experienced with the 99Mo/99mTc generator may show their after effects through development of additional isotope production facilities or alternative methods to the production of 99Mo. These shortages will likely also show their effects through the development of other radionuclides and radiopharmaceuticals as alternatives to 99mTc based diagnostic imaging agents. There is much recent interest in 18F and 68Ga based agents as PET alternatives to 99mTc. The development of multimodality imaging agents (MRI and PET/SPECT)[169, 170] and radiolabeled nanoparticle imaging agents[171] were not included in this paper, however these are two of the new up and coming areas about which we will likely see much more in the future.
Radiotherapeutic agents hold hope as alternatives to chemotherapeutic drugs in that the side effects can be significantly less due to the very low concentrations of actual drug administered (nM versus mM concentrations). However, delivery of sufficient radiation dose to tumors without overburdening the non-target tissues continues to be an issue that needs to be overcome.
All of the radiometals discussed have both advantages and disadvantages regarding their routine use. The nuclear properties (Table 1) of the radiometals make them useful for radiopharmaceutical applications. Their availability in high specific activity (high activity/unit mass) and their chemistry at the radiotracer level (often nM or less) have made their routine use challenging. The interest in using a commercially available BFCA (i.e., DOTA or DTPA analogs) rather than synthesizing an appropriate BFCA has increased interest in the radiometals for which these BFCAs may be useful (e.g., radiolanthanides, 90Y, 111In, 67/68Ga, 64Cu) even when the resultant radiometal complex is less than optimal (e.g., DOTA for Cu) in vivo. Suitable chelates as the basis of BFCAs are lacking for the less used radiometals for which DOTA or DTPA will not serve as a useful BFCA (e.g., 199Au, 105Rh, 89Zr, 67/68Ga). Addressing the issues of isotope availability, high specific activity, and appropriate BFCA technology in this field for the various radiometals will be critical for the development of the next generation of radiopharmaceuticals for use in nuclear medicine.
Acknowledgments
We would like to acknowledge NIBIB Training Grant 5 T32 EB004822 (V.C and D.W.D.), support from the U.S. Department of Veterans Affairs Research Career Scientist Award (T.J.H.) and editorial/technical support from Tammy L. Rold.
References
- 1.Brucer M, Harris CC, MacIntyre WJ, Taplin GV, editors. The Heritage of Nuclear Medicine: Commemorating the 25th Anniversary of the Society of Nuclear Medicine, 1954–1979. New York: Society of Nuclear Medicine; 1979. p. 166. [Google Scholar]
- 2.The Technetium-99m Generator in Brookhaven History by Brookhaven National Laboratories http://www.bnl.gov/bnlweb/history/tc-99m.asp
- 3.Mausner LF, Mirzadeh S. In: Reactor production of radionuclides, in Handbook of Radiopharmaceuticals: Radiochemistry and Applications. Welch MJ, Redvanly CS, editors. New York: John Wiley & Sons, Ltd; 2003. pp. 87–118. [Google Scholar]
- 4.Ballinger JR. 99Mo shortage in nuclear medicine: Crisis or challenge? Journal of Labelled Compounds and Radiopharmaceuticals. 2010;53:167–168. [Google Scholar]
- 5.Bhattacharyya S, Dixit M. Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals. Dalton Transactions. 2011;40:6112–6128. doi: 10.1039/c1dt10379b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQuade P, Rowland DJ, Lewis JS, Welch MJ. Positron-emitting isotopes produced on biomedical cyclotrons. Current Medicinal Chemistry. 2005;12:807–818. doi: 10.2174/0929867053507397. [DOI] [PubMed] [Google Scholar]
- 7.Ruth TJ. The uses of radiotracers in the life sciences. Reports on Progress in Physics. 2009;72:1–23. [Google Scholar]
- 8.Volkert WA, Goeckeler WF, Ehrhardt GJ, Ketring AR. Therapeutic radionuclides: Production and decay property considerations. Journal of Nuclear Medicine. 1991;32:174–185. [PubMed] [Google Scholar]
- 9.Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals. Radiochemistry and Applications. New York: John Wiley & Sons Ltd; 2003. p. 848. [Google Scholar]
- 10.Qaim SM. Cyclotron production of medical radionuclides, in Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences. In: Vertes A, Nagy S, Klencsar Z, editors. 2nd edn. Dordrecht/Boston/London: Kluwer Academic Publishers; 2003. pp. 47–79. [Google Scholar]
- 11.Anderson CJ, Welch MJ. Radiometal-labeled agents (non-technetium) for diagnostic imaging. Chemical Reviews. 1999;99:2219–2234. doi: 10.1021/cr980451q. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee SR, Maresca KP, Francesconi L, Valliant J, Babich JW, Zubieta J. New directions in the coordination chemistry of 99mTc: A reflection on technetium core structures and a strategy for new chelate design. Nuclear Medicine and Biology. 2005;32:1–20. doi: 10.1016/j.nucmedbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Blower P. Towards molecular imaging and treatment of disease with radionuclides: the role of inorganic chemistry. Dalton Transactions. 2006:1705–1711. doi: 10.1039/b516860k. [DOI] [PubMed] [Google Scholar]
- 14.Jurisson S, Berning D, Jia W, Ma D. Coordination compounds in nuclear medicine. Chemical Reviews. 1993;93:1137–1156. [Google Scholar]
- 15.Jurisson SS, Lydon JD. Potential technetium small molecule radiopharmaceuticals. Chemical Reviews. 1999;99:2205–2218. doi: 10.1021/cr980435t. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Edwards DS. 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chemical Reviews. 1999;99:2235–2268. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 17.Reichert DE, Lewis JS, Anderson CJ. Metal complexes as diagnostic tools. Coordination Chemistry Reviews. 1999;184:3–66. [Google Scholar]
- 18.Schibli R, Schubiger AP. Current use and future potential of organometallic radiopharmaceuticals. European Journal of Nuclear Medicine. 2002;29:1529–1542. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 19.Schubiger PA, Alberto R, Smith A. Vehicles, chelators, and radionuclides: Choosing the "building blocks" of an effective therapeutic radioimmunoconjugate. Bioconjugate Chemistry. 1996;7:X1–X179. doi: 10.1021/bc950097s. [DOI] [PubMed] [Google Scholar]
- 20.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chemical Reviews. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 21.Zeglis BM, Lewis JS. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Transactions. 2011;40:6168–6195. doi: 10.1039/c0dt01595d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey AM. Radiometal complexes in molecular imaging and therapy. Current Medicinal Chemistry. 2010;17:3673–3683. doi: 10.2174/092986710793213733. [DOI] [PubMed] [Google Scholar]
- 23.Gotthardt M, Boermann OC, Behr TM, Behe MP, Oyen WJ. Development and clinical application of peptide-based radiopharmaceuticals. Current Medicinal Chemistry. 2004;10:2951–2963. doi: 10.2174/1381612043383502. [DOI] [PubMed] [Google Scholar]
- 24.Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. Journal of Nuclear Medicine. 2011;52:841–844. doi: 10.2967/jnumed.110.084236. [DOI] [PubMed] [Google Scholar]
- 25.Qaim SM. Development of novel positron emitters for medical applications: Nuclear and radiochemical aspects. Radiochimica Acta. 2011;99:611–625. [Google Scholar]
- 26.National Nuclear Data Center, Brookhaven National Laboratory, Last Update: January 12, 2012, http://www.nndc.bnl.gov/
- 27.Liu S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Advanced Drug Delivery Reviews. 2008;60:1347–1370. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Advanced Drug Delivery Reviews. 2010;62:1005–1022. doi: 10.1016/j.addr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Correia JDG, Paulo A, Raposinho PD, Santos I. Radiometallated peptides for molecular imaging and targeted therapy. Dalton Transactions. 2011;40:6144–6167. doi: 10.1039/c0dt01599g. [DOI] [PubMed] [Google Scholar]
- 30.De León-Rodríguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA - peptide conjugates. Bioconjugate Chemistry. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. Journal of Nuclear Medicine. 2008;49:158–163. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 32.Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treatment Reviews. 2008;34:13–26. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Deutscher SL. Phage display in molecular imaging and diagnosis of cancer. Chemical Reviews. 2010;110:3196–3211. doi: 10.1021/cr900317f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chemical Reviews. 2010;110:3087–3111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Xie J, Chen X. Peptide-based probes for targeted molecular imaging. Biochemistry. 2010;49:1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanda PK, Lane SR, Retzloff LB, Pandey US, Smith CJ. Radiolabeled regulatory peptides for imaging and therapy. Current Opinion in Endocrinology, Diabetes and Obesity. 2010;17:69–76. doi: 10.1097/MED.0b013e32833392ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schottelius M, Wester HJ. Molecular imaging targeting peptide receptors. Methods. 2009;48:161–177. doi: 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Tweedle MF. Peptide-targeted diagnostics and radiotherapeutics. Accounts of Chemical Research. 2009;42:958–968. doi: 10.1021/ar800215p. [DOI] [PubMed] [Google Scholar]
- 39.Yan Y, Chen X. Peptide heterodimers for molecular imaging. Amino Acids. 2010:1–12. doi: 10.1007/s00726-010-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chemical Reviews. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole WC, DeNardo SJ, Meares CF, McCall MJ, DeNardo GL, Epstein AL, O'Brien HA, Moi MK. Comparative serum stability of radiochelates for antibody radiopharmaceuticals. Journal of Nuclear Medicine. 1987;28:83–90. [PubMed] [Google Scholar]
- 42.Moi MK, DeNardo SJ, Meares CF. Stable bifunctional chelates of metals used in radiotherapy. Cancer Research. 1990;50:789s–793s. [PubMed] [Google Scholar]
- 43.Davison A. In: The coordination chemistry of technetium, in Technetium in Chemistry and Nuclear Medicine. Deutsch E, Nicolini M, Wagner HN, editors. Verona: Cortina International; 1983. pp. 3–14. [Google Scholar]
- 44.Davison A, Jones AG, Orvig C, Sohn M. A new class of oxotechnetium(5+) chelate complexes containing a TcON2S2 core. Inorganic Chemistry. 1981;20:1629–1632. [Google Scholar]
- 45.Deutsch E, Bushong W, Glavan K, Elder R, Sodd V, Scholz K, Fortman D, Lukes S. Heart imaging with cationic complexes of technetium. Science. 1981;214:85–86. doi: 10.1126/science.6897930. [DOI] [PubMed] [Google Scholar]
- 46.Deutsch E, Libson K. In: Application of technetium chemistry to the practice of nuclear medicine, in Technetium in Chemistry and Nuclear Medicine. Deutsch E, Nicolini M, Wagner HN, editors. Verona: Cortina International; 1983. pp. 29–36. [Google Scholar]
- 47.Holman BL, Jones AG, Lister-James J, Davison A, Abrams MJ, Kirshenbaum JM, Tumeh SS, English RJ. A new Tc-99m–labeled myocardial imaging agent, hexakis(t-Butylisonitrile)-technetium(I) [Tc-99m TBI]: Initial experience in the human. Journal of Nuclear Medicine. 1984;25:1350–1355. [PubMed] [Google Scholar]
- 48.Vanderheyden JL, Ketring AR, Libson K, Heeg MJ, Roecker L, Motz P, Whittle R, Elder RC, Deutsch E. Synthesis and characterization of cationic technetium complexes of 1,2-bis(dimethylphosphino)ethane (DMPE). Structure determinations of trans-[TcV(DMPE)2(OH)(O)](F3CSO3)2, trans-[TcIII(DMPE)2Cl2]F3CSO3, and [TcI(DMPE)3]+ using x-ray diffraction, EXAFS, and technetium-99 NMR. Inorganic Chemistry. 1984;23:3184–3191. [Google Scholar]
- 49.Bartholoma M, Valliant J, Maresca KP, Babich J, Zubieta J. Single amino acid chelates (SAAC): a strategy for the design of technetium and rhenium radiopharmaceuticals. Chemical Communications. 2009;5:493–512. doi: 10.1039/b814903h. [DOI] [PubMed] [Google Scholar]
- 50.Tisato F, Porchia M, Bolzati C, Refosco F, Vittadini A. The preparation of substitution-inert 99Tc metal-fragments: Promising candidates for the design of new 99mTc radiopharmaceuticals. Coordination Chemistry Reviews. 2006;250:2034–2045. [Google Scholar]
- 51.Pasqualini R, Duatti A. Synthesis and characterization of the new neutral myocardial imaging agent (99mTcN(NOET)2) (NOET = N-Ethyl-N-ethoxydithiocarbamato) ChemInform. 1992;23:1354–1355. [Google Scholar]
- 52.Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M. Bis(Dithiocarbamato) nitrido technetium-99m radiopharmaceuticals: A class of neutral myocardial imaging agents. Journal of Nuclear Medicine. 1994;35:334–341. [PubMed] [Google Scholar]
- 53.Bolzati C, Cavazza-Ceccato M, Agostini S, Tokunaga S, Casara D, Bandoli G. Subcellular distribution and metabolism studies of the potential myocardial imaging agent [99mTc(N)(DBODC)(PNP5)]+ . Journal of Nuclear Medicine. 2008;49:1336–1344. doi: 10.2967/jnumed.108.051482. [DOI] [PubMed] [Google Scholar]
- 54.Hatada K, Riou LM, Ruiz M, Yamamichi Y, Duatti A, Lima RL, Goode AR, Watson DD, Beller GA, Glover DK. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: Comparison with 99mTc-sestamibi and 99mTc-tetrofosmin in rats. Journal of Nuclear Medicine. 2004;45:2095–2101. [PubMed] [Google Scholar]
- 55.Kim Y-S, Wang J, Broisat A, Glover DK, Liu S. Tc-99m-N-MPO: Novel cationic Tc-99m radiotracer for myocardial perfusion imaging. Journal of Nuclear Cardiology. 2008;15:535–546. doi: 10.1016/j.nuclcard.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 56.Liu S. Ether and crown ether-containing cationic 99mTc complexes useful as radiopharmaceuticals for heart imaging. Dalton Transactions. 2007:1183–1193. doi: 10.1039/b618406e. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Chen L, Liu S, Barber C, Stevenson G, Furenlid L, Barrett H, Woolfenden J. Kinetic characterization of a novel cationic 99mTc(I)-tricarbonyl complex, 99mTc-15C5-PNP, for myocardial perfusion imaging. Journal of Nuclear Cardiology. 2010;17:858–867. doi: 10.1007/s12350-010-9262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendes F, Gano L, Fernandes C, Paulo A, Santos I. Studies of the myocardial uptake and excretion mechanisms of a novel 99mTc heart perfusion agent. Nuclear Medicine and Biology. 2012;39:207–213. doi: 10.1016/j.nucmedbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Meszaros LK, Dose A, Biagini SCG, Blower PJ. Synthesis and evaluation of analogues of HYNIC as bifunctional chelators for technetium. Dalton Transactions. 2011;40:6260–6267. doi: 10.1039/c0dt01608j. [DOI] [PubMed] [Google Scholar]
- 60.Abiraj K, Mansi R, Tamma M-L, Forrer F, Cescato R, Reubi JC, Akyel KG, Maecke HR. Tetraamine-derived bifunctional chelators for technetium-99m labelling: synthesis, bioconjugation and evaluation as targeted SPECT imaging probes for GRP-receptor-positive tumours. Chemistry - A European Journal. 2010;16:2115–2124. doi: 10.1002/chem.200902011. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee SR, Maresca KP, Stephenson KA, Valliant JF, Babich JW, Graham WA, Barzana M, Dong Q, Fischman AJ, Zubieta J. N,N-Bis(2-mercaptoethyl)methylamine: A new coligand for Tc-99m labeling of hydrazinonicotinamide peptides. Bioconjugate Chemistry. 2005;16:885–902. doi: 10.1021/bc050040y. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y-S, He Z, Hsieh W-Y, Liu S. A novel ternary ligand system useful for preparation of cationic 99mTc-diazenido complexes and 99mTc-labeling of small biomolecules. Bioconjugate Chemistry. 2006;17:473–484. doi: 10.1021/bc0502715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King R, Surfraz MB-U, Finucane C, Biagini SCG, Blower PJ, Mather SJ. 99mTc-HYNIC-gastrin peptides: Assisted coordination of 99mTc by amino acid side chains results in improved performance both in vitro and in vivo. Journal of Nuclear Medicine. 2009;50:591–598. doi: 10.2967/jnumed.108.058289. [DOI] [PubMed] [Google Scholar]
- 64.Rose DJ, Maresca KP, Nicholson T, Davison A, Jones AG, Babich J, Fischman A, Graham W, DeBord JRD, Zubieta J. Synthesis and characterization of organohydrazino complexes of technetium, rhenium, and molybdenum with the {M(η1-HxNNR)(η2-HyNNR)} core and their relationship to radiolabeled organohydrazine-derivatized chemotactic peptides with diagnostic applications. Inorganic Chemistry. 1998;37:2701–2716. doi: 10.1021/ic970352f. [DOI] [PubMed] [Google Scholar]
- 65.Cyr JE, Pearson DA, Wilson DM, Nelson CA, Guaraldi M, Azure MT, Lister-James J, Dinkelborg LM, Dean RT. Somatostatin receptor-binding peptides suitable for tumor radiotherapy with Re-188 or Re-186: Chemistry and initial biological studies. Journal of Medicinal Chemistry. 2007;50:1354–1364. doi: 10.1021/jm061290i. [DOI] [PubMed] [Google Scholar]
- 66.Boschi A, Bolzati C, Benini E, Malagò E, Uccelli L, Duatti A, Piffanelli A, Refosco F, Tisato F. A novel approach to the high-specific-activity labeling of small peptides with the technetium-99m fragment [99mTc(N)(PXP)]2+ (PXP = Diphosphine Ligand) Bioconjugate Chemistry. 2001;12:1035–1042. doi: 10.1021/bc0155162. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes C, Kniess T, Gano L, Seifert S, Spies H, Santos I. Synthesis and biological evaluation of silylated mixed-ligand 99mTc complexes with the [PNS/S] donor atom set. Nuclear Medicine and Biology. 2004;31:785–793. doi: 10.1016/j.nucmedbio.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Pietzsch H-J, Tisato F, Refosco F, Leibnitz P, Drews A, Seifert S, Spies H. Synthesis and characterization of novel trigonal bipyramidal technetium(III) mixed-ligand complexes with SES/S/P coordination (E = O, N(CH3), S) Inorganic Chemistry. 2000;40:59–64. doi: 10.1021/ic000828m. [DOI] [PubMed] [Google Scholar]
- 69.Alberto R, Schibli R, Abram U, Egli A, Knapp FF, Schubiger PA. Potential of the "[M(CO)3]+" (M = Re, Tc) moiety for the labeling of biomolecules. Radiochimica Acta. 1997;79:99–103. [Google Scholar]
- 70.Alberto R, Schibli R, Egli A, Schubiger AP, Herrmann WA, Artus G, Abram U, Kaden TA. Metal carbonyl syntheses XXII. Low pressure carbonylation of [MOCl4] and [MO4]: the technetium(I) and rhenium(I) complexes [NEt4]2[MCl3(CO)3] Journal of Organometallic Chemistry. 1995;493:119–127. [Google Scholar]
- 71.Alberto R, Schibli R, Egli A, Schubiger AP, Abram U, Kaden TA. A novel organometallic aqua complex of technetium for the labeling of biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4] in aqueous solution and its reaction with a bifunctional ligand. Journal of the American Chemical Society. 1998;120:7987–7988. [Google Scholar]
- 72.Alberto R, Schibli R, Schubiger AP, Abram U, Pietzsch HJ, Johannsen B. First application of fac-[99mTc(OH2)3(CO)3]+ in bioorganometallic chemistry: Design, structure, and in vitro affinity of a 5-HT(1A) receptor ligand labeled with 99mTc. Journal of the American Chemical Society. 1999;121:6076–6077. [Google Scholar]
- 73.Garcia R, Paulo A, Santos I. Rhenium and technetium complexes with anionic or neutral scorpionates: An overview of their relevance in biomedical applications. Inorganica Chimica Acta. 2009;362:4315–4327. [Google Scholar]
- 74.Bowen ML, Lim NC, Ewart CB, Misri R, Ferreira CL, Hafeli U, Adam MJ, Orvig C. Glucosamine conjugates bearing N,N,O-donors: potential imaging agents utilizing the [M(CO)3]+ core (M = Re, Tc) Dalton Transactions. 2009:9216–9227. doi: 10.1039/b914310f. [DOI] [PubMed] [Google Scholar]
- 75.Mindt TL, Müller C, Melis M, Jong Md, Schibli R. “Click-to-chelate”: In vitro and in vivo comparison of a 99mTc(CO)3-labeled N(τ)-histidine folate derivative with its isostructural, clicked 1,2,3-triazole analogue. Bioconjugate Chemistry. 2008;19:1689–1695. doi: 10.1021/bc800183r. [DOI] [PubMed] [Google Scholar]
- 76.Retzloff LB, Heinzke L, Figureoa SD, Sublett SV, Ma L, Sieckman GL, Rold TL, Santos I, Hoffman TJ, Smith CJ. Evaluation of [99mTc-(CO)3-X-Y-bombesin(7–14)NH2] conjugates for targeting gastrin-releasing peptide receptors overexpressed on breast carcinoma. Anticancer Research. 2010;30:19–30. [PubMed] [Google Scholar]
- 77.Taylor AT, Lipowska M, Marzilli LG. 99mTc(CO)3(NTA): A 99mTc renal tracer with pharmacokinetic properties comparable to those of 131I-OIH in healthy volunteers. Journal of Nuclear Medicine. 2010;51:391–396. doi: 10.2967/jnumed.109.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tzanopoulou S, Sagnou M, Paravatou-Petsotas M, Gourni E, Loudos G, Xanthopoulos S, Lafkas D, Kiaris H, Varvarigou A, Pirmettis IC, Papadopoulos M, Pelecanou M. Evaluation of Re and 99mTc complexes of 2-(4'-Aminophenyl)benzothiazole as potential breast cancer radiopharmaceuticals. Journal of Medicinal Chemistry. 2010;53:4633–4641. doi: 10.1021/jm1001293. [DOI] [PubMed] [Google Scholar]
- 79.Vitor RF, Esteves T, Marques F, Raposinho P, Paulo A, Rodrigues S, Rueff J, Casimiro S, Costa L, Santos I. 99mTc-tricarbonyl complexes functionalized with anthracenyl fragments: Synthesis, characterization, and evaluation of their radiotoxic effects in murine melanoma cells. Cancer Biotherapy & Radiopharmaceuticals. 2009;24:551–563. doi: 10.1089/cbr.2009.0647. [DOI] [PubMed] [Google Scholar]
- 80.Struthers H, Spingler B, Mindt TL, Schibli R. “Click-to-Chelate”: Design and incorporation of triazole-containing metal-chelating systems into biomolecules of diagnostic and therapeutic interest. Chemistry - A European Journal. 2008;14:6173–6183. doi: 10.1002/chem.200702024. [DOI] [PubMed] [Google Scholar]
- 81.Moore AL, Bucar D-K, MacGillivray LR, Benny PD. “Click” labeling strategy for M(CO)3 (M = Re, 99mTc) prostate cancer targeted Flutamide agents. Dalton Transactions. 2010;39:1926–1928. doi: 10.1039/b921413e. [DOI] [PubMed] [Google Scholar]
- 82.Benny PD, Green JL, Engelbrecht HP, Barnes CL, Jurisson SS. Reactivity of rhenium(V) oxo Schiff base complexes with phosphine ligands: Rearrangement and reduction reactions. Inorganic Chemistry. 2005;44:2381–2390. doi: 10.1021/ic048670j. [DOI] [PubMed] [Google Scholar]
- 83.Liu G, Hnatowich DJ. Labeling biomolecules with radiorhenium - a review of the bifunctional chelators. Anti-Cancer Agents in Medicinal Chemistry. 2007;7:367–377. doi: 10.2174/187152007780618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liepe K, Kotzerke J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nuclear Medicine Communications. 2007;28:623–630. doi: 10.1097/MNM.0b013e32825a6adc. [DOI] [PubMed] [Google Scholar]
- 85.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Seminars in Nuclear Medicine. 2010;40:89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Syed R, Bomanji JB, Nagabhushan N, Kayani I, Groves A, Waddington W, Cassoni A, Ell PJ. 186Re-HEDP in the treatment of patients with inoperable osteosarcoma. Journal of Nuclear Medicine. 2006;47:1927–1935. [PubMed] [Google Scholar]
- 87.Zafeirakis A. Can response to palliative treatment with radiopharmaceuticals be further enhanced? Hellenic Journal of Nuclear Medicine. 2009;12:151–157. [PubMed] [Google Scholar]
- 88.Edelman MJ, Clamon G, Kahn D, Magram M, Lister-James J, Line BR. Targeted radiopharmaceutical therapy for advanced lung cancer: Phase 1 trial of rhenium Re188 P2045, a somatostatin analog. Journal of Thoracic Oncology. 2009;4:1550–1554. doi: 10.1097/JTO.0b013e3181bf1070. [DOI] [PubMed] [Google Scholar]
- 89.Bernal P, Raoul J-L, Stare J, Sereegotov E, Sundram FX, Kumar A, Jeong J-M, Pusuwan P, Divgi C, Zanzonico P, Vidmar G, Buscombe J, Chau TTM, Saw MM, Chen S, Ogbac R, Dondi M, Padhy AK. International Atomic Energy Agency-sponsored multination study of intra-arterial rhenium-188-labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: Results with special emphasis on prognostic value of dosimetric study. Seminars in Nuclear Medicine. 2008;38:S40–S45. doi: 10.1053/j.semnuclmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Cantorias MV, Howell RC, Todaro L, Cyr JE, Berndorff D, Rogers RD, Francesconi LC. MO tripeptide diastereomers (M = 99/99mTc, Re): Models to identify the structure of 99mTc peptide targeted radiopharmaceuticals. Inorganic Chemistry. 2007;46:7326–7340. doi: 10.1021/ic070077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schiller E, Seifert S, Tisato F, Refosco F, Kraus W, Spies H, Pietzsch H-J. Mixed-ligand rhenium-188 complexes with tetradentate/monodentate NS3/P (4' + 1') coordination: Relation of structure with antioxidation stability. Bioconjugate Chemistry. 2005;16:634–643. doi: 10.1021/bc049745a. [DOI] [PubMed] [Google Scholar]
- 92.Binkley SL, Barone NV, Underwood AC, Milsted A, Franklin BR, Herrick RS, Ziegler CJ. The synthesis and toxicity of tripodal tricarbonyl rhenium complexes as radiopharmaceutical models. Journal of Inorganic Biochemistry. 2010;104:632–638. doi: 10.1016/j.jinorgbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Torres Martin De Rosales R, Finucane C, Foster J, Mather SJ, Blower PJ. 188Re(CO)3-dipicolylamine-alendronate: A new bisphosphonate conjugate for the radiotherapy of bone metastases. Bioconjugate Chemistry. 2010;21:811–815. doi: 10.1021/bc100071k. [DOI] [PubMed] [Google Scholar]
- 94.Armstrong AF, Valliant JF. Differences between the macroscopic and tracer level chemistry of rhenium and technetium: Contrasting cage isomerisation behaviour of Re(I) and Tc(I) carborane complexes. Dalton Transactions. 2010;39:8128–8131. doi: 10.1039/c0dt00490a. [DOI] [PubMed] [Google Scholar]
- 95.Bonardi M, Groppi F, Manenti S, Persico E, Gini L, Abbas K, Holzwarth U, Simonelli F, Alfassi Z. Production study of high specific activity NCA Re-186g by proton and deuteron cyclotron irradiation. Journal of Radioanalytical and Nuclear Chemistry. 2010;286:1–7. doi: 10.1016/j.apradiso.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 96.Lapi S, Mills WJ, Wilson J, McQuarrie S, Publicover J, Schueller M, Schyler D, Ressler JJ, Ruth TJ. Production cross-sections of 181–186Re isotopes from proton bombardment of natural tungsten. Applied Radiation and Isotopes. 2007;65:345–349. doi: 10.1016/j.apradiso.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 97.Moustapha ME, Ehrhardt GJ, Smith CJ, Szajek LP, Eckelman WC, Jurisson SS. Preparation of cyclotron-produced 186Re and comparison with reactor-produced 186Re and generator-produced 188Re for the labeling of bombesin. Nuclear Medicine and Biology. 2006;33:81–89. doi: 10.1016/j.nucmedbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Szelecsényi F, Steyn G, Kovács Z, Aardaneh K, Vermeulen C, van der Walt T. Production possibility of 186Re via the 192Os(p,α3n) 186Re nuclear reaction. Journal of Radioanalytical and Nuclear Chemistry. 2009;282:261–263. [Google Scholar]
- 99.Zhang X, Li Q, Li W, Sheng R, Shen S. Production of no-carrier-added 186Re via deuteron induced reactions on isotopically enriched 186W. Applied Radiation and Isotopes. 2001;54:89–92. doi: 10.1016/s0969-8043(00)00268-2. [DOI] [PubMed] [Google Scholar]
- 100.Anderson CJ, Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biotherapy and Radiopharmaceuticals. 2009;24:379–393. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones-Wilson TM, Deal KA, Anderson CJ, McCarthy DW, Kovacs Z, Motekaitis RJ, Sherry AD, Martell AE, Welch MJ. The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nuclear Medicine and Biology. 1998;25:523–530. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 102.Kukis DL, Diril H, Greiner DP, DeNardo SJ, DeNardo GL, Salako QA, Meares CF. A comparative study of copper-67 radiolabeling and kinetic stabilities of antibody-macrocyle chelate conjugates. Cancer. 1994;73:779–786. doi: 10.1002/1097-0142(19940201)73:3+<779::aid-cncr2820731306>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 103.Motekaitis RJ, Sun Y, Martell AE, Welch MJ. Synthesis, characterization, and Cu(II) solution chemistry of dioxotetraazamacrocycles. Canadian Journal of Chemistry. 1999;77:614–623. [Google Scholar]
- 104.Sun X, Wuest M, Kovacs Z, Sherry D, Motekaitis R, Wang Z, Martell A, Welch M, Anderson C. In vivo behavior of copper-64-labeled methanephosphonate tetraaza macrocyclic ligands. Journal of Biological Inorganic Chemistry. 2003;8:217–225. doi: 10.1007/s00775-002-0408-5. [DOI] [PubMed] [Google Scholar]
- 105.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. Journal of Medicinal Chemistry. 2001;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 106.Boswell CA, Regino CAS, Baidoo KE, Wong KJ, Bumb A, Xu H, Milenic DE, Kelley JA, Lai CC, Brechbiel MW. Synthesis of a cross-bridged cyclam derivative for peptide conjugation and 64Cu radiolabeling. Bioconjugate Chemistry. 2008;19:1476–1484. doi: 10.1021/bc800039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoffman TJ, Smith CJ. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nuclear Medicine and Biology. 2009;36:579–585. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 108.Liu Z, Li Z-B, Cao Q, Liu S, Wang F, Chen X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J Nucl Med. 2009;50:1168–1177. doi: 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjugate Chemistry. 2009;20:1016–1025. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prasanphanich AF, Retzloff L, Lane SR, Nanda PK, Sieckman GL, Rold TL, Ma L, Figueroa SD, Sublett SV, Hoffman TJ, Smith CJ. In vitro and in vivo analysis of [64Cu-NO2A-8-Aoc-BBN(7–14)NH2]: A site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors. Nuclear Medicine and Biology. 2009;36:171–181. doi: 10.1016/j.nucmedbio.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshii Y, Yoneda M, Ikawa M, Furukawa T, Kiyono Y, Mori T, Yoshii H, Oyama N, Okazawa H, Saga T, Fujibayashi Y. Radiolabeled Cu-ATSM as a novel indicator of overreduced intracellular state due to mitochondrial dysfunction: studies with mitochondrial DNA-less ρ0 cells and cybrids carrying MELAS mitochondrial DNA mutation. Nuclear Medicine and Biology. 2012;39:177–185. doi: 10.1016/j.nucmedbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 112.Good S, Walter M, Waser B, Wang X, Müller-Brand J, Béhé M, Reubi J-C, Maecke H. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35:1868–1877. doi: 10.1007/s00259-008-0803-4. [DOI] [PubMed] [Google Scholar]
- 113.Motekaitis RJ, Martell AE, Koch SA, Hwang, Quarless DA, Welch MJ. The gallium(III) and indium(III) complexes of tris(2-mercaptobenzyl)amine and tris(2-hydroxybenzyl)amine. Inorganic Chemistry. 1998;37:5902–5911. [Google Scholar]
- 114.Bartholoma M, Louie AS, Valliant J, Zubieta J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chemical Reviews. 2010;110:2903–2920. doi: 10.1021/cr1000755. [DOI] [PubMed] [Google Scholar]
- 115.Heppeler A, André JP, Buschmann I, Wang X, Reubi J-C, Hennig M, Kaden TA, Maecke HR. Metal-ion-dependent biological properties of a chelator-derived somatostatin analogue for tumour targeting. Chemistry - A European Journal. 2008;14:3026–3034. doi: 10.1002/chem.200701264. [DOI] [PubMed] [Google Scholar]
- 116.Cutler CS, Smith CJ, Ehrhardt GJ, Tyler TT, Jurisson SS, Deutsch E. Current and potential therapeutic uses of lanthanide radioisotopes. Cancer Biotherapy & Radiopharmaceuticals. 2000;15:531–545. doi: 10.1089/cbr.2000.15.531. [DOI] [PubMed] [Google Scholar]
- 117.Jurisson SS, Cutler CS, Smith SV. Radiometal complexes: characterization and relevant in vitro studies. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2008;52:222–234. [PubMed] [Google Scholar]
- 118.Li WP, Ma DS, Higginbotham C, Hoffman T, Ketring AR, Cutler CS, Jurisson SS. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nuclear Medicine and Biology. 2001;28:145–154. doi: 10.1016/s0969-8051(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 119.Aime S, Botta M, Fasano M, Marques MPM, Geraldes CFGC, Pubanz D, Merbach AE. Conformational and coordination equilibria on DOTA complexes of lanthanide metal ions in aqueous solution studied by 1H–NMR spectroscopy. Inorganic Chemistry. 1997;36:2059–2068. doi: 10.1021/ic961364o. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y, Martell AE, Reibenspies JH, Reichert DE, Welch MJ. Synthesis and characterization of racemic mixture and meso isomers of bis(trans-2-aminocyclohexyl)aminepentaacetic acid and the stabilities of their Gd(III) complexes. Inorganic Chemistry. 2000;39:1480–1486. doi: 10.1021/ic991189m. [DOI] [PubMed] [Google Scholar]
- 121.Li WP, Smith CJ, Cutler CS, Hoffman TJ, Ketring AR, Jurisson SS. Aminocarboxylate complexes and octreotide complexes with no carrier added 177Lu, 166Ho, and 149Pm. Nuclear Medicine and Biology. 2003;30:241–251. doi: 10.1016/s0969-8051(02)00418-3. [DOI] [PubMed] [Google Scholar]
- 122.Kobayashi H, Wu C, Yoo TM, Sun B-F, Drumm D, Pastan I, Paik CH, Gansow OA, Carrasquillo JA, Brechbiel MW. Evaluation of the in vivo biodistribution of yttrium-labeled Isomers of CHX-DTPA-conjugated monoclonal antibodies. Journal of Nuclear Medicine. 1998;39:829–836. [PubMed] [Google Scholar]
- 123.Kozak RW, Raubitschek A, Mirzadeh S, Brechbiel MW, Junghaus R, Gansow OA, Waldmann TA. Nature of the bifunctional chelating agent used for radioimmunotherapy with yttrium-90 monoclonal antibodies: Critical factors in determining in vivo survival and organ toxicity. Cancer Research. 1989;49:2639–2644. [PubMed] [Google Scholar]
- 124.McMurry TJ, Pippin CG, Wu C, Deal KA, Brechbiel MW, Mirzadeh S, Gansow OA. Physical parameters and biological stability of yttrium(III) diethylenetriaminepentaacetic acid derivative conjugates. Journal of Medicinal Chemistry. 1998;41:3546–3549. doi: 10.1021/jm980152t. [DOI] [PubMed] [Google Scholar]
- 125.Wu C, Brechbiel MW, Gansow OA, Kobayashi H, Carrasquillo JA, Pastan I. Stability of the four 2-(p-nitrobenzyl)-trans-CyDTPA 88Y complexes. Radiochimica Acta. 1997;79:123–126. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 126.Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radiometal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorganic & Medicinal Chemistry. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 127.Cheson BD. Radioimmunotherapy of non-Hodgkin lymphomas. Blood. 2003;101:391–398. doi: 10.1182/blood-2002-06-1793. [DOI] [PubMed] [Google Scholar]
- 128.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clinical Radiology. 2010;65:500–516. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brechbiel MW. Targeted alpha-therapy: past, present, future? Dalton Transactions. 2007:4918–4928. doi: 10.1039/b704726f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted alpha-particle therapy. The Journal of Nuclear Medicine. 2005;46:199S–204S. [PubMed] [Google Scholar]
- 131.Zalutsky MR, Pozzi OR. Radioimmunotherapy with alpha-particle emitting radionuclides. The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2004;48:289–296. [PubMed] [Google Scholar]
- 132.Song HA, Kang CS, Baidoo KE, Milenic DE, Chen Y, Dai A, Brechbiel MW, Chong H-S. Efficient bifunctional decadentate ligand 3p–C-DEPA for targeted α-radioimmunotherapy applications. Bioconjugate Chemistry. 2011;22:1128–1135. doi: 10.1021/bc100586y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holland JP, Williamson MJ, Lewis JS. Unconventional nuclides for radiopharmaceuticals. Molecular Imaging. 2010;9:1–20. [PMC free article] [PubMed] [Google Scholar]
- 134.Jurcic JG, Caron PC, Nikula TK, Papadopoulos EB, Finn RD, Gansow OA, Miller WH, Jr, Geerlings MW, Warrell RP, Jr, Larson SM, Scheinberg DA. Radiolabeled anti-CD33 monoclonal antibody M195 for myeloid leukemias. Cancer Research. 1995;55:5908S–5910S. [PubMed] [Google Scholar]
- 135.Wild D, Frischknecht M, Zhang H, Morgenstern A, Bruchertseifer F, Boisclair J, Provencher-Bolliger A, Reubi J-C, Maecke HR. Alpha- versus beta-particle radiopeptide therapy in a human prostate cancer model (213Bi-DOTA-PESIN and 213Bi-AMBA versus 177Lu-DOTA-PESIN) Cancer Research. 2011;71:1009–1018. doi: 10.1158/0008-5472.CAN-10-1186. [DOI] [PubMed] [Google Scholar]
- 136.Miederer M, Scheinberg DA, McDevitt MR. Realizing the potential of the actinium-225 radionuclide generator in targeted alpha particle therapy applications. Advanced Drug Delivery Reviews. 2008;60:1371–1382. doi: 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Couturier O, Supiot S, Degraef-Mougin M, Faivre-Chauvet A, Carlier T, Chatal J-F, Davodeau F, Cherel M. Cancer radioimmunotherapy with alpha-emitting nuclides. European Journal of Nuclear Medicine and Molecular Imaging. 2005;32:601–614. doi: 10.1007/s00259-005-1803-2. [DOI] [PubMed] [Google Scholar]
- 138.Henriksen G, Bruland OS, Larsen RH. Thorium and actinium polyphosphonate compounds as bone-seeking alpha particle-emitting agents. Anticancer Research. 2004;24:101–105. [PubMed] [Google Scholar]
- 139.Roesch F, Riss PJ. The Renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Current Topics in Medicinal Chemistry. 2010;10:1633–1668. doi: 10.2174/156802610793176738. [DOI] [PubMed] [Google Scholar]
- 140.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Transactions. 2011;40:6104–6111. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- 141.Berry DJ, Ma Y, Ballinger JR, Tavare R, Koers A, Sunassee K, Zhou T, Nawaz S, Mullen GED, Hider RC, Blower PJ. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chemical Communications. 2011;47:7068–7070. doi: 10.1039/c1cc12123e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Notni J, Šimeček J, Hermann P, Wester H-J. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chemistry - A European Journal. 2011;17:14718–14722. doi: 10.1002/chem.201103503. [DOI] [PubMed] [Google Scholar]
- 143.Rösch F. Radiolanthanides in endoradiotherapy: an overview. Radiochimica Acta. 2007;95:303–311. [Google Scholar]
- 144.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nuclear Medicine and Biology. 2009;36:729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for ImmunoPET of prostate-specific membrane antigen expression in vivo. The Journal of Nuclear Medicine. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, De Jong JR, Van Dongen GA, Schröder CP, Lub-De Hooge MN, De Vries EG. Biodistribution of 89Zr-trastuzumab and PET Imaging of HER2-positive lesions in patients with metastatic breast cancer. Clinical Pharmacology and Therapeutics. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 147.Holland JP, Caldas-Lopes E, Divilov V, Longo VA, Taldone T, Zatorska D, Chiosis G, Lewis JS. Measuring the pharmacodynamic effects of a novel HSP90 inhibitor on HER2/ neu expression in mice using 89Zr-DFO-trastuzumab. PLoS ONE. 2010;5:1–11. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCabe KE, Wu AM. Positive progress in ImmunoPET—not just a coincidence. Cancer Biotherapy & Radiopharmaceuticals. 2007;25:253–261. doi: 10.1089/cbr.2010.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E, Vandlen R, Darwish M, Junutula JR, Williams S-P, Marik J. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nuclear Medicine and Biology. 2010;37:289–297. doi: 10.1016/j.nucmedbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 150.Bottenus BN, Kan P, Jenkins T, Ballard B, Rold TL, Barnes C, Cutler C, Hoffman TJ, Green MA, Jurisson SS. Gold(III) bis-thiosemicarbazonato complexes: synthesis, characterization, radiochemistry and X-ray crystal structure analysis. Nuclear Medicine and Biology. 2010;37:41–49. doi: 10.1016/j.nucmedbio.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 151.Chanda N, Kan P, Watkinson LD, Shukla R, Zambre A, Carmack TL, Engelbrecht H, Lever JR, Katti K, Fent GM, Casteel SW, Smith CJ, Miller WH, Jurisson S, Boote E, Robertson JD, Cutler C, Dobrovolskaia M, Kannan R, Katti KV. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine: Nanotechnology, Biology, and Medicine. 2010;6:201–209. doi: 10.1016/j.nano.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 152.Kannan R, Rahing V, Cutler C, Pandrapragada R, Katti KK, Kattumuri V, Robertson JD, Casteel SJ, Jurisson S, Smith C, Boote E, Katti KV. Nanocompatible chemistry toward fabrication of target-specific gold nanoparticles. Journal of the American Chemical Society. 2006;128:11342–11343. doi: 10.1021/ja063280c. [DOI] [PubMed] [Google Scholar]
- 153.Katti KV, Kannan R, Katti K, Kattumori V, Pandrapraganda R, Rahing V, Cutler C, Boote EJ, Casteel SW, Smith CJ, Robertson JD, Jurisson SS. Hybrid gold nanoparticles in molecular imaging and radiotherapy. Czechoslovak Journal of Physics. 2006;56:D23–D34. [Google Scholar]
- 154.Helm L, Merbach AE. Applications of advanced experimental techniques: High pressure NMR and computer simulations. Dalton Transactions. 2002:633–641. [Google Scholar]
- 155.Rund JV, Basolo F, Pearson RG. Catalysis of substitution reactions of rhodium(III) complexes. The reaction of aquopentachlororhodate(III) ion with pyridine. Inorganic Chemistry. 1964;3:658–661. [Google Scholar]
- 156.Efe GE, Pillai MRA, Schlemper EO, Troutner DE. Rhodium complexes of two bidentate secondary amine oxime ligands and application to the labelling of proteins. Polyhedron. 1991;10:1617–1624. [Google Scholar]
- 157.Lo JM, Pillai MRA, John CS, Troutner DE. Radiochemical purity evaluation of rhodium-105 complexes by magnesium oxide. Applied Radiation and Isotopes. 1990;41:103–105. [Google Scholar]
- 158.Goswami N. Dissertation. University of Missouri; 1996. 105Rh(III) complexes with acyclic tetrathioether ligands: Potential radiotherapeutic agents. [Google Scholar]
- 159.Goswami N, Alberto R, Barnes CL, Jurisson S. Rhodium(III) complexes with acyclic tetrathioether ligands. Effects of backbone chain length on the conformation of the Rh(III) complex. Inorganic Chemistry. 1996;35:7546–7555. [Google Scholar]
- 160.Goswami N, Higginbotham C, Volkert W, Alberto R, Nef W, Jurisson S. Rhodium-105 tetrathioether complexes: radiochemistry and initial biological evaluation. Nuclear Medicine and Biology. 1999;26:951–957. doi: 10.1016/s0969-8051(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 161.Jurisson SS, Ketring AR, Volkert WA. Rhodium-105 complexes as potential radiotherapeutic agents. Transition Metal Chemistry. 1997;22:315–317. [Google Scholar]
- 162.Li N, Struttman M, Higginbotham C, Grall AJ, Skerlj JF, Vollano JF, Bridger SA, Ochrymowycz LA, Ketring AR, Abrams MJ, Volkert WA. Biodistribution of model 105Rh-labeled tetradentate thiamacrocycles in rats. Nuclear Medicine and Biology. 1997;24:85–92. doi: 10.1016/s0969-8051(96)00177-1. [DOI] [PubMed] [Google Scholar]
- 163.Venkatesh M, Goswami N, Volkert WA, Schlemper EO, Ketring AR, Barnes CL, Jurisson S. An Rh-105 complex of tetrathiacyclohexadecane diol with potential for formulating bifunctional chelates. Nuclear Medicine and Biology. 1996;23:33–40. doi: 10.1016/0969-8051(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 164.Akgun Z. Dissertation. University of Missouri; 2006. Synthesis and evaluation of 105Rhodium(III) complexes derived from diaminodithioether (DADTE) ligands. [Google Scholar]
- 165.Akgun Z, Engelbrecht H, Fan K-H, Barnes CL, Cutler CS, Jurisson SS, Lever SZ. The complexation of rhodium(III) with acyclic diaminedithioether (DADTE) ligands. Dalton Transactions. 2010;39:10169–10178. doi: 10.1039/c0dt00813c. [DOI] [PubMed] [Google Scholar]
- 166.Li N. Dissertation. University of Missouri; 1996. Synthesis and characterization of rhodium-105-labeled thiamacrocycles for use to formulate peptide receptor agents. [Google Scholar]
- 167.Li N, Eberlein CM, Volkert WA, Ochrymowycz L, Barnes C, Ketring AR. Comparisons of 105Rh(III) Chloride Complexation with [14]aneNS3, [14]aneN2S2 and [14]aneN4 Macrocycles in Aqueous Solution. Radiochimica Acta. 1996;75:83–95. [Google Scholar]
- 168.Cagnolini A, Ballard B, Engelbrecht HP, Rold TL, Barnes C, Cutler C, Hoffman TJ, Kannan R, Katti K, Jurisson SS. Tetradentate bis-phosphine ligands (P2N2 and P2S2) and their Rh(III), Ni(II) and 105Rh Complexes: X-ray crystal structures of trans-[RhCl2(L2)]PF6, [Ni(L2)](PF6)2 and µ-O2SO2-[Ni(L5)]2(PF6)2 . Nuclear Medicine and Biology. 2011;38:63–76. doi: 10.1016/j.nucmedbio.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 169.Jennings LE, Long NJ. 'Two is better than one'-probes for dual-modality molecular imaging. Chemical Communications. 2009:3511–3524. doi: 10.1039/b821903f. [DOI] [PubMed] [Google Scholar]
- 170.Pascu SI, Waghorn PA, Conry T, Lin B, James C, Zayed JM. Design considerations towards simultaneously radiolabeled and fluorescent imaging probes incorporating metallic species, in Advances in Inorganic Chemistry. In: van Rudi E, Colin DH, editors. Academic Press; 2009. pp. 131–178. [Google Scholar]
- 171.Huang W-Y, Davis JJ. Multimodality and nanoparticles in medical imaging. Dalton Transactions. 2011;40:6087–6103. doi: 10.1039/c0dt01656j. [DOI] [PMC free article] [PubMed] [Google Scholar]