Abstract
Purpose. One of the common side effects experienced by head and neck cancer patients on chemoradiotherapy is mucositis. Severe mucositis may be controllable by limiting cancer therapy, but it has resulted in decreasing the completion rate of chemoradiotherapy. The efficacy of royal jelly (RJ) as prophylaxis against chemoradiotherapy-induced mucositis was evaluated through clinical scoring of oral and pharyngeal mucositis. Methods. In this randomized, single-blind (physician-blind), clinical trial, 13 patients with head and neck cancer requiring chemoradiation were randomly assigned to two groups. Seven patients assigned to the study group received RJ, and 6 patients were assigned to the control group. RJ group patients took RJ three times per day during treatment. The patients in both groups were evaluated twice a week for the development of mucositis using Common Terminology Criteria for Adverse Events version 3.0. Results. A significant reduction in mucositis was seen among RJ-treated patients compared with controls (P < 0.001). Conclusion. This study demonstrated that prophylactic use of RJ was effective in reducing mucositis induced by chemoradiotherapy in head and neck cancer patients. However, further studies are needed because of the small sample size and the absence of double blinding.
1. Introduction
Chemoradiotherapy for head and neck cancer induces mucositis, and it can also cause ulcers. Patients may experience pain, dysphagia, and dysphonia. As patients lose their oral feeding ability, they require external nutrition support. Strong early side effects of chemoradiotherapy may be controllable by limiting cancer therapy, but this has resulted in decreasing the chemoradiotherapy completion rate.
Many authors have reported the prophylactic use of bee products such as honey, royal jelly (RJ), and propolis for oral mucositis [1–7]. Kohno et al. reported the prophylactic use of honey extract as concurrent chemoradiotherapy for head and neck cancer patients [8]. Suemaru et al. also evaluated their effects on 5-fluorouracil-induced experimental oral mucositis in hamsters [9]. Erdem and Gungormus evaluated the effect of RJ on oral mucositis in patients undergoing radiotherapy and chemotherapy, and they reported that the mean time to resolution of oral mucositis was significantly shorter in the RJ group than in the control group [10].
The results suggested that the topical application of royal jelly may have a healing effect on severe oral mucositis induced by chemotherapy. Therefore, the efficacy of RJ as prophylaxis against chemoradiotherapy-induced mucositis was evaluated.
2. Materials and Methods
2.1. Design
The objective of this study was to evaluate the efficacy of RJ as prophylaxis against chemoradiotherapy-induced mucositis through clinical scoring of oral and pharyngeal mucositis in head and neck cancer patients.
This study was approved by the Ethics Committee of Kyorin University. All patients provided their written, informed consent. Head and neck squamous cell carcinoma patients were enrolled. Eligible patients were aged >18 years with a performance status of 0 to 1. Patients were randomly assigned to the control and RJ groups in this single-blind (physician-blind), clinical trial.
2.2. Induction Chemotherapy
The regimen was as follows. Nedaplatin (80 mg/m2) was administered on day 1, and S-1 was simultaneously administered to patients orally twice daily at an initial dose of 65 mg/m2/day (patients with body surface area (BSA) > 1.5 m2 received 100 mg/day; patients with 1.25 m2 < BSA < 1.5 m2 received 80 mg/day) for 2 weeks (days 1–14).
2.3. Concomitant Chemotherapy and Radiotherapy
Three chemotherapy regimens were used.
Weekly Nedaplatin and Docetaxel Regimen. During radiotherapy, weekly nedaplatin (15 mg/m2) and docetaxel (10 mg/m2) were administered for 6 courses intravenously.
S-1 Regimen. S-1 was administered at a dose of 80 mg/day on alternate days for 6 weeks.
Cisplatin Regimen. This was a form of selective arterial chemotherapy. Cisplatin (5 mg/m2) was administered with the catheter in the vessel feeding the tumor on Monday to Friday (5 days/week) for 6 weeks.
Radiotherapy was administered to both groups: 2.0 Gy/day fractions on Monday to Friday for 33 to 35 fractions, for a total dose of 66 to 70 Gy by Linac.
2.4. RJ Group
RJ was prepared by Yamada Apiculture Center, Inc. (Okayama, Japan). RJ was collected from Apis mellifera L. that fed primarily on nectar and pollen from several flowers in Zhejiang, China. This product complies with the organic standards of the European Union. The product name is “organic royal jelly-gen nyu.” It has the consistency of an ointment and contains a 1 gram measuring spoon that patients used to apply the RJ. The RJ group took 1 gram of RJ three times a day (3 g/day) during radiation treatment.
2.5. Control Group
The control group did not take any RJ.
2.6. Evaluation
Evaluation was done during the radiation period and 1 month after radiation. Patients were evaluated twice a week from the mouth to the pharynx by inspection and fiberscope examination. The reaction of the mucosa was graded using the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE). The mucositis was graded as follows: Grade 1, erythema of the mucosa; Grade 2, patchy ulcerations or pseudomembranes; Grade 3, confluent ulcerations or pseudomembranes, bleeding with minor trauma; Grade 4, tissue necrosis, significant spontaneous bleeding, life-threatening consequences; and Grade 5, death.
2.7. Statistical Analysis
Data are shown as means ± standard deviation. Statistical significance was analyzed using the nonparametric Mann-Whitney U test for 2 groups. P < 0.05 was considered significant.
3. Results
3.1. Patients' Characteristics
This study was done at Kyorin University Hospital, Japan, between 2009 and 2010. Thirteen patients (12 males, 1 female; median age 65.0 years; age range, 51–84 years) diagnosed with head and neck cancer were enrolled in the trial. The primary cancer site was hypopharyngeal in 5 patients, oropharyngeal in 4, laryngeal in 2, oral cavity in 1, and maxillary sinus in 1. The patients' characteristics are shown in Table 1. Seven patients were assigned to the RJ group and 6 to the control group.
Table 1.
Patients' profile.
| Case | Group | Tumor site | Sex | Age (year) | TNM stage | IC | CRT |
|---|---|---|---|---|---|---|---|
| 1 | RJ | Larynx | M | 59 | T2N0M0 | — | S-1 |
| 2 | RJ | Oropharynx | M | 84 | T3N2cM0 | — | S-1 |
| 3 | RJ | Hypopharynx | M | 62 | TXN3M0 | S1 + N | N + T |
| 4 | RJ | Hypopharynx | M | 58 | T1N3M0 | S1 + N | N + T |
| 5 | RJ | Larynx | F | 75 | T2N0M0 | — | S-1 |
| 6 | RJ | Oropharynx | M | 65 | T4aN2bM0 | — | CDDP |
| 7 | RJ | Hypopharynx | M | 51 | T2N2bM0 | — | N + T |
| 8 | CONT | Hypopharynx | M | 84 | T2N0M0 | — | S-1 |
| 9 | CONT | Oropharynx | M | 63 | T1N2bM0 | — | N + T |
| 10 | CONT | Oropharynx | M | 62 | T1N2aM0 | — | CDDP |
| 11 | CONT | Oropharynx | M | 65 | T4aN0M0 | S1 + N | N + T |
| 12 | CONT | Hypopharynx | M | 66 | T2N1M0 | S1 + N | S-1 |
| 13 | CONT | Maxillary sinus | M | 78 | T4aN0M0 | — | CDDP |
RJ: royal jelly, CONT: control, M: male, and F: female.
IC: induction chemotherapy.
CRT: chemoradiotherapy.
S1 + N: S1 + nedaplatin.
S1: S-1 regimen.
N + T: Weekly nedaplatin and docetaxel regimen.
CDDP: Cisplatin regimen.
There were no side effects such as allergy, irritation, or toxicity during treatment. All patients of both groups completed planned chemoradiotherapy.
3.2. Mucositis
Four patients received induction chemotherapy. All had no sign of mucositis at the beginning of concomitant chemoradiotherapy.
At the end of radiation, in the RJ group, Grade 3 mucositis was observed in 71.4% (5/7), and Grade 2 was seen in 28.6% (2/7). In the control group, Grade 3 mucositis was seen in 100% (6/6). In the control group, one case progressed to Grade 4 one month after treatment.
Figure 1 shows the grades of mucositis at the beginning of radiation, after 20 Gy, after 40 Gy, at the end of radiation, and 1 month after radiation. A significant difference was observed between the groups in the grade of mucositis at each point.
Figure 1.
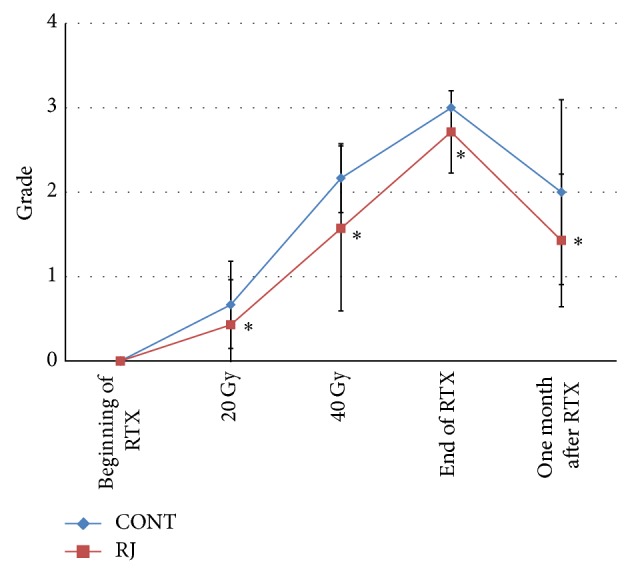
Grades of mucositis. Each data point represents the mean ± SD. Mann-Whitney U test: * P < 0.001 versus control. RTX: radiotherapy.
Figure 2 shows the average time to progress to Grade 2 mucositis from the beginning of radiation in both groups. The average time was 25.9 ± 9.6 days in the RJ group and 19.0 ± 4.1 days in the control group. The average time was significantly longer in the RJ group (P < 0.001).
Figure 2.
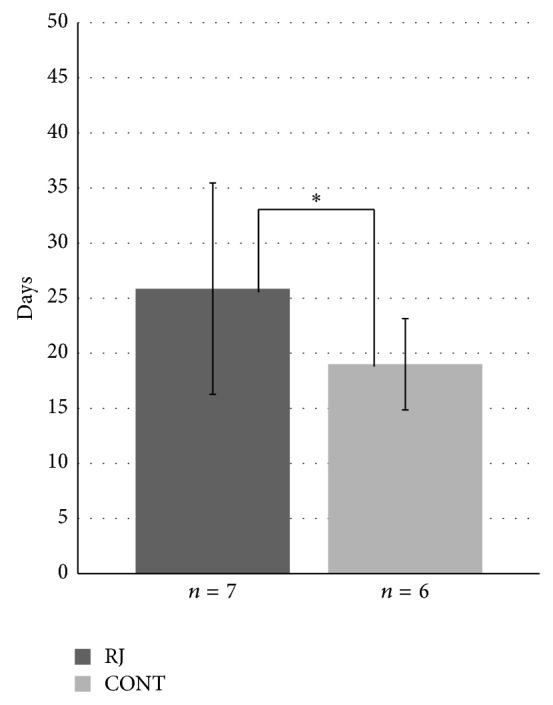
Time to progress to G2 mucositis. Each data point represents the mean ± SD. Mann-Whitney U test: * P < 0.001 versus control.
Figure 3 shows the average time to progress to grade 3 mucositis from the beginning of radiation in both groups. The average time was 37.4 ± 11.8 days in the RJ group and 31.0 ± 5.8 days in the control group. The average time was significantly longer in the RJ group (P < 0.001).
Figure 3.
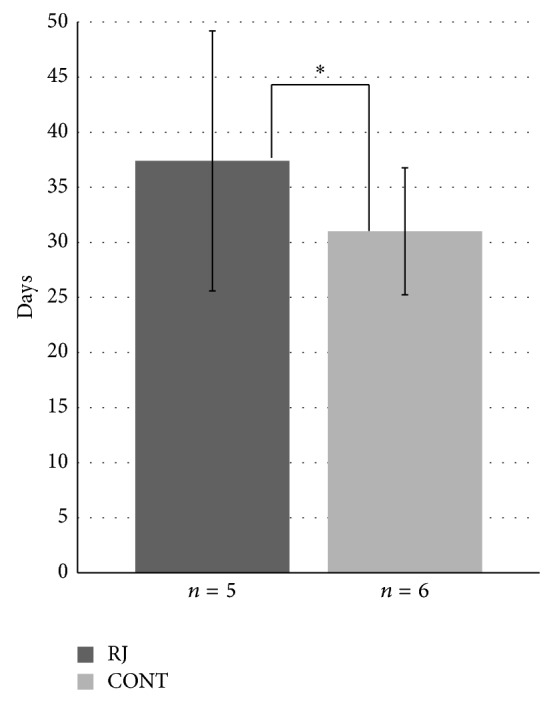
Time to progress to G3 mucositis. Each data point represents the mean ± SD. Mann-Whitney U test: * P < 0.001 versus control.
The results shown in Figures 1 and 2 suggest that applying RJ may prevent or reduce the severity of chemoradiotherapy-induced mucositis.
4. Discussion
Oral mucositis occurs in 15–40% of patients receiving standard chemotherapy, and 100% of patients receiving radiation therapy for head and neck cancer develop oral mucositis of varying degrees [11, 12]. The mechanism by which mucositis occurs is based on the fact that the oral mucosa has a high level of mitotic activity and high cell turnover. Due to the high degree of cell desquamation, there is a continuous need for cell multiplication to recover the oral mucosa. Tissues with high levels of mitotic activity respond rapidly to radiation, since the most sensitive phases of the cell cycle are G2 and mitosis. Thus, the mucosa is rapidly affected [13]. The same is true for chemotherapeutic drugs such as cisplatin, S-1, nedaplatin, and docetaxel. Chemoradiotherapy causes various changes in normal tissues, depending on the closely interrelated factors of total dose, fractionation schedule, and volume treated. Until now, there has been no way to prevent chemoradiotherapy-induced mucositis using only gargling and analgesics.
Bee products are commonly used traditionally to treat not only mucositis, but also skin disorders like cuts and burns as traditional wound healing agents [14–17]. Bee products are not artificial, but natural resources. This point results in easy acceptance among many cancer patients because they must always take so many artificial medicines. Therefore, this study evaluated the efficacy of bee products for mucositis induced by chemoradiotherapy.
The first issue that needed to be resolved was which kind of bee product would be best for mucositis. Suemaru et al. already evaluated three bee products (honey, RJ, and propolis) for 5-fluorouracil-induced experimental oral mucositis in hamsters [9]. They reported that only the RJ ointments significantly improved recovery from chemotherapy-induced mucositis in a dose-dependent manner. These results suggested that topical application of RJ has a healing effect on severe oral mucositis induced by chemotherapy. This is the reason why RJ was selected for this study.
RJ is mainly secreted by the hypopharyngeal and mandibular glands of worker honeybees between the sixth and twelfth days of their life, and it is an essential food for the development of the queen honeybee. RJ is a complex substance containing a unique combination of proteins (12–15%), sugars (10–12%), lipids (3–7%), amino acids, vitamins, and minerals [18]. RJ has also been demonstrated to possess many pharmacological activities in experimental animals, including antitumor [19], antioxidant [20, 21], anti-inflammatory [22], antibacterial [23], antiallergic [24], antiaging [25], and antihypertensive properties [26]. Recently, many authors have reported that the antioxidant effect is important for wound healing [27–29]. In this respect, RJ is an ideal agent. Inoue et al. reported the effect of dietary RJ on tissue DNA oxidative damage in mice [20]. In mice that were fed a dietary supplement of RJ, the levels of a marker of oxidative stress, 8-hydroxy-2-deoxyguanosine, were significantly reduced in kidney DNA and serum.
Furthermore, Kohno et al. suggested that RJ has anti-inflammatory actions through inhibiting proinflammatory cytokine production by activated macrophages [22]. They named the factor honeybee RJ-derived anti-inflammatory factor.
Watanabe et al. reported that RJ showed scavenging activity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, superoxide radicals, and hydroxylradicals. Therefore, in the healing effect of RJ on mucositis, radical scavenging activity is more important than keratinocyte growth factor generation [30].
RJ also has antibacterial actions. Royalisin found in the RJ of Apis mellifera is an antimicrobial peptide. It plays an important role in protecting wounds from being infected [23].
The present study showed that RJ prevented progression of mucositis from the early phase, and the average time to progress to Grade 2 mucositis was 25.9 ± 9.6 days versus 19.0 ± 4.1 days (RJ versus control). The average time to progress to Grade 3 was 37.4 ± 11.8 days versus 31.0 ± 5.8 days (RJ versus control). A significant reduction in mucositis occurred among RJ-treated patients compared with controls (P < 0.001).
At the end of radiation, Grade 3 mucositis was observed in 71.4% (5/7) in the RJ group and 100% (6/6) in the control group. These results suggest that topical application of RJ is effective in preventing accelerated mucositis induced by chemoradiotherapy.
In this study, only RJ was evaluated for mucositis, but Nakajima et al. reported that, of all bee products, propolis is the most powerful antioxidant [21]. An antioxidant effect is important in the mucositis healing process. Although we would have liked to evaluate propolis, propolis extracted with water was not available. Propolis extracted with ethanol is not good for mucositis due to stimulation by alcohol. In the future, we would like to evaluate propolis extracted with water.
Thus, further studies are needed to evaluate the effect of RJ on mucositis and elucidate the precise mechanisms of action. Nevertheless, it is possible to say that RJ tends to prevent progression of mucositis.
Acknowledgment
This research was supported by Yamada Research Grant (2009) from Yamada Bee Farm in Japan.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Abdulrhman M., Samir El Barbary N., Ahmed Amin D., Saeid Ebrahim R. Honey and a mixture of honey, beeswax, and olive oilpropolis extract in treatment of chemotherapy-induced oral mucositis: a randomized controlled pilot study. Pediatric Hematology and Oncology. 2012;29(3):285–292. doi: 10.3109/08880018.2012.669026. [DOI] [PubMed] [Google Scholar]
- 2.Bardy J., Molassiotis A., Ryder W. D., Mais K., Sykes A., Yap B., Lee L., Kaczmarski E., Slevin N. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. British Journal of Oral and Maxillofacial Surgery. 2012;50(3):221–226. doi: 10.1016/j.bjoms.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B. M., Zakaria A., Ahmad N. M. Topical application of honey in the management of radiation mucositis: a preliminary study. Supportive Care in Cancer. 2003;11(4):242–248. doi: 10.1007/s00520-003-0443-y. [DOI] [PubMed] [Google Scholar]
- 4.Khanal B., Baliga M., Uppal N. Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. International Journal of Oral and Maxillofacial Surgery. 2010;39(12):1181–1185. doi: 10.1016/j.ijom.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Motallebnejad M., Akram S., Moghadamnia A., Moulana Z., Omidi S. The effect of topical application of pure honey on radiation-induced mucositis: a randomized clinical trial. Journal of Contemporary Dental Practice. 2008;9(3):40–47. [PubMed] [Google Scholar]
- 6.Rashad U. M., Al-Gezawy S. M., El-Gezawy E., Azzaz A. N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. Journal of Laryngology and Otology. 2009;123(2):223–228. doi: 10.1017/S0022215108002478. [DOI] [PubMed] [Google Scholar]
- 7.Song J. J., Twumasi-Ankrah P., Salcido R. Systematic review and meta-analysis on the use of honey to protect from the effects of radiation-induced oral mucositis. Advances in Skin and Wound Care. 2012;25(1):23–28. doi: 10.1097/01.ASW.0000410687.14363.a3. [DOI] [PubMed] [Google Scholar]
- 8.Kohno N., Kitahara S., Tamura E., Tanabe T. Concurrent chemoradiotherapy with low-dose cisplatin plus 5-fluorouracil for the treatment of patients with unresectable head and neck cancer. Oncology. 2002;63(3):226–231. doi: 10.1159/000065469. [DOI] [PubMed] [Google Scholar]
- 9.Suemaru K., Cui R., Li B., Watanabe S., Okihara K., Hashimoto K., Yamada H., Araki H. Topical application of royal jelly has a healing effect on 5-fluorouracil-induced experimental oral mucositis in hamsters. Methods and Findings in Experimental and Clinical Pharmacology. 2008;30(2):103–106. doi: 10.1358/mf.2008.30.2.1159655. [DOI] [PubMed] [Google Scholar]
- 10.Erdem O., Gungormus Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holistic Nursing Practice. 2014;28:242–246. doi: 10.1097/HNP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 11.Das D., Agarwal S. K., Chandola H. M. Protective effect of Yashtimadhu (Glycyrrhiza glabra) against side effects of radiation/chemotherapy in head and neck malignancies. Ayu. 2012;32:196–199. doi: 10.4103/0974-8520.92579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suresh A. V. S., Varma P. P., Sinha S., Deepika S., Raman R., Srinivasan M., Mandapal T., Reddy C. O., Anand B. B. Risk-scoring system for predicting mucositis in patients of head and neck cancer receiving concurrent chemoradiotherapy [rssm-hn] Journal of Cancer Research and Therapeutics. 2010;6(4):448–451. doi: 10.4103/0973-1482.77100. [DOI] [PubMed] [Google Scholar]
- 13.Santos R. C. S., Dias R. S., Giordani A. J., Segreto R. A., Segreto H. R. C. Mucositis in head and neck cancer patients undergoing radiochemotherapy. Revista da Escola de Enfermagem. 2011;45(6):1338–1344. doi: 10.1590/S0080-62342011000600009. [DOI] [PubMed] [Google Scholar]
- 14.Boekema B. K., Pool L., Ulrich M. M. The effect of a honey based gel and silver sulphadiazine on bacterial infections of in vitro burn wounds. Burns. 2013;39(4):754–759. doi: 10.1016/j.burns.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Farsaei S., Khalili H., Farboud E. S. Potential role of statins on wound healing: review of the literature. International Wound Journal. 2012;9(3):238–247. doi: 10.1111/j.1742-481X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S. S., Singh O., Bhagel P. S., Moses S., Shukla S., Mathur R. K. Honey dressing versus silver sulfadiazene dressing for wound healing in burn patients: a retrospective study. Journal of Cutaneous and Aesthetic Surgery. 2011;4(3):183–187. doi: 10.4103/0974-2077.91249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan M. K., Hasan Adli D. S., Tumiran M. A., Abdulla M. A., Yusoff K. M. The efficacy of Gelam honey dressing towards excisional wound healing. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/805932.805932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita H., Ikeda T., Kajita K., Fujioka K., Mori I., Okada H., Uno Y., Ishizuka T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutrition Journal. 2012;11(1, article 77) doi: 10.1186/1475-2891-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend G. F., Morgan J. F., Tolnai S., Hazlett B., Morton H. J., Shuel R. W. Studies on the in vitro antitumor activity of fatty acids. I. 10-Hydroxy-2-decenoic acid from royal jelly. Cancer research. 1960;20:503–510. [PubMed] [Google Scholar]
- 20.Inoue S.-I., Koya-Miyata S., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Royal Jelly prolongs the life span of C3H/HeJ mice: correlation with reduced DNA damage. Experimental Gerontology. 2003;38(9):965–969. doi: 10.1016/S0531-5565(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima Y., Tsuruma K., Shimazawa M., Mishima S., Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complementary and Alternative Medicine. 2009;9, article 4 doi: 10.1186/1472-6882-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohno K., Okamoto I., Sano O., Arai N., Iwaki K., Ikeda M., Kurimoto M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Bioscience, Biotechnology and Biochemistry. 2004;68(1):138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 23.Tseng J.-M., Huang J.-R., Huang H.-C., Tzen J. T. C., Chou W.-M., Peng C.-C. Facilitative production of an antimicrobial peptide royalisin and its antibody via an artificial oil-body system. Biotechnology Progress. 2011;27(1):153–161. doi: 10.1002/btpr.528. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto I., Taniguchi Y., Kunikata T., Kohno K., Iwaki K., Ikeda M., Kurimoto M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sciences. 2003;73(16):2029–2045. doi: 10.1016/S0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- 25.Park H. M., Cho M. H., Cho Y., Kim S. Y. Royal jelly increases collagen production in rat skin after ovariectomy. Journal of Medicinal Food. 2012;15(6):568–575. doi: 10.1089/jmf.2011.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokunaga K.-H., Yoshida C., Suzuki K.-M., Maruyama H., Futamura Y., Araki Y., Mishima S. Antihypertensive effect of peptides from Royal Jelly in spontaneously hypertensive rats. Biological and Pharmaceutical Bulletin. 2004;27(2):189–192. doi: 10.1248/bpb.27.189. [DOI] [PubMed] [Google Scholar]
- 27.Deniz M., Borman H., Seyhan T., Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 2013;39(2):320–325. doi: 10.1016/j.burns.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Hsiao C.-Y., Hung C.-Y., Tsai T.-H., Chak K.-F. A study of the wound healing mechanism of a traditional chinese medicine, Angelica sinensis, using a proteomic approach. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/467531.467531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y.-H., Chang J.-J., Chien C.-T., Yang M.-C., Chien H.-F. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Experimental Diabetes Research. 2012;2012 doi: 10.1155/2012/504693.504693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe S., Suemaru K., Takechi K., Kaji H., Imai K., Araki H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil-induced oral mucositis. Journal of Pharmacological Sciences. 2013;121(2):110–118. doi: 10.1254/jphs.12181FP. [DOI] [PubMed] [Google Scholar]


