Abstract
Aging is associated with common conditions, including cancer, diabetes, cardiovascular disease, and Alzheimer's disease. The type of multi-targeted pharmacological approach necessary to address a complex multifaceteddisease such as aging might take advantage of pleiotropic natural polyphenols affecting a wide variety of biological processes. We have recently postulated that the secoiridoids oleuropein aglycone (OA) and decarboxymethyl oleuropein aglycone (DOA), two complex polyphenols present in health-promoting extra virgin olive oil (EVOO), might constitute anew family of plant-produced gerosuppressant agents. This paper describes an analysis of the biological activity spectra (BAS) of OA and DOA using PASS (Prediction of Activity Spectra for Substances) software. PASS can predict thousands of biological activities, as the BAS of a compound is an intrinsic property that is largely dependent on the compound's structure and reflects pharmacological effects, physiological and biochemical mechanisms of action, and specific toxicities. Using Pharmaexpert, a tool that analyzes the PASS-predicted BAS of substances based on thousands of “mechanism-effect” and “effect-mechanism” relationships, we illuminate hypothesis-generating pharmacological effects, mechanisms of action, and targets that might underlie the anti-aging/anti-cancer activities of the gerosuppressant EVOO oleuropeins.
Keywords: PASS, Biological activity spectra, oleuropein aglycone, decarboxymethyl oleuropein aglycone, cancer, aging
INTRODUCTION
The inter-species hormesis, or xenohormesis, hypothesis originally proposed by Dr. David A. Sinclair [1, 2] states that stress-induced synthesis of plant polyphenols and many other phytochemicals provides an environmental chemical signature that upregulates stress resistance pathways in plant consumers, including humans [1-5]. The existence of xenohormesis might explain how chemical compounds produced by plants and other autotrophs to defend against adverse environmental conditions can generate beneficial effects in the heterotrophs (animals and fungi) that consume them. Thus, we can take advantage of the healthy benefits that are chemically encrypted within plant-derived biocompounds. The natural polyphenolic compound resveratrol (3,5,4′-trihydroxystilbene) has emerged as an archetypal xenohormetic mediator of longevity that clearly delays or attenuates many age-related chronic diseases in animal models [6-12].
Dr. Mikhail V. Blagosklonny has recently proposed that “hormesis does not make sense except in the light of TOR-driven aging” [6]. In this scenario, aging-related disease (e.g., atherosclerosis, diabetes, cancer, and other diseases) can be understood as the product of synergistic interactions between our evolutionary path to sedentarism, which increases a number of gero-promoting factors (e.g., nutrients, growth factors, cytokines, insulin) that upregulate key gerogenes (e.g., the nutrient-sensing mammalian target of rapamycin [mTOR]), and the “defective design” of central energy metabolism sensors that function either as metabolic gerogenes (e.g., mTOR) or metabolic gerosuppressors (e.g., AMP-activated protein kinase [AMPK], which antagonizes the gerogenic activity of mTOR) [13-29]. This “defective design” refers to (a) the ability of metabolic gerogenes to continue, in an aimless but harmful manner, a developmental program that was beneficial early in life but was not switched off upon its completion and therefore drives aging; and (b) the weakness of the metabolic gerosuppressors that antagonize the metabolo-gerogenic pathway. In this metabolic framework for aging-related diseases, upregulation of metabolic gerogenes limits lifespan by accelerating age-related diseases, whereas the responsiveness of metabolo-gerosuppressor signaling should decline with aging because robust and continuous activation of metabolic suppressors in response to metabolic stresses results in accelerated aging [30, 31]. In other words, the ability of metabolic gerogenes to drive aging can be triggered or accelerated by the loss of responsiveness of critical metabolic gerosuppressors to their appropriate activation.
If the upregulation of metabolic gerogenes limits lifespan by accelerating the progression of age-related diseases, such as atherosclerosis or cancer, the behavioral and/or pharmacological suppression of metabolic gerogene-driven aging (e.g., via non-permanent activation of metabolic gerosuppressors) should increase healthy lifespan. We have recently explored, for the first time, the putative AMPK/mTOR-related xenohormetic nature of complex polyphenols that are naturally present in extra virgin olive oil (EVOO), a pivotal component of the Mediterranean-style diet that has been repeatedly associated with a reduction in age-related morbidity and a longer life expectancy [29]. Using a crude EVOO phenolic extract that is highly enriched in the secoiridoids oleuropein aglycone (OA) and decarboxymethyl oleuropein aglycone (DOA), we have shown that EVOO oleuropeins, which provide an effective defense against the attack of plants by herbivores and pathogens, are bona fide xenohormetins that are able to activate the gerosuppressor AMPK and trigger numerous resveratrol-like anti-aging transcriptomic signatures in biologically aggressive cancer cells. As such, we postulated that EVOO secoiridoids constitute a new family of plant-produced gerosuppressant agents that molecularly “repair” the aimless (and harmful) AMPK/mTOR-driven quasi-program that leads to aging and aging-related diseases, including cancer [29, 32].
As a compound's biological activity spectrum (BAS) is an intrinsic property that is representative of different pharmacological effects, physiological and biochemical mechanisms of action, and specific toxicities, and the BAS is largely dependent on the structural nature of a compound [33-43], we recently hypothesized that computerized prediction of BAS might help us to elucidate hypothesis-generating pharmacological effects, mechanisms of action, and targets underlying the anti-aging/anti-cancer activity of gerosuppressant oleuropeins present in EVOO. Here, we employed Prediction of Activity Spectra for Substances (PASS) software [33-43] (www.pharmaexpert.ru/passonline/index.php), which is hosted by the V. N. Orechovich Institute of Biomedical Chemistry (www.ibmc.msk.ru/en) under the aegis of the Russian Foundation of Basic Research. Using Pharmaexpert (www.genexplain.com/pharmaexpert), a tool developed to analyze the BAS of substances predicted by PASS, we characterized EVOO oleuropeins with different types of biological activities based on multiple mechanisms of action, analyses of activity-activity relationships, and drug-drug interactions. We now report on how a multi-targeted pharmacological approach needed for a complex multifaceted disease, such as aging, might take advantage of EVOO-derived polyphenols, pleiotropically impacting a wide variety of biological processes.
RESULTS
Description of the chemical structures of EVOO oleuropeins
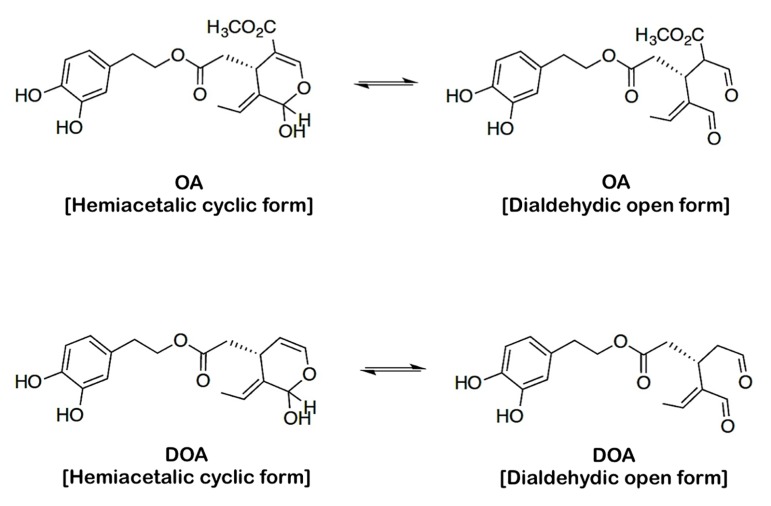
OA and DOA are structurally related secoiridoids with identical dihydroxylated aromatic moieties that differ only due to the presence of a methoxycarbonyl group on C-5 of the dihydropyrane ring of OA (Fig. 1). As the PASS tool interprets the BAS based on the 2D structure of molecules, the hemiacetalic (“cyclic”) and dialdehidic (“open”) structures of OA and DOA were drawn using ACD/ChemSketch version 12, then saved as MDL Molfiles (*.mol) and directly uploaded into the PASS prediction program to predict the biologically active spectra of the molecules.
Figure 1. Structures of oleuropein aglycone (OA) and decarboxymethyl oleuropein aglycone (DOA).
PASS estimation of the BAS of EVOO oleuropeins
Predictions were made using the PASS12 refined version of the program (www.genexplain.com/pass), which predicts 1,105 types of biological activities with a mean prediction accuracy in leave-one-out cross-validation (LOOCV) of 96%. PASS prediction tools are constructed using principal compounds from the MDDR database (produced by Accelrys and Prous Science), which is continuously updated with biologically relevant compounds. The PASS training set consisted of 287,633 known biologically active substances (e.g., drugs, drug-candidates, lead compounds, toxic compounds) compiled from various sources, including publications, patents, chemical databases, and “gray” literature [33-43].
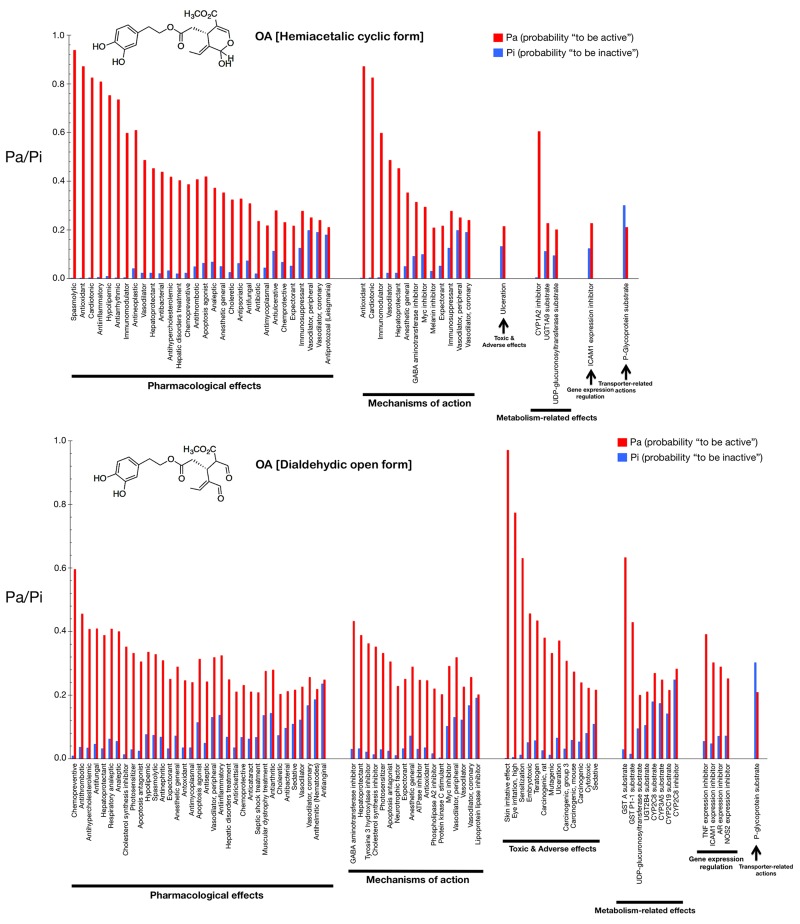
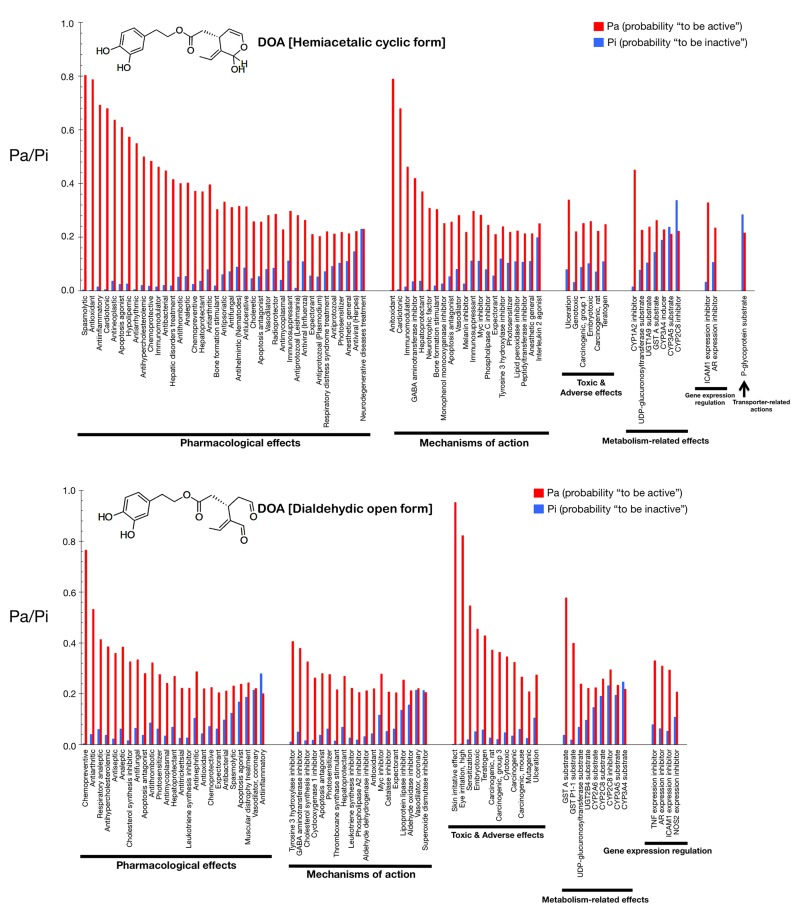
Figs. 2 and 3 show only the activities predicted at a Pa (probability “to be active”) > 0.200 for the hemiacetalic and dialdehidic forms of OA (Fig. 2) and DOA (Fig. 3), grouped using PharmaExpert (www.genexplain.com/pharmaexpert), a tool that was developed to analyze the biological activity spectra of substances predicted by PASS. PharmaExpert software analyzes the relationships between biological activities (“mechanism-effect(s)” and “effect-mechanism(s)”), identifies probable drug-drug interactions, and searches for compounds acting on multiple targets [33-43].
Figure 2. Biological activity spectra of the gerosuppressant olive oil oleuropein OA.
The results of predicted activity spectra generated by PASS are presented as a bar graph of biological activities with the probabilities “to be active” (Pa) and “to be inactive” (Pi) calculated for each activity. The values vary from 0.000 to 1.000; the higher a Pa value is the lower is the predicted probability of obtaining false positives in biological testing. The lists are arranged in descending order of Pa-Pi; therefore, more probable biological activities are at the top of the list. The list can be shortened at any desirable cutoff value, but PASS uses the criteria Pa=Pi as the as the default threshold, i.e., only biological activities with Pa > Pi are considered as probable for a particular compound. If we choose to use rather high value of Pa as cutoff for selection of probable activities, the chance to confirm the predicted activities is high too, but many existing activities will be lost. For instance, if one selects for consideration particular biological activities predicted with Pa > 0.9, then about 90% of actual activities will be lost (i.e., the expected probability to find inactive compounds in the selected set is very low but about 90% of active compounds will be missed). If one lowers the Pa threshold to 0.8, the probability to find inactive compounds is still low, but about 80% of active compounds will be missed, etc. Another important aspect of PASS predictions is the compounds' novelty. If one limits to high Pa values, one may find close analogues of known biologically active substances among the tested compounds. For instance, for Pa > 0.7, the chance to experimentally find the biological activity is high, but some of the activities may be close analogue of known pharmaceutical agents. If one chooses 0.5<Pa<0.7 values, the chances of obtaining activity in the experiment are lower, but the compound may be less similar to known pharmaceutical agents. For Pa < 0.5, the chances of obtaining activity in the experiment are even lower, but if activity is found, the compound might happen to be a new chemical entity. Nevertheless, it is important to keep in mind that the probability Pa reflects the similarity of a molecule under prediction with the structures of molecules, which are the most typical in a sub-set of “actives” in the training set. Therefore, there is no usually direct correlation between the Pa vales and quantitative characteristics of biological activities.
Figure 3. Biological activity spectra of the gerosuppressant olive oil oleuropein DOA.
(see Fig. 2 text for details).
Different types of biological activities are divided into six classes: mechanisms of action, pharmacological effects, metabolism-related actions, transporter terms, gene expression terms, and toxic/adverse effects.
The mechanisms of action reflect the interactions of biologically active compounds with biological entities at the macromolecular level. In addition to the expected antioxidant mechanism of action of OA (Pa = 0.872), PASS predicts some new mechanisms for the cyclic hemiacetalic form of OA, including cardiotonic (Pa = 0.826), immunomodulator (Pa = 0.598), vasodilator (Pa = 0.487), and hepatoprotectant (Pa = 0.453) activities. The cyclic hemiacetalic form of DOA is similarly predicted to exhibit an expected antioxidant mechanism of action (Pa = 0.790) while lacking all of the cardiotonic, immunomodulator, and hepatoprotectant mechanisms predicted for the cyclic hemiacetalic form of OA.
The open dialdehydic form of OA is predicted to lack all of the cardiotonic, immunomodulator, and hepatoprotectant mechanisms of action suggested for the cyclic hemiacetalic form of OA. Moreover, the expected antioxidant mechanism of OA is notably reduced when PASS predictions are used to estimate the probable mechanisms of action of the open dialdehydic form of OA (Pa = 0.247). The top predicted mechanism of action for the open dialdehydic form of OA is GABA aminotransferase inhibitor activity (Pa = 0.433). The open dialdehydic form of DOA is predicted to lack (Pa = 0.221) the highly predicted antioxidant mechanism of action of the cyclic hemiacetalic form of DOA, while gaining new mechanisms such as acting as a tyrosine 3 hydroxylase inhibitor (Pa = 0.407) and cholesterol synthesis inhibitor (Pa = 0.380).
The pharmacological effects reflect the pharmacological action or pharmacotherapeutic application of the compound. Some of the most likely anti-aging/anti-cancer pharmacological effects, showing higher Pa values in the predictions for the cyclic hemiacetalic form of OA, include anti-antioxidant (Pa = 0.872), anti-inflammatory (Pa = 0.809), anti-neoplastic (Pa = 0.610), apoptosis agonist (Pa = 0.419), and chemopreventive (Pa = 0.387) effects. The cyclic hemiacetalic form of DOA conserves all the anti-aging/anti-cancer pharmacological effects observed in the prediction for the cyclic hemiacetalic form OA, but displaying somewhat lower Pa values (i.e., anti-oxidant [Pa = 0.790], anti-inflammatory [Pa = 0.693], and anti-neoplastic [Pa = 0.637] effects). The sole exception is the apoptosis agonist effect, which is predicted to occur with a higher probability (Pa = 0.610) for the cyclic hemiacetalic form of DOA.
Remarkably, the open dialdehydic form of OA exclusively conserves the chemopreventive effect of the cyclic hemiacetalic form of OA, and with an even higher Pa value (0.596). Similarly, the open dialdehydic form of DOA notably gains a strong anti-aging/anti-cancer-related chemopreventive effect (Pa = 0.766) compared with the weaker effect observed in the activity prediction for the cyclic hemiacetalic form of DOA (Pa = 0.372).
The metabolism-related actions reflect interactions of chemical compounds with metabolic enzymes. The cyclic hemiacetalic forms of OA and DOA are predicted to function as CYP1A2 inhibitors (Pa = 0.605 and Pa = 0.451, respectively).
The open dialdehydic form of OA is predicted to function as a substrate of GST A and GST P1-1 (Pa = 0.633 and 0.430, respectively). Similarly, the open dialdehydic form of DOA is predicted to function as a GST A and GST P1-1 substrate (Pa = 0.579 and 0.400, respectively).
The transporter terms reflect the interaction of chemical compounds with transporters (e.g., P-glycoprotein substrate, P-glycoprotein inhibitor, P-glycoprotein inducer). The cyclic and open forms of OA as well as the cyclic form of DOA are all predicted to function as P-glycoprotein substrates, but with a low Pa (~0.220); this activity is not predicted for the open dialdehydic form of DOA.
The gene expression terms reflect the influence of chemical compounds on the expression of certain genes. The cyclic hemiacetalic form of OA is predicted to negatively regulate ICAM1 gene expression with a low Pa (0.228). The cyclic hemiacetalic form of DOA is similarly predicted to inhibit the expression of ICAM1 (Pa = 0.329) and AR (Pa = 0.235) genes.
In addition to inhibiting the expression of the ICAM1 gene (Pa = 0.303) and AR (Pa = 0.290), as indicated for the cyclic hemiacetalic form of OA, the open dialdehydic form is predicted to inhibit the expression of the TNF (Pa = 0.392) and NOS2 (Pa = 0.253) genes. The open dialdehydic form of DOA, in addition to inhibiting the ICAM1 (Pa = 0.295) and AR (Pa = 0.310) genes, as indicated for its cyclic hemiacetalic form, is also predicted to inhibit the expression of the TNF (Pa = 0.331) and NOS2 (Pa = 0.208) genes.
The toxic/adverse effects reflect the specific toxicities or adverse reactions of the chemical compounds. No highly predicted toxic/adverse effects are indicated for the cyclic hemiacetalic form of OA beyond ulceration (Pa = 0.215). Interestingly, other indirectly related anti-aging/anti-cancer pharmacological effects with low Pa values are predicted for the cyclic hemiacetalic form of DOA, specifically embryotoxic (Pa = 0.260) and teratogen (Pa = 0.248) effects.
The open dialdehydic form of OA, in addition to showing skin irritation (Pa = 0.971) and eye irritation (Pa = 0.774) effects, is predicted to exhibit embryotoxic (Pa = 0.457) and teratogenic (Pa = 0.435) toxicities with Pa values that are somewhat higher than those predicted for its cyclic hemiacetalic form. A very similar pattern of toxic and adverse effects is predicted for the open dialdehydic form of DOA, including skin irritation (Pa = 0.954) and eye irritation (Pa = 0.823) effects as well as embryotoxic (Pa = 0.456), and teratogenic (Pa = 0.429) activities.
Contributions of particular atoms to the anti-aging/anti-cancer activities of EVOO oleuropeins
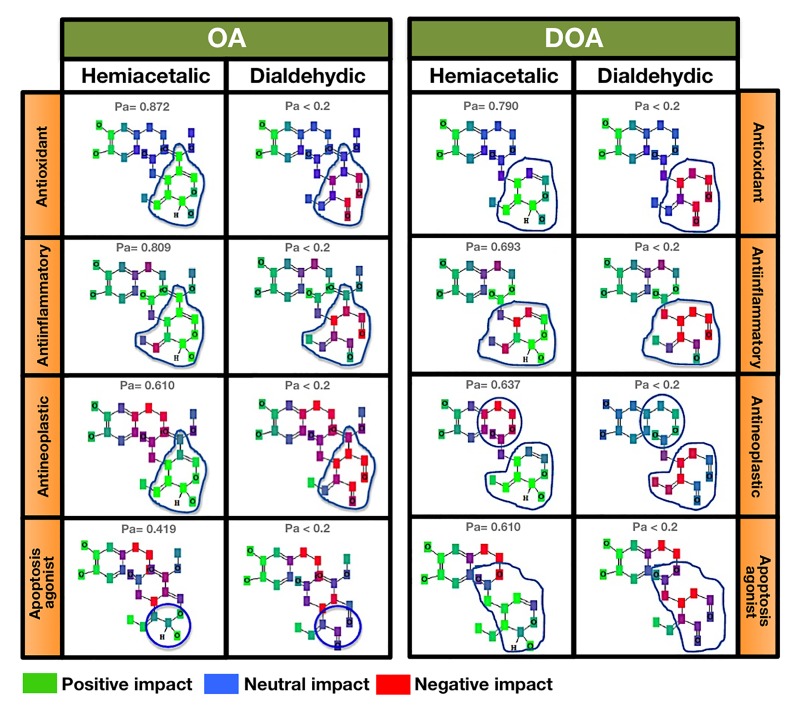
To preliminarily elucidate hypothesis-generating structural requirements of gerosuppressant oleuropeins, we assessed how the naturally occurring chemical structures of OA and DOA (i.e., the hemiacetalic closed forms versus dialdehydic open forms) impact some anti-aging/anti-cancer-related effects predicted with probabilities of Pa > 0.500 by PASS (i.e., among the top 10 pharmacological effects predicted for each polyphenol isomeric form). Some of the pharma-cological effects assumed to be closely related to the previously reported anti-aging and anti-cancer effects of OA and DOA (i.e., anti-oxidant, anti-inflammatory, and anti-neoplastic effects) are exclusively restricted to the hemiacetalic cyclic forms of OA and DOA (Fig. 4). The apoptosis agonist effect is predicted to occur with a notably higher probability in the cyclic hemiacetalic form of DOA (Pa = 0.610) than in the cyclic hemiacetalic form of OA (Pa = 0.419). Fig. 4 allows assessment of the impact of particular atoms on particular activities of the oleuropein isomers (green indicates a “positive impact”, blue a “neutral impact”, and red a “negative impact”). As green-colored atoms reflect a positive impact on a given activity while the red-colored atoms reflect a negative impact on the same activity, it should be noted that the chemopreventive mechanisms of action that are significantly predicted for the open dialdehydic forms of OA and DOA, but not for their cyclic hemiacetalic isomers mostly involve all of the atoms involved in the polyphenolic structure of DOA. Remarkably, as observed for many drugs with potential antineoplastic and chemopreventive properties, including curcumin and resveratrol [44-50], the open dialdehydic forms of OA and DOA are predicted to exhibit teratogenic and embryotoxic properties.
Figure 4. Contributions of particular atoms to the gerosuppressant activities of OA and DOA.
Figures provides a detailed comparison of the atomic groups that are likely to be responsible for the differences (blue circles) in the predictions of antioxidant, anti-inflammatory, antineoplastic, and apoptosis agonist activities with a Pa > 0.4 obtained for the hemiacetalic closed forms of OA and DOA that are not predicted (Pa < 0.2) for the dialdehydic open forms of OA and DOA.
DISCUSSION
Aging is associated with common conditions, including cancer, diabetes, cardiovascular disease, and Alzheimer's disease. A multi-targeted pharmacological approach necessary for addressing a complex multifaceted disease such as aging might take advantage of pleiotropic natural polyphenols affecting a wide range of biological processes. We recently postulated that the secoiridoids OA and DOA, two complex polyphenols present in health-promoting EVOO, might constitute a new family of plant-produced gerosuppressant agents [29, 32]. This paper describes, for the first time, the analysis of BAS for OA and DOA using PASS software.
The PASS algorithm is based on the concept of the BAS, which should be viewed as an intrinsic property of a compound. The BAS reflects all of the various biological activities that arise from the interactions of a compound with biological entities. Because in PASS, the biological activity spectrum represents a theoretical estimate for the general biological potential of the compound under study, this definition differs significantly from some other definitions of a “biological activity profile” or “biological activity spectrum” that are commonly published in the literature. PASS can then simultaneously predict pharmacological effects, mechanisms of action, mutagenicities, carcinogenicities, teratogenicities, and embryotoxicities based exclusively on the structural formula of a substance [33-43]. Using Pharmaexpert, a tool that analyzes the BAS of substances predicted by PASS based on a knowledgebase including thousands of “mechanism-effect(s)” and “effect-mechanism(s)” relationships, we have elucidated hypothesis-generating pharmacological effects, mechanisms of action, and targets that might underlie the anti-aging/anti-cancer activities of EVOO oleuropeins for the first time.
The results of the application of PASS to EVOO oleuropeins strongly support the notion that plant-derived dietary polyphenols may improve some disease states and promote health by operating as pleiotropic molecules that are capable of interacting with multiple molecular targets involved in aging and cancer-related processes, such as oxidation, inflammation, cholesterol and lipid synthesis, and immunosuppression. Although forthcoming studies should mechanistically confirm the predicted ability of the EVOO oleuropein DOA to exert anti-inflammatory, antihypercholesterolemic, and/or hepatoprotectant effects as part of the anti-aging and/or anti-cancer responses of DOA in in vivo models, we note that several studies have confirmed preventive effects of OA in experimental models of inflammation, inflammatory angiogenesis, and arthritis, in addition to hepatoprotective effects of OA in mice, chemopreventive effects in endothelial dysfunction-associated vascular diseases, and cardioprotective and neuroprotective effects [51-66].
Importantly, the results of our application of PASS to the EVOO oleuropeins OA and DOA, which exhibit two naturally occurring isomeric structures, strongly suggest that a different biological activity spectrum can be expected from hemiacetalic versus dialdehydic forms. In aqueous or physiological media, it should be assumed that both types of compounds exist in an equilibrium mixture consisting of a hemiacetal (i.e., the cyclic form) and a dialdehyde isomer (i.e., the open form). Although this equilibrium could be shifted in practice, for instance, by the preferential interaction of one of the isomers with a target protein, the dialdehyde form appears to be more conformationally free and adaptable to interact with a suitable target. It should be noted that the presence of the methoxycarbonyl constituent in OA clearly favors the cyclic hemiacetalic form due to the conjugation of the ester group with the internal double bond. In sharp contrast, a much greater ratio favoring the open dialdehydic form should be expected for DOA. Accordingly, the reported 1H-NMR of DOA shows that the dialdehyde form largely predominates [67-69]. In this regard, it is intriguing that application of PASS software to the isomeric forms of OA and DOA exclusively predicted (at least with high Pa values) chemopreventive activity for the open dialdehydic forms of OA, and more significantly, DOA. Chemopreventive agents derived from edible plants have been a part of the daily intake of many humans and animals since ancient times. There are multiple lines of compelling evidence from epidemiological, clinical, and laboratory studies that these dietary constituents are associated with a reduction of cancer risk [70-72]. It is reasonable to suggest that the gerosuppressant secoiridoids OA and DOA found in EVOO might be added to the list of plant-derived chemopreventive agents, not only based on their predicted antineoplastic effects, which have been unambiguously confirmed by our group in in vitro studies with cultured cancer cells, but also based on their predicted toxicities. Archetypal anti-aging/anti-cancer polyphenols, such as curcumin and resveratrol, have been shown to operate as “double-edged swords”, such that in vitro experiments reveal carcinogenic and pro-oxidant effects, in addition to anticancer and antioxidant effects. Accordingly, these polyphenols have been shown to exert embryotoxic and teratogenic effects using zebrafish embryos as disease models [44-50]. The PASS predictions obtained in the present study similarly predict carcinogenic, embryotoxic, and teratogenic effects for the dialdehydic forms of OA and DOA. Under the assumption that inhibition of developmentally regulated genes in vitro might also predict developmental toxicity under in vivo conditions [72], experiments are currently underway in our laboratory to examine the effect of the chemopreventive EVOO secoiridoid DOA on both the induction and differentiation of mouse induced pluripotent stem cells (iPSCs), as an in vitro model of embryotoxicity and the tumor-initiation properties of cancer stem cells.
We have recently postulated that the secoiridoids OA and DOA, two complex polyphenols present in health-promoting EVOO, might constitute a new family of plant-produced gerosuppressant agents [29, 32]. We demonstrated that the anticancer activity of EVOO phenolic extracts that were highly enriched in the secoiridoids OA and DOA was intriguingly related to the activation of anti-aging/cellular stress-like gene signatures (e.g., associated with endoplasmic reticulum stress, the unfolded protein response, spermidine and polyamine metabolism, AMPK activation, suppression of crucial genes involved in the Warburg effect and the self-renewal capacity of “immortal” cancer stem cells), while the same OA- and DOA-enriched phenolic extracts significantly prevented age-related changes in cell size, morphological heterogeneity, and senescence-associated β-galactosidase staining of normal diploid human fibroblasts at the end of their proliferative lifespans [29]. Because the exploration of putative therapeutic mechanisms using conventional wet laboratory experiments to obtain a better understanding of the ultimate pharmacological behavior of these polyphenolic gerosuppressants was expected to be tedious, time consuming, and expensive, we were encouraged to develop in silico techniques. The results of the present study confirm that PASS software is extremely useful for the elucidation of unknown therapeutic mechanisms in plant-based drug discovery and, more importantly, reveals hypothesis-generating pharmacological effects, mechanisms of action, and targets that likely underlie the anti-aging/anti-cancer activities of complex polyphenols that are naturally present in EVOO.
Acknowledgments
This work was financially supported by the Ministerio de Ciencia e Innovación (SAF2012-38914), Plan Nacional de I+D+I, MICINN, Spain.
Footnotes
Conflict of interest statement
The authors of this manuscript declare no conflict of interests.
REFERENCES
- 1.Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- 2.Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper PL, Hooper PL, Tytell M, Vígh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surh YJ. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann N Y Acad Sci. 2011;1229:1–6. doi: 10.1111/j.1749-6632.2011.06097.x. [DOI] [PubMed] [Google Scholar]
- 5.Testa G, Biasi F, Poli G, Chiarpotto E. Calorie Restriction and Dietary Restriction Mimetics: a Strategy for Improving Healthy Aging and Longevity. Curr Pharm Des. 2014;20:2950–2977. doi: 10.2174/13816128113196660699. [DOI] [PubMed] [Google Scholar]
- 6.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchal J, Pifferi F, Aujard F. Resveratrol in mammals: effects on aging biomarkers, age-related diseases, and life span. Ann N Y Acad Sci. 2013;1290:67–73. doi: 10.1111/nyas.12214. [DOI] [PubMed] [Google Scholar]
- 8.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY) 2011;3:821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández AF, Fraga MF. The effects of the dietary polyphenol resveratrol on human healthy aging and lifespan. Epigenetics. 2011;6:870–874. doi: 10.4161/epi.6.7.16499. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 12.Mouchiroud L, Molin L, Dallière N, Solari F. Life span extension by resveratrol, rapamycin, and metformin: The promise of dietary restriction mimetics for an healthy aging. Biofactors. 2010;36:377–382. doi: 10.1002/biof.127. [DOI] [PubMed] [Google Scholar]
- 13.Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–62. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008;7:1520–1524. doi: 10.4161/cbt.7.10.6663. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–1887. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 16.Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8:4055–4059. doi: 10.4161/cc.8.24.10310. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 18.Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle. 2010;9:4788–4794. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011;3:1130–1141. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagosklonny MV. Rapalogs in cancer prevention: anti-aging or anticancer? Cancer Biol Ther. 2012;13:1349–1354. doi: 10.4161/cbt.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–1146. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagosklonny MV. Answering the ultimate question “what is the proximal cause of aging?”. Aging (Albany NY) 2012;4:861–877. doi: 10.18632/aging.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blagosklonny MV. Common drugs and treatments for cancer and age-related diseases: revitalizing answers to NCI's provocative questions. Oncotarget. 2012;3:1711–1724. doi: 10.18632/oncotarget.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blagosklonny MV. MTOR-driven quasi-programmed aging as a disposable soma theory: blind watchmaker vs intelligent designer. Cell Cycle. 2013;12:1842–1847. doi: 10.4161/cc.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013;5:592–598. doi: 10.18632/aging.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blagosklonny MV. Aging is not programmed: Genetic pseudo-program is a shadow of developmental growth. Cell Cycle. 2013;12:3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menendez JA, Joven J, Aragonès G, Barrajón-Catalán E, Beltrán-Debón R, Borrás-Linares I, Camps J, Corominas-Faja B, Cufí S, Fernández-Arroyo S, Garcia-Heredia A, Hernández-Aguilera A, Herranz-López M, Jiménez-Sánchez C, López-Bonet E, Lozano-Sánchez J, et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: a new family of gerosuppressant agents. Cell Cycle. 2013;12:555–578. doi: 10.4161/cc.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariño G, Ugalde AP, Salvador-Montoliu N, Varela I, Quirós PM, Cadiñanos J, van der Pluijm I, Freije JM, López-Otín C. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008;17:2196–2211. doi: 10.1093/hmg/ddn120. [DOI] [PubMed] [Google Scholar]
- 31.Mariño G, López-Otín C. Autophagy and aging: new lessons from progeroid mice. Autophagy. 2008;4:807–809. doi: 10.4161/auto.6478. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Martin A, Fernández-Arroyo S, Cufí S, Oliveras-Ferraros C, Lozano-Sánchez J, Vellón L, Micol V, Joven J, Segura-Carretero A, Menendez JA. Phenolic secoiridoids in extra virgin olive oil impede fibrogenic and oncogenic epithelial-to-mesenchymal transition: extra virgin olive oil as a source of novel antiaging phytochemicals. Rejuvenation Res. 2012;15:3–21. doi: 10.1089/rej.2011.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filimonov DA, Poroĭkov VV, Karaicheva EI, Kazarian RK, Budunova AP, Mikhaĭlovskiĭ EM, Rudnitskikh AV, Goncharenko LV, Burov IuV. The computerized prediction of the spectrum of biological activity of chemical compounds by their structural formula: the PASS system. Prediction of Activity Spectra for Substance. Eksp Klin Farmakol. 1995;58:56–62. [PubMed] [Google Scholar]
- 34.Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics. 2000;16:747–748. doi: 10.1093/bioinformatics/16.8.747. [DOI] [PubMed] [Google Scholar]
- 35.Poroikov VV, Filimonov DA, Ihlenfeldt WD, Gloriozova TA, Lagunin AA, Borodina YV, Stepanchikova AV, Nicklaus MC. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J Chem Inf Comput Sci. 2003;43:228–236. doi: 10.1021/ci020048r. [DOI] [PubMed] [Google Scholar]
- 36.Stepanchikova AV, Lagunin AA, Filimonov DA, Poroikov VV. Prediction of biological activity spectra for substances: evaluation on the diverse sets of drug-like structures. Curr Med Chem. 2003;10:225–233. doi: 10.2174/0929867033368510. [DOI] [PubMed] [Google Scholar]
- 37.Varnek Alexandre, Tropsha Alexander., editors. Chemoinformatics Approaches to Virtual Screening. Cambridge (UK): RSC Publishing; 2008. pp. 182–216. [Google Scholar]
- 38.Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, Alam I, Saxena AK. Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem. 2008;51:1601–1609. doi: 10.1021/jm701496h. [DOI] [PubMed] [Google Scholar]
- 39.Benaamane N, Nedjar-Kolli B, Bentarzi Y, Hammal L, Geronikaki A, Eleftheriou P, Lagunin A. Synthesis and in silico biological activity evaluation of new N-substituted pyrazolo-oxazin-2-one systems. Bioorg Med Chem. 2008;16:3059–3066. doi: 10.1016/j.bmc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Lagunin A, Filimonov D, Poroikov V. Multi-targeted natural products evaluation based on biological activity prediction with PASS. Curr Pharm Des. 2010;16:1703–1717. doi: 10.2174/138161210791164063. [DOI] [PubMed] [Google Scholar]
- 41.Singh D, Gawande DY, Singh T, Poroikov V, Goel RK. Revealing pharmacodynamics of medicinal plants using in silico approach: a case study with wet lab validation. Comput Biol Med. 2014;47:1–6. doi: 10.1016/j.compbiomed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Ivanov SM, Lagunin AA, Zakharov AV, Filimonov DA, Poroĭkov VV. Computer search for molecular mechanisms of ulcerogenic action of nonsteroidal antiinflammatory drugs. Biomed Khim. 2014;60:7–16. doi: 10.18097/pbmc20146001007. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Lohan P, Aneja DK, Gupta GK, Kaushik D, Prakash O. Design, synthesis, computational and biological evaluation of some new hydrazino derivatives of DHA and pyranopyrazoles. Eur J Med Chem. 2012;50:81–89. doi: 10.1016/j.ejmech.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 44.Wu JY, Lin CY, Lin TW, Ken CF, Wen YD. Curcumin affects development of zebrafish embryo. Biol Pharm Bull. 2007;30:1336–1339. doi: 10.1248/bpb.30.1336. [DOI] [PubMed] [Google Scholar]
- 45.Huang FJ, Lan KC, Kang HY, Liu YC, Hsuuw YD, Chan WH, Huang KE. Effect of curcumin on in vitro early post-implantation stages of mouse embryo development. Eur J Obstet Gynecol Reprod Biol. 2013;166:47–51. doi: 10.1016/j.ejogrb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Hossain DM, Bhattacharyya S, Das T, Sa G. Curcumin: the multi-targeted therapy for cancer regression. Front Biosci (Schol Ed) 2012;4:335–355. doi: 10.2741/272. [DOI] [PubMed] [Google Scholar]
- 47.Khan HY, Zubair H, Ullah MF, Ahmad A, Hadi SM. A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols. Curr Drug Targets. 2012;13:1738–1749. doi: 10.2174/138945012804545560. [DOI] [PubMed] [Google Scholar]
- 48.Szekeres T, Saiko P, Fritzer-Szekeres M, Djavan B, Jäger W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann N Y Acad Sci. 2011;1215:89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- 49.Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 50.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puel C, Quintin A, Agalias A, Mathey J, Obled C, Mazur A, Davicco MJ, Lebecque P, Skaltsounis AL, Coxam V. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br J Nutr. 2004;92:119–127. doi: 10.1079/BJN20041181. [DOI] [PubMed] [Google Scholar]
- 52.Giamarellos-Bourboulis EJ, Geladopoulos T, Chrisofos M, Koutoukas P, Vassiliadis J, Alexandrou I, Tsaganos T, Sabracos L, Karagianni V, Pelekanou E, Tzepi I, Kranidioti H, Koussoulas V, Giamarellou H. Oleuropein: a novel immunomodulator conferring prolonged survival in experimental sepsis by Pseudomonas aeruginosa. Shock. 2006;26:410–416. doi: 10.1097/01.shk.0000226342.70904.06. [DOI] [PubMed] [Google Scholar]
- 53.Poudyal H, Campbell F, Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y, Choi Y, Park T. Hepatoprotective effect of oleuropein in mice: mechanisms uncovered by gene expression profiling. Biotechnol J. 2010;5:950–960. doi: 10.1002/biot.201000068. [DOI] [PubMed] [Google Scholar]
- 55.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Morittu VM, Procopio A, Britti D, Cuzzocrea S. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin Nutr. 2011;30:533–540. doi: 10.1016/j.clnu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, De Caterina R, Carluccio MA. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–89. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Domitrović R, Jakovac H, Marchesi VV, Šain I, Romić Ž, Rahelić D. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012;65:451–64. doi: 10.1016/j.phrs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Morittu VM, Procopio A, Britti D, Cuzzocrea S. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J Pharmacol Exp Ther. 2011;339:859–869. doi: 10.1124/jpet.111.182808. [DOI] [PubMed] [Google Scholar]
- 59.Visioli F, Galli C. Antiatherogenic components of olive oil. Curr Atheroscler Rep. 2001;3:64–7. doi: 10.1007/s11883-001-0012-0. [DOI] [PubMed] [Google Scholar]
- 60.Manna C, Migliardi V, Golino P, Scognamiglio A, Galletti P, Chiariello M, Zappia V. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem. 2004;15:461–466. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Turner R, Etienne N, Alonso MG, de Pascual-Teresa S, Minihane AM, Weinberg PD, Rimbach G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int J Vitam Nutr Res. 2005;75:61–70. doi: 10.1024/0300-9831.75.1.61. [DOI] [PubMed] [Google Scholar]
- 62.Omar SH. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm J. 2010;18:111–121. doi: 10.1016/j.jsps.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rigacci S, Guidotti V, Bucciantini M, Nichino D, Relini A, Berti A, Stefani M. Aβ(1-42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr Alzheimer Res. 2011;8:841–852. doi: 10.2174/156720511798192682. [DOI] [PubMed] [Google Scholar]
- 64.Diomede L, Rigacci S, Romeo M, Stefani M, Salmona M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit. PLoS One. 2013;8:e58893. doi: 10.1371/journal.pone.0058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grossi C, Rigacci S, Ambrosini S, Dami TE, Luccarini I, Traini C, Failli P, Berti A, Casamenti F, Stefani M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS One. 2013;8:e71702. doi: 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luccarini I, Ed Dami T, Grossi C, Rigacci S, Stefani M, Casamenti F. Oleuropein aglycone counteracts Aβ42 toxicity in the rat brain. Neurosci Lett. 2014;558:67–72. doi: 10.1016/j.neulet.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 67.Karkoula E, Skantzari A, Melliou E, Magiatis P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative (1)H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J Agric Food Chem. 2012;60:11696–11703. doi: 10.1021/jf3032765. [DOI] [PubMed] [Google Scholar]
- 68.Vougogiannopoulou K, Lemus C, Halabalaki M, Pergola C, Werz O, Smith AB, 3rd, Michel S, Skaltsounis L, Deguin B. One-step semisynthesis of oleacein and the determination as a 5-lipoxygenase inhibitor. J Nat Prod. 2014;77:441–445. doi: 10.1021/np401010x. [DOI] [PubMed] [Google Scholar]
- 69.Karkoula E, Skantzari A, Melliou E, Magiatis P. Quantitative Measurement of Major Secoiridoid Derivatives in Olive Oil Using qNMR. Proof of the Artificial Formation of Aldehydic Oleuropein and Ligstroside Aglycon Isomers. J Agric Food Chem. 2014 Jan 13; doi: 10.1021/jf404421p. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 71.Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res (Phila) 2009;2:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- 72.Wagh V, Jagtap S, Meganathan K, Potta SP, Winkler J, Hescheler J, Sachinidis A. Effect of chemopreventive agents on differentiation of mouse embryonic stem cells. Front Biosci (Elite Ed) 2012;4:156–168. doi: 10.2741/e366. [DOI] [PubMed] [Google Scholar]