Abstract
Alopecia is an exceedingly prevalent problem effecting men and women of all ages. The standard of care for alopecia involves either transplanting existing hair follicles to bald areas or attempting to stimulate existing follicles with topical and/or oral medication. Yet, these treatment options are fraught with problems of cost, side effects, and, most importantly, inadequate long-term hair coverage. Innovative cell-based therapies have focused on the dermal papilla cell as a way to grow new hair in previously bald areas. However, despite this attention, many obstacles exist, including retention of dermal papilla inducing ability and maintenance of dermal papilla productivity after several passages of culture. The use of adipocyte lineage cells, including adipose-derived stem cells, has shown promise as a cell-based solution to regulate hair regeneration and may help in maintaining or increasing dermal papilla cells inducing hair ability. In this review, we highlight recent advances in the understanding of the cellular contribution and regulation of dermal papilla cells and summarize adipocyte lineage cells in hair regeneration.
Keywords: Adipocyte lineage cells, dermal papilla cells, hair regeneration, tissue engineering
Introduction
Alopecia, regardless of the etiology, can result in devastating physical and psychological sequelae. Clinical conditions causing hair loss include but are not limited to androgenic alopecia, alopecia areata, and scarring alopecia. These frequently occurring conditions can be progressive and irreversible with no treatment available to reverse the underlying hair loss. The current management strategy focuses on stimulating existing follicles to grow new hair or recreating a fuller head of hair by transplantation. The two most frequently used treatments include drug therapies (minoxidil and finasteride) and human hair transplantation. Other treatment options include laser therapy, dietary supplementation, and exercise training.1 The drug therapies enhance hair growth and minimize future hair loss. The topical drug minoxidil is associated with problems such as skin irritation, as well as unwanted hair growth elsewhere on the body, and the oral medication finasteride is associated with sexual side effects including fertility problems.2 Both medications are associated with accelerated hair loss when the medications are stopped after prolonged use.3 In human hair transplantation, hair follicles from areas of the scalp that are not within the bald area are excised and re-implanted within the bald area to create the illusion of a fuller head of hair. The number of follicles that can be harvested and re-implanted into a bald area limits hair transplant surgery. Additionally, this therapy requires a costly, time-consuming procedure that is often only temporary due to the progressive nature of many hair loss conditions. Artificial hair transplantation has proved to be ineffective, associated with recurrent infections, rejection, and periodic loss of fibers needing frequent replacement, which lead the Food and Drug Administration (FDA) to ban the procedure in 1983.4 While many patients benefit from the current treatment options for alopecia, there is a significant fraction of patients who realize little benefit and deserve improved alternative therapies.
Thus, the currently available treatments for hair loss have numerous limitations, and alternative therapies need to be incorporated into clinical practice. Cell-based hair regeneration has been investigated as a possible alternative. Dermal papilla cells (DPCs) have been noted to be a key element-cell for regenerating hair growth in previously bald skin.5 Dermal papillas (DPs) are located at the base of the hair follicle, which is a unique tissue surrounded by epithelial matrix cells. DPCs are thought to provide regulating factors and nutrients to support proliferation and differentiation of the epithelial matrix cells in hair cycle progression and also in follicle formation in embryonic skin. Different from rodent hair follicle DPCs, human DPCs (hDPCs) show lower inducing hair growth ability after several passages of culture. There is an ongoing effort to try to overcome this shortcoming of hDPCs and adapt cell-based hair reconstitution techniques into clinical settings.
Besides the microenvironment involving epithelial–mesenchymal interactions inside the hair follicle, macroenvironment outside the hair follicle should also be paid attention to. Recent basic research has revealed that the dermal macroenvironment is important in the maintenance of the bulge cells population and hair follicle growth. The subcutaneous adipocytes, together with dermis and adjacent follicles, are defined as the interfollicular dermal macroenvironment, which have been shown to be relevant for epidermal homeostasis during hair follicle regeneration. Adipose-derived stem cells (ASCs) (mesenchymal stem cells reserved in the white adipose tissue) are believed to have vast applications in regenerative medicine. In addition, ASCs and their secretomes mediate diverse skin-regeneration effects. ASCs may become a useful tool to stimulate tissue-engineered hair follicle growth and modulate its regenerative behavior.
In this review, we highlight recent advances in the understanding of the cellular contribution and regulation of DPCs and summarize the role of adipocyte lineage cells, including ASCs, in hair regeneration.
DPCs and hair follicle tissue engineering
The hair follicle is a mini-organ that is unique compared to other tissues in the human body, as it is characterized by cycles, in which all of the hair growth machinery is lost, and then fully reconstituted.6 They are composed of an outer root sheath, an inner root sheath, and a hair shaft (Figure 1). The growth activity of hair follicles is controlled by a highly coordinated series of bidirectional epithelial–mesenchymal interactions.7 DPCs play a pivotal role in hair formation, growth, and cycling.
Figure 1.
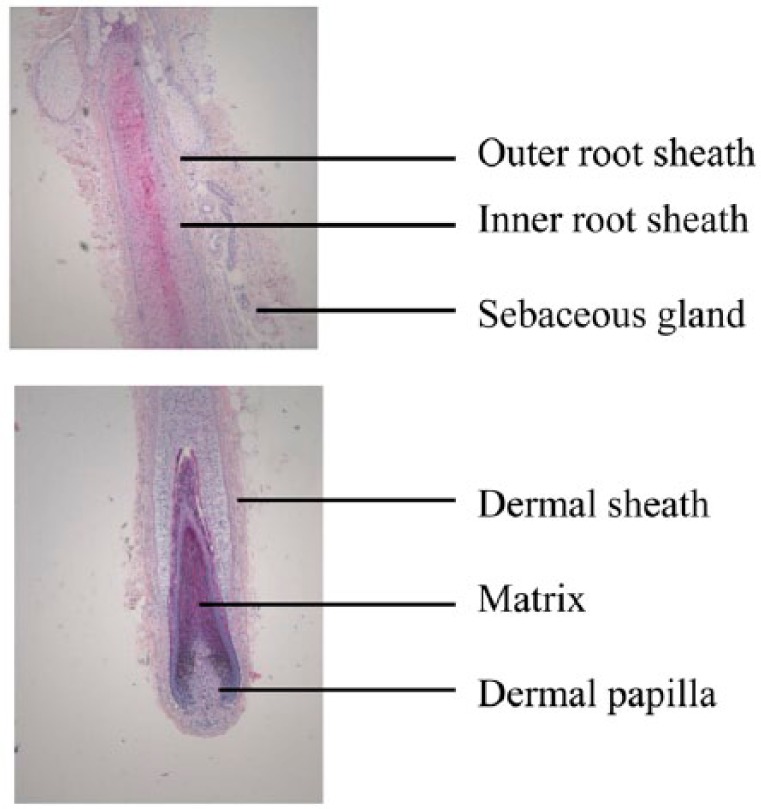
Histological analyses of a natural hair follicle isolated from an adult human scalp skin. H&E staining shows the whole structures of hair follicle.
H&E: hematoxylin and eosin.
Role of DP in hair formation
The DP is the major dermal compartment which has roles in hair formation during embryonic morphogenesis and hair cycling after birth.8 The epithelial part of a hair follicle needs DPCs to maintain its growth, and dissociated epidermal cells require the guidance of dermal cells to be organized into complicated hair structures.9 The hair cycle consists of phases of growth (anagen), degeneration (catagen), and rest (telogen). During late telogen to early anagen transition, signals from DP stimulate the hair germ and quiescent bulge stem cells to become activated.10 During anagen, cells at the base of the follicle start to proliferate, which results in the formation of a new hair filament, while stem cells in the bulge give rise to hair germs. In catagen, follicles undergo apoptosis, but the DP remains intact and migrates upwards, until it comes to rest next to the stem cells of the hair follicle bulge. This situation persists during telogen.11 Both the hair-forming ability of epithelium and the hair inductivity of DP change during the hair cycle and affect the frequency of hair formation in transplantation surgery.12 DPCs not only retain the ability to form new DPCs but they can also contribute to dermal sheath cells and non-follicle-associated fibroblasts during skin reconstitution wound healing.13 The dermal sheath is the second dermal compartment which is contiguous with the DP at its base. Studies suggest that it has a role in hair induction and may contain DPC progenitor cells.14 Yamao et al. co-transplanted DPCs of high passage number with cultured dermal sheath cells in a graft chamber assay onto the dorsum of nude mice. The results showed that dermal sheath cells participate in the process of DPC-induced hair formation and dermal sheath formation is critical to the normal development of hairs with hair shafts.15
Characteristic of DPCs
DPCs are believed to be derived from cells forming a dermal condensation at the start of follicle development. With the interaction between epithelial cells and underlying mesenchymal cells, a portion of the condensed dermal cells differentiates into DPCs.16 DPCs express a unique set of genes and proteins compared with other cells, such as dermal sheath cells and dermal fibroblasts, in the dermal condensation. For example, intense expressions of laminin and fibronectin proteins are detected in the DP.17 The activity of alkaline phosphatase (ALP) has been used as a marker to detect the presence of DP18,19 and as a marker of cell differentiation and an index for the hair-inductive capacity of DPCs.20 ALP activity is also exploited as a simple and reliable method to distinguish DPCs from other hair follicle structures.21 Cells from DP that express ALP can be cultured and implanted to induce hair follicle formation.22 α-Smooth muscle actin (α-SMA) can be detected in cultured DPCs but not in vivo, whereas dermal fibroblasts did not produce α-SMA proteins.23 α-SMA correlates with cells originating from hair follicles24 and myofibroblastic transition.25 It is also shown that DPCs produced more versican during anagen.26 Versican is not expressed until the hair follicle is beginning to produce fibers. With follicle maturation, versican expression reaches a maximum at the height of the growth phase, after which it diminishes as the end of this phase approaches.27 CD133, a hematopoietic stem cell marker, is strongly expressed in DPCs during stage 3–4 of hair follicle development and during early anagen phase in mouse skin.28 Interestingly, DPCs possess a multi-lineage potential as these cells can be induced to differentiate into adipogenic and osteogenic lineages.29 There are also reports of DPCs being reprogrammed into the pluripotent state to generate induced pluripotent stem (iPS) cells.30,31
Isolation of DPCs
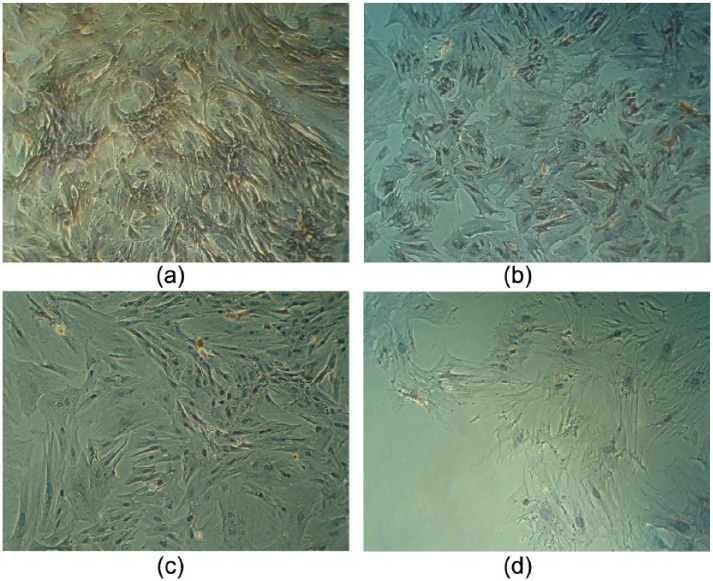
The most widely used method to isolate DP is surgical microdissection, which has been successfully established in rodent whisker follicles as well as in human hair follicles.32,33 For the purpose of hair follicle tissue engineering, an efficient method has been developed, which involves applying dispase and subsequent collagenase to the lower part of hair follicles.34 As contamination of DP cultures with other mesenchymal cells, epithelial cells, matrix, or adipose tissue is undesirable, the use of microdissection techniques over those of enzymatic digestion is preferred. The isolated DPs are seeded onto a culture flask and within 1 week, the outgrowth of DPCs can be observed with flattened and polygonal morphology (Figure 2).
Figure 2.
Establishment of primary dermal papilla cells culture. (a) Hair follicles were identified in scalp skin and dissected free under a stereomicroscope. (b) After digestion in 0.25% collagenase at 37°C for 30 min, a whole hair follicle with full structure can be extracted with a forceps. Using the tip of a needle, pressure was applied to the base of the follicle dermal sheath which was cut open, and the dermal papilla was revealed. (c) The dermal papilla was peeled off with a small portion of dermal sheath tissue at its base and was transferred to a 35-mm dish and cultured undisturbed for 1 week. (d) After 7 days, early migration of dermal papilla cells from the explants was evident.
Hair reconstruction with DPCs: in vivo model
In vivo hair reconstruction models are used to assess the hair inductivity of isolated cell populations. Various experimental assays have shown the successful construction of hair follicles in rodent animals.35,36 By grafting a mixture of epidermal cells and DPCs onto the backs of nude mice, hair-forming capacities of DPCs can be assessed.37 Furthermore, regenerating hair follicles with passaged DPCs from adult pelage follicles and adult footpad skin has shown that embryonic-like inductive potential may be a widespread property shared by all adult hair follicle DPCs.32 Additionally, co-grafted human keratinocytes and murine DP-enriched cells in chamber assays produce hair follicle–like structures consisting of multiple epidermal cell layers with a well-keratinized innermost region, but the hair follicles do not possess the normal hair follicle structures.38 Human scalp DPCs co-cultured with mouse epidermal keratinocytes have also been shown to induce hair formation in a flap graft assay.39 “Patch” assays, formed by injecting hDPCs, grown as spheroids, together with mouse epidermal cells in reconstitution, have been shown to increase the ability of cultured hDPCs to induce hair follicles from mouse epidermal cells.40 A key factor for generating hair follicle–like structures is the use of a mesenchymal component (murine DPCs), which is the same species as the recipient. However, the use of immunodeficient host mice in these in vivo models possesses drawbacks especially when applying these systems to the regeneration of human hair follicles.
Hair reconstruction with DPCs: in vitro model
The in vitro environment is more controllable with fewer unknown factors; however, it is difficult to simulate the complicated niche where hair follicles reside. Collagen gel has been used as the matrix with epidermal and dermal cells on the surface or incorporated within the gel, but the structures formed are still far from the normal hair follicle.41,42
Regeneration of human hair follicles
Most of the studies using rodent DPCs demonstrate hair follicle regeneration. Only a few reports have shown that hDPCs maintained hair-inducing ability after in vitro expansion.43,44 However, complete and entirely human hair follicles formed from normal cultured cells have not been reported because after a few passages, cultured DPCs lose their trichogenic properties.45,46 To reconstitute human hair follicles that are not chimeric with other species, hDPCs with an inductive property will be needed. How to efficiently expand DPCs while maintaining their hair-inductive capacity by optimizing culture media or adding growth factors has been a major challenge and is a prerequisite for future tissue-engineering hair follicle therapies. Cultured with glycogen synthase kinase-3β (GSK-3β) inhibition, hDPCs showed Wnt/β-catenin signaling activation and displayed constant hair induction ability when transplanted with murine epidermal cell fraction.47 Inamatsu et al.48 found that co-cultures of DPCs and keratinocytes could support the rapid growth of the DPCs. The feeder effect of keratinocytes could be partly replaced with conditioned medium (CM) from keratinocytes. By adding CM of keratinocytes into culture medium, DPCs could be cultured for more than 90 passages without losing their hair-inductive ability; however, high passage DPCs were unable to induce complete hair follicles but were able to induce incomplete hair follicles.15 Reports have also shown that basic fibroblast growth factor (bFGF) may also enhance experimental hair growth.49 Recently, Higgins reported that hDPCs in culture will change their molecular signatures very rapidly and profoundly from a three-dimensional to a two-dimensional environment and lose their hair-inducing capacity. Therefore, they reconstructed the three-dimensional environment by culturing the DPCs in hanging drops and forming spheroids. The results show that DPCs in three-dimensional spheroid cultures can restore their inductive ability partially and are capable of inducing de novo hair follicles in human skin.50 A similar report by Thangapazham et al.51 showed that inserting adult DPCs into dermal–epidermal composites could improve hair-inducing properties and then achieve human hair reconstitution.
Pathways involved in maintaining hair inductivity in DPC culture
The Wnt pathway contributes to the initiation of folliculogenesis. Activation of the Wnt pathway is thought to be the first mesenchymal signal involved in the epithelial–mesenchymal interaction of folliculogenesis.52 Bone morphogenetic protein-2 (BMP2) is a multifunctional growth factor and was originally defined by its ability to induce ectopic bone and cartilage formation in vivo. In the hair formation process, it controls not only the differentiation process of hair matrix cells but also the activity of dermal fibroblasts.53 Transforming growth factor-β (TGF-β) is a well-known inducer of extracellular matrix components such as collagen and fibronectin. It is involved in promotion of the hair placode, contributes to anagen induction,54 is highly expressed in hDPCs compared with human hair follicle cells, and is suggested to mediate the hair-inductive capacity of hDPCs.55 It also acts as intercellular modulator of growth factor signaling exchange between the hair follicle epithelium and the mesenchyme.
Adipocyte lineage cells and hair follicle regeneration
Besides the microenvironment of epithelial–mesenchymal interactions inside the hair follicle, the macroenvironment around it is also essential for the construction and regeneration of hair follicle. Intradermal adipose tissue is an essential macroenvironment to hair follicles.
Adipocyte lineage cells are closely related with hair growth
Adipose tissue comprises mature adipocytes and stromal vascular cells, including adipocyte precursor cells. Adipocyte precursor cells are also referred to as preadipocytes or ASCs, depending on their potential to differentiate in adipocytes only or to additional cell types such as osteoblasts and chondrocytes.56 Previous studies have revealed a close correlation between subcutaneous adipose tissue and hair follicle formation and function.57 In fact, numerous studies have shown that the hair follicle’s regenerative cycle, induced by follicular stem cells, is closely associated with adipose tissue and adipocyte lineage cells. For example, the thickness of the intradermal adipocyte layer in the hair follicle active cycle (anagen) increases significantly compared with the thickness of that in the resting phase of the hair cycle.58
Adipocyte lineage cells in different stages expose different functions involved in the development of skin and hair follicles. Functional analysis of adipocyte lineage cells in mice with defects in adipogenesis and in transplantation experiments revealed that immature adipocyte cells are necessary and sufficient to drive follicular stem cell activation. The data showed that adipocyte precursor cells are necessary and sufficient for the activation of skin epidermal stem cells; these studies highlighted the importance of cells in the adipocyte lineage as niche cells within individual tissues. In particular, Festa et al.59 reported that adipose lineage cells, including mature adipocyte and preadipocytes, have been defined as skin niche cells that regulate hair follicle stem cell activity. The number of adipocyte precursor cells change with the hair cycle: the cell number peaks in the skin during follicular stem cell activation (anagen) and decreases during catagen stage.
Mature adipocyte cells, different from the preadipocytes, show a negative effect on the proliferation of hair follicle cells. Misago et al.60 studied the influence of fat cells (i.e. adipocytes) on the proliferation and differentiation of organoid hair follicle cells in a three-dimensional collagen gel matrix culture system. They found that the outgrowth of epithelial cells from organoid hair follicles was distinctly inhibited by co-culture with fat cells in close apposition, and that the organoid hair follicles morphologically showed a rounded contour like that of hair follicles in the skin, rather than of amoeboid appearance. They suggested that fat cells have an inhibitory effect on the proliferation of perifollicular fibroblasts in this co-culture system, which resulted in no outgrowth of epithelial cells of hair follicle organoids. The direct accelerating effect on hair follicle differentiation might reflect the inhibition of proliferation of organoid hair follicle cells by fat cells. The fat cells would have the capability of inhibiting the proliferation of hair follicles penetrating deep into the dermis with the development of the hair or hair cycles; after downward growth of the hair follicle into the subcutaneous fat tissue, a new hair bulb is formed with subsequent differentiation. This seems to be convincing physiological evidence that fat cells possess the capacity to accelerate the differentiation of hair follicles. Furthermore, Plikus et al.61 showed that during the hair cycle, mature intradermal adipocytes expressed BMP2 messenger RNA (mRNA) which was an inhibitory signal for bulge cell activity. The activation of hair follicle stem cells was subject to the effects of the macroenvironment of the surrounding dermis. The expression of BMP2 might coordinate the function of subcutaneous fat and DP of hair follicle in response to the external environment, and may have implications for the evolution of integuments.
A change of the characteristics or the number of adipocyte lineage cells may cause disorders of skin and hair. Abnormal lipid metabolism in transgenic mice models result in malformations in skin structure and function. For example, apolipoprotein C1 (APOC1) transgenic mice, which overexpress human apolipoprotein C1 and have hyperlipidemia, exhibit abnormalities of hair growth that are correlated to the level of human APOC1 gene expression in the skin.62 On the contrary, mice lacking diacylglycerol acyltransferase 2 (DGAT2), which demonstrate reduced triglycerides in tissues, demonstrate skin barrier abnormalities.63
The possibilities of ASCs used in hair follicle regeneration
During the past decade, ASCs have become one of the most widely studied adult stem cell populations for research in soft tissue–engineering and regenerative medicine applications. ASCs were initially identified as “preadipocytes.” While further research found these cells to have the ability to differentiate into several mesenchymal lineages and characteristics of stem cells. The acronym “ASC” is the standard nomenclature proposed by the International Federation for Adipose Therapeutics and Science to describe the plastic-adherent, proliferative, multipotent cell population isolated from adipose tissue.64
ASCs and bone marrow–derived stem cells (BMSCs) share similar immunophenotypic and immunomodulatory characteristics, gene profiles, and functions.65–68 In contrast with BMSCs, ASCs possess several advantages as a promising cell source for tissue regeneration and clinical therapy. For example, ASCs can be obtained from an abundant autologous source with minor invasive harvesting (liposuction); ASCs also have significant proliferative capacity in culture and multi-lineage potential, such as adipogenic, osteogenic, and chondrogenic lineage differentiations.69–72 ASCs can be identified by means of fluorescence-activated cell sorting, as they have several specific surface markers: CD117, human leukocyte antigen–DR (HLA-DR), and stem cell–associated markers such as CD34.73
ASCs possess several important metabolic properties which suggest their potential positive role in hair regeneration. An essential function of ASCs is the production and secretion of growth factors that activate neighboring cells. These growth factors include vascular endothelial growth factor (VEGF), TGF-β, hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), placental growth factor, and bFGF. The expression of these potent growth factors allows ASCs to have an angiogenic capacity and the ability to induce tissue neovascularization, which show that ASCs may contribute a macroenvironment with an abundant blood supply for hair cells to regenerate hair follicles. ASCs are also immunomodulatory and immunosuppressive via direct cell-to-cell interaction or secreted cytokine profile such as prostaglandin E2 (PGE2), leukemia inhibitory factor (LIF), and kynurenine.74 ASCs and their secreto-mes mediate diverse skin-regenerative effects, such as wound-healing, antioxidant protection, anti-wrinkling, and whitening effects.75–78
Examination of the role of ASCs in hair regeneration
Although there is currently no conclusive data to indicate that ASCs play an essential role in hair regeneration, several assays have been assessed to demonstrate a potential role between ASCs and hair regeneration. To investigate whether ASCs are capable of directly participating in hair follicle morphogenesis, CD34+ ASCs were grafted with mouse fetal epidermal and dermal cells in a nude mouse model.73 The results showed that CD34+ ASCs could be detected to participate in forming hair follicle, blood vessel, and fat tissue. Another in vivo study involved injecting conditioned medium of ASCs (ASC-CM) subcutaneously and monitoring the darkening of the shaved skin. It was concluded that ASC-CM could promote hair growth, and the effect could be enhanced under hypoxia.79
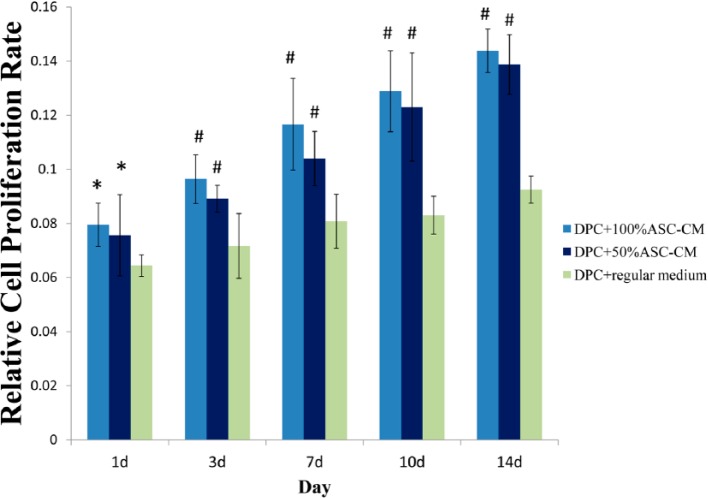
To study the promotion effect of ASCs on hair regeneration in vitro, two models are commonly used: one is directly contacting ASCs with hair cells,59,60 and the other is using the transwell technique80 or conditional medium from ASCs79,81 to simulate the situation of hair cells influenced by ASCs. Previous studies also suggested that the paracrine function of ASCs should be considered as an important mechanism to stimulate hair growth. The ASC-CM contains secretory factors derived from ASCs and might be a promising tool for hair growth promotion. Recently, we examined the influence of ASC-CM on the hair-inducing ability of rat DPCs. By culturing DPCs in ASC-CM, it was found that ASC-CM could significantly enhanced proliferation of DPCs in a concentration gradient manner (Figure 3). We also found that DPCs treated with 100% ASC-CM could increase the expression of ALP than those with 50% ASC-CM or regular DP medium. This may indicate ASC-CM could increase the hair-inducing ability of DPCs (Figure 4).
Figure 3.
The influence of adipose stem cell conditional medium (ASC-CM) on proliferation of cultured dermal papilla cells (DPCs). Proliferation of passage 6 DPCs cultured for 1–14 days in various ratio of ASC-CM (100%, 50%, or 0%/regular medium). Data are displayed in means ± s.d. of at least three (n = 9) independent experiments of each ratio or time point. One-way ANOVA, followed by Bonferroni’s multiple comparison test, compared with the DPC + regular medium group.
ANOVA: analysis of variance.
*p < 0.05; #p < 0.01. Bar, ISD.
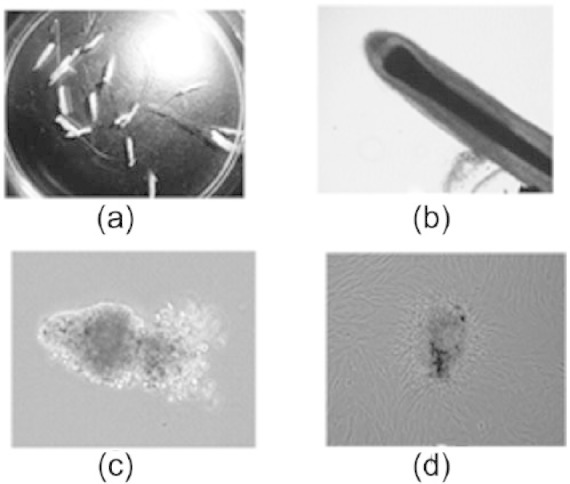
Figure 4.
Immunocytochemistry for alkaline phosphatase (ALP) in dermal papilla (DP) cells. Rat whisker DP cells in passage 6 cultured for 14 days in various ratio of adipose stem cell conditional medium (ASC-CM) (100%, 50%, or 0%/regular medium). (10×) The brown signal was positive staining of ALP. Blue color was nuclear stain with hematoxylin. (a) DP cells in 100% ASC-CM. (b) DP cells in 50% ASC-CM. (c) DP cells in 0% ASC-CM/regular medium. (d) The blank control.
The signal pathways involved in the adipocyte macroenvironment and hair growth regulation
Bone morphogenic proteins and Wnt/β-catenin pathways are the two important pathways involved in the adipocyte extrafollicle macroenvironment to hair growth. Plikus et al.82 found that BMP cycling in the intradermal adipose tissue did not correlate with the β-catenin cycling within the hair follicle. They also found that some Wnt inhibitors, Dkk1 and Sfrp4, could inhibit hair stem cell activation. The interfollicular macroenvironment could also induce anagen re-entry through releasing the activator platelet-derived growth factor–alpha (PDGFA) by the adipocyte precursor cells located in the interfollicular dermal region.59 A lack of adipocyte precursor cells resulted in bulge stem cell activation defects in Ebf1 null mice; this defect is rescued by transplanting of adipocyte precursor cells.83 These data support that a fat/follicle axis is important to the hair regeneration cycle.84
Conclusion
DPCs are essential for the growth and regeneration of hair follicles. For the purpose of hair follicle engineering, important issues including expansion of the number of DPCs and maintenance of their hair-inductive ability should be further investigated. Adipocyte lineage cells have been reported to participate in the regulation of hair follicles growth. ASCs, regarded as a promising cell source in regenerative medicine, show positive effects on DPCs hair-inducing ability. But further work needs to be completed before clinical applications of ASC-based therapeutic approaches in hair regeneration become a clinical reality. For example, the mechanisms of ASCs in the participation of the formation of hair and other skin appendages must be explored. Although there have been several studies suggesting that ASCs may stimulate the pathways enrolled in hair growth, there is little known about the role of ASCs in the epithelial–mesenchymal interaction. Additionally, despite recent progress in the lab and in clinic, there are still many gaps in the understanding of basic DPC and ASC biology that must be addressed before cell therapy can be applied to its fullest potential in clinic. Therefore, if we can understand what regulates the fate of ASCs, we can potentially enhance the function of the graft, and we can select target-specific differentiation pathways that lead to the formation of sebaceous and sweat glands, hair follicles, and interfollicular epidermis. Finally, the contribution of growth factors should be further explored. In conclusion, the potential of hair regeneration using ASCs is promising.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was supported by the National Science and Technology Support Program special funds of China (2009BAI87B03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Draelos ZD. Camouflage technique for alopecia areata: what is a patient to do? Dermatol Ther 2011; 24: 305–310. [DOI] [PubMed] [Google Scholar]
- 2. Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry 2012; 73: 1220–1223. [DOI] [PubMed] [Google Scholar]
- 3. Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov 2012; 6: 130–136. [DOI] [PubMed] [Google Scholar]
- 4. Mysore V. Synthetic hairs: should they be used? Indian J Dermatol Venereol Leprol 2006; 72: 5–7. [DOI] [PubMed] [Google Scholar]
- 5. Yoon SY, Yoon JS, Jo SJ, et al. A role of placental growth factor in hair growth. J Dermatol Sci 2014; 74: 125–134. [DOI] [PubMed] [Google Scholar]
- 6. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med 1999; 341: 491–497. [DOI] [PubMed] [Google Scholar]
- 7. Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg 2006; 25(1): 2–10. [DOI] [PubMed] [Google Scholar]
- 8. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol 2005; 3: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuzaki T, Yoshizato K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair Regen 1998; 6: 524–530. [DOI] [PubMed] [Google Scholar]
- 10. Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009; 4: 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011; 144: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ 2007; 49: 185–195. [DOI] [PubMed] [Google Scholar]
- 13. Biernaskie J, Paris M, Morozova O, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 2009; 5: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development 1992; 116: 563–571. [DOI] [PubMed] [Google Scholar]
- 15. Yamao M, Inamatsu M, Ogawa Y, et al. Contact between dermal papilla cells and dermal sheath cells enhances the ability of DPCs to induce hair growth. J Invest Dermatol 2010; 130: 2707–2718. [DOI] [PubMed] [Google Scholar]
- 16. Driskell RR, Clavel C, Rendl M, et al. Hair follicle dermal papilla cells at a glance. J Cell Sci 2011; 124: 1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messenger AG, Elliott K, Westgate GE, et al. Distribution of extracellular matrix molecules in human hair follicles. Ann N Y Acad Sci 1991; 642: 253–262. [DOI] [PubMed] [Google Scholar]
- 18. McElwee KJ, Kissling S, Wenzel E, et al. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol 2003; 121: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 19. Lee SH, Yoon J, Shin SH, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One 2012; 7: e34152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 2008; 22: 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paus R, Müller-Röver S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999; 113: 523–532. [DOI] [PubMed] [Google Scholar]
- 22. Miyake T, Cameron AM, Hall BK. Stage-specific expression patterns of alkaline phosphatase during development of the first arch skeleton in inbred C57BL/6 mouse embryos. J Anat 1997; 190: 239–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jahoda CA, Reynolds AJ, Chaponnier C, et al. Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci 1991; 99: 627–636. [DOI] [PubMed] [Google Scholar]
- 24. Hill RP, Gledhill K, Gardner A, et al. Generation and characterization of multipotent stem cells from established dermal cultures. PLoS One 2012; 7: e50742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds AJ, Chaponnier C, Jahoda CA, et al. A quantitative study of the differential expression of alpha-smooth muscle actin in cell populations of follicular and non-follicular origin. J Invest Dermatol 1993; 101: 577–583. [DOI] [PubMed] [Google Scholar]
- 26. Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci 2005; 39: 147–154. [DOI] [PubMed] [Google Scholar]
- 27. Du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol 1995; 105: 426–431. [DOI] [PubMed] [Google Scholar]
- 28. Ito Y, Hamazaki TS, Ohnuma K, et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol 2007; 127: 1052–1060. [DOI] [PubMed] [Google Scholar]
- 29. Jahoda CA, Whitehouse J, Reynolds AJ, et al. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol 2003; 12: 849–859. [DOI] [PubMed] [Google Scholar]
- 30. Tsai SY, Clavel C, Kim S, et al. Oct4 and Klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells 2010; 28: 221–228. [DOI] [PubMed] [Google Scholar]
- 31. Tsai SY, Bouwman BA, Ang YS, et al. Single transcription factor reprogramming of hair follicle dermal papilla cells to induced pluripotent stem cells. Stem Cells 2011; 29: 964–971. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development 1992; 115: 587–593. [DOI] [PubMed] [Google Scholar]
- 33. Roh C, Tao Q, Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics 2004; 19: 207–217. [DOI] [PubMed] [Google Scholar]
- 34. Zheng G, Zhu Z, Zhu K, et al. Cultivation and identification of follicular papilla cells from back skin of actual rat in vitro. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012; 26: 603–607. [PubMed] [Google Scholar]
- 35. Kamimura J, Lee D, Baden HP, et al. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J Invest Dermatol 1997; 109: 534–540. [DOI] [PubMed] [Google Scholar]
- 36. Ferraris C, Bernard BA, Dhouailly D. Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int J Dev Biol 1997; 41: 491–498. [PubMed] [Google Scholar]
- 37. Weinberg WC, Goodman LV, George C, et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol 1993; 100: 229–236. [DOI] [PubMed] [Google Scholar]
- 38. Ehama R, Ishimatsu-Tsuji Y, Iriyama S, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol 2007; 127: 2106–2115. [DOI] [PubMed] [Google Scholar]
- 39. Qiao J, Zawadzka A, Philips E, et al. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen Med 2009; 4: 667–676. [DOI] [PubMed] [Google Scholar]
- 40. Kang BM, Kwack MH, Kim MK, et al. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol 2012; 132: 237–239. [DOI] [PubMed] [Google Scholar]
- 41. Qiao J, Turetsky A, Kemp P, et al. Hair morphogenesis in vitro: formation of hair structures suitable for implantation. Regen Med 2008; 3: 683–692. [DOI] [PubMed] [Google Scholar]
- 42. Havlickova B, Bíró T, Mescalchin A, et al. Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial-mesenchymal interactions. Br J Dermatol 2004; 151: 753–765. [DOI] [PubMed] [Google Scholar]
- 43. Wu JJ, Zhu TY, Lu YG, et al. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch Dermatol Res 2006; 298: 183–190. [DOI] [PubMed] [Google Scholar]
- 44. Inoue K, Kato H, Sato T, et al. Evaluation of animal models for the hair-inducing capacity of cultured human dermal papilla cells. Cells Tissues Organs 2009; 190: 102–110. [DOI] [PubMed] [Google Scholar]
- 45. Ohyama M, Zheng Y, Paus R, et al. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol 2010; 19: 89–99. [DOI] [PubMed] [Google Scholar]
- 46. Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 2000; 14: 1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 47. Soma T, Fujiwara S, Shirakata Y, et al. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp Dermatol 2012; 21: 307–309. [DOI] [PubMed] [Google Scholar]
- 48. Inamatsu M, Matsuzaki T, Iwanari H, et al. Establishment of rat dermal papilla cell lines that sustain the potency to hair follicles from afollicular skin. J Invest Dermatol 1998; 111: 767–775. [DOI] [PubMed] [Google Scholar]
- 49. Osada A, Iwabuchi T, Kishimoto J, et al. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng 2007; 13: 975–982. [DOI] [PubMed] [Google Scholar]
- 50. Higgins CA, Chen JC, Cerise JE, et al. Microenvironmental reprogramming by three dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A 2013; 110: 19679–19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thangapazham RL, Klover P, Wang JA, et al. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J Invest Dermatol 2014; 134: 538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011; 145: 941–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002; 118: 216–225. [DOI] [PubMed] [Google Scholar]
- 54. Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012; 10: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Inoue K, Aoi N, Yamauchi Y, et al. TGF-beta is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med 2009; 13: 4643– 4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dani C, Billon N. Adipocyte precursors: developmental origins, self-renewal, and plasticity. In:Symonds ME. (ed.) Adipose tissue biology. London: Springer, 2012, pp. 1–16. [Google Scholar]
- 57. Chen HC, Smith SJ, Tow B, et al. Leptin modulates the effects of acyl CoA: diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest 2002; 109: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansen LS, Coggle JE, Wells J, et al. The influence of the hair cycle on the thickness of mouse skin. Anat Rec 1984; 210: 569–573. [DOI] [PubMed] [Google Scholar]
- 59. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011; 146: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Misago N, Toda S, Sugihara H, et al. Proliferation and differentiation of organoid hair follicle cells co-cultured with fat cells in collagen gel matrix culture. Br J Dermatol 1998; 139: 40–48. [DOI] [PubMed] [Google Scholar]
- 61. Plikus MV, Mayer JA, de la, Cruz D, et al. Cyclic dermal BMP signaling regulates stem cell activation during hair regeneration. Nature 2008; 451: 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jong MC, Gijbels MJ, Dahlmans VE, et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J Clin Invest 1998; 101: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stone SJ, Myers HM, Watkins SM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 2004; 279: 11767–11776. [DOI] [PubMed] [Google Scholar]
- 64. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007; 100: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 1992; 13: 69–80. [DOI] [PubMed] [Google Scholar]
- 66. Kim CG, Lee JJ, Jung DY, et al. Profiling of differentially expressed genes in human stem cells by cDNA microarray. Mol Cells 2006; 21: 343–355. [PubMed] [Google Scholar]
- 67. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 69. Philips BJ, Marra KG, Rubin JP. Adipose stem cell-based soft tissue regeneration. Expert Opin Biol Ther 2012; 12: 155–163. [DOI] [PubMed] [Google Scholar]
- 70. Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001; 189: 54–63. [DOI] [PubMed] [Google Scholar]
- 71. Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004; 6: 7–14. [DOI] [PubMed] [Google Scholar]
- 72. Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev 2007; 16: 91–104. [DOI] [PubMed] [Google Scholar]
- 73. He J, Duan H, Xiong Y, et al. Participation of CD34-enriched mouse adipose cells in hair morphogenesis. Mol Med Rep 2013; 7: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 74. Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab 2012; 23: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim WS, Park BS, Kim HK, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 2008; 49: 133–142. [DOI] [PubMed] [Google Scholar]
- 76. Kim WS, Park BS, Park SH, et al. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 2009; 53: 96–102. [DOI] [PubMed] [Google Scholar]
- 77. Kim WS, Park SH, Ahn SJ, et al. Whitening effect of adipose-derived stem cells: a critical role of TGF-beta 1. Biol Pharm Bull 2008; 31: 606–610. [DOI] [PubMed] [Google Scholar]
- 78. Park BS, Jang KA, Sung JH, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 2008; 34: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 79. Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res 2010; 31: 27–34. [DOI] [PubMed] [Google Scholar]
- 80. Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007; 48: 15–24. [DOI] [PubMed] [Google Scholar]
- 81. Won CH, Yoo HG, Kwon OS, et al. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci 2010; 57: 134–137. [DOI] [PubMed] [Google Scholar]
- 82. Plikus MV, Widelitz RB, Maxson R, et al. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol 2009; 53: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell 2011; 146: 678–681. [DOI] [PubMed] [Google Scholar]
- 84. Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci 2012; 66: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]