Abstract
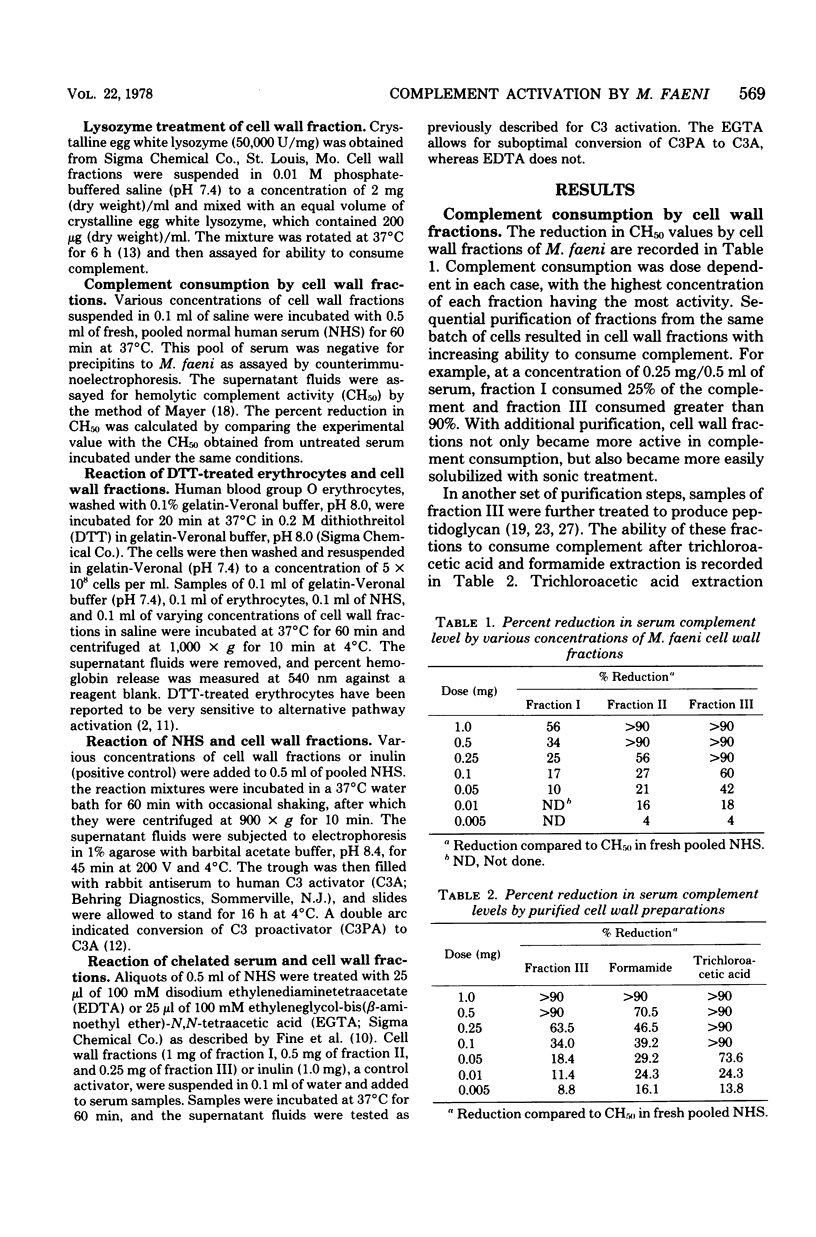
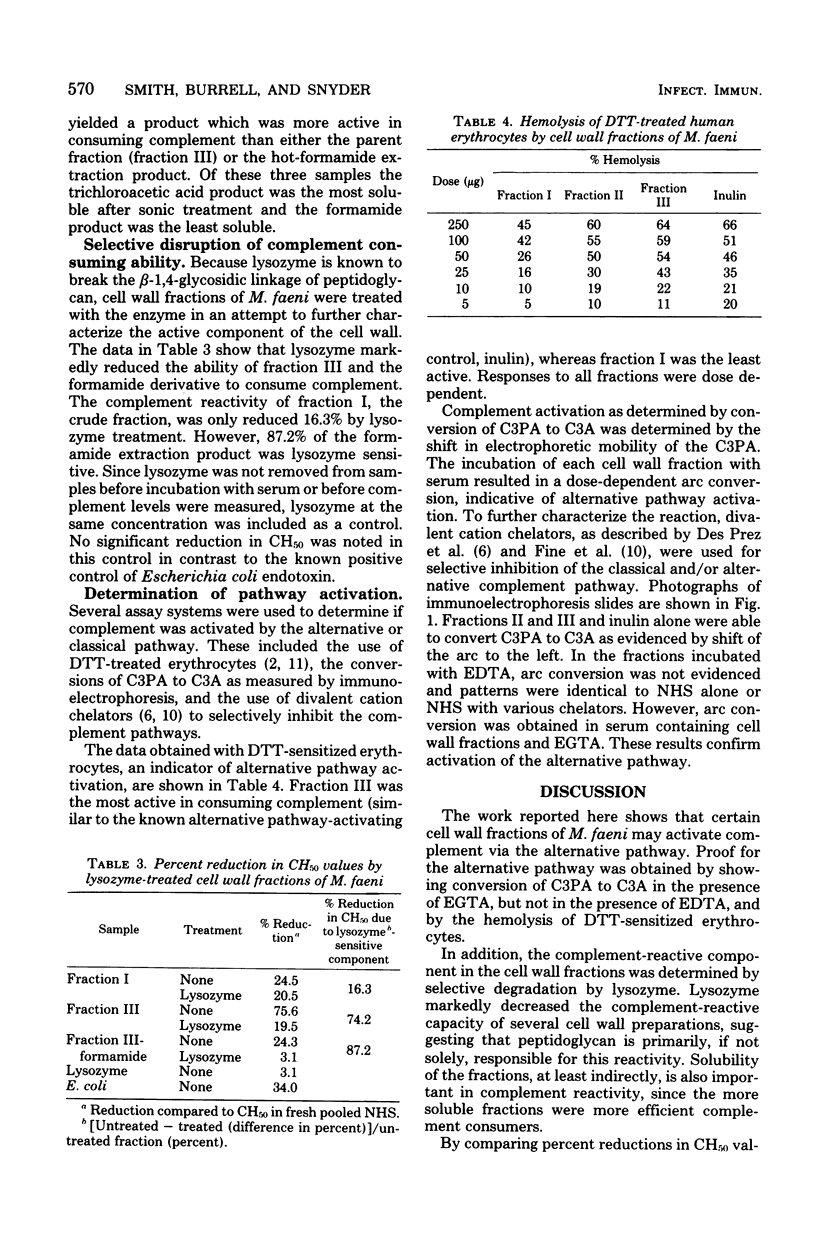
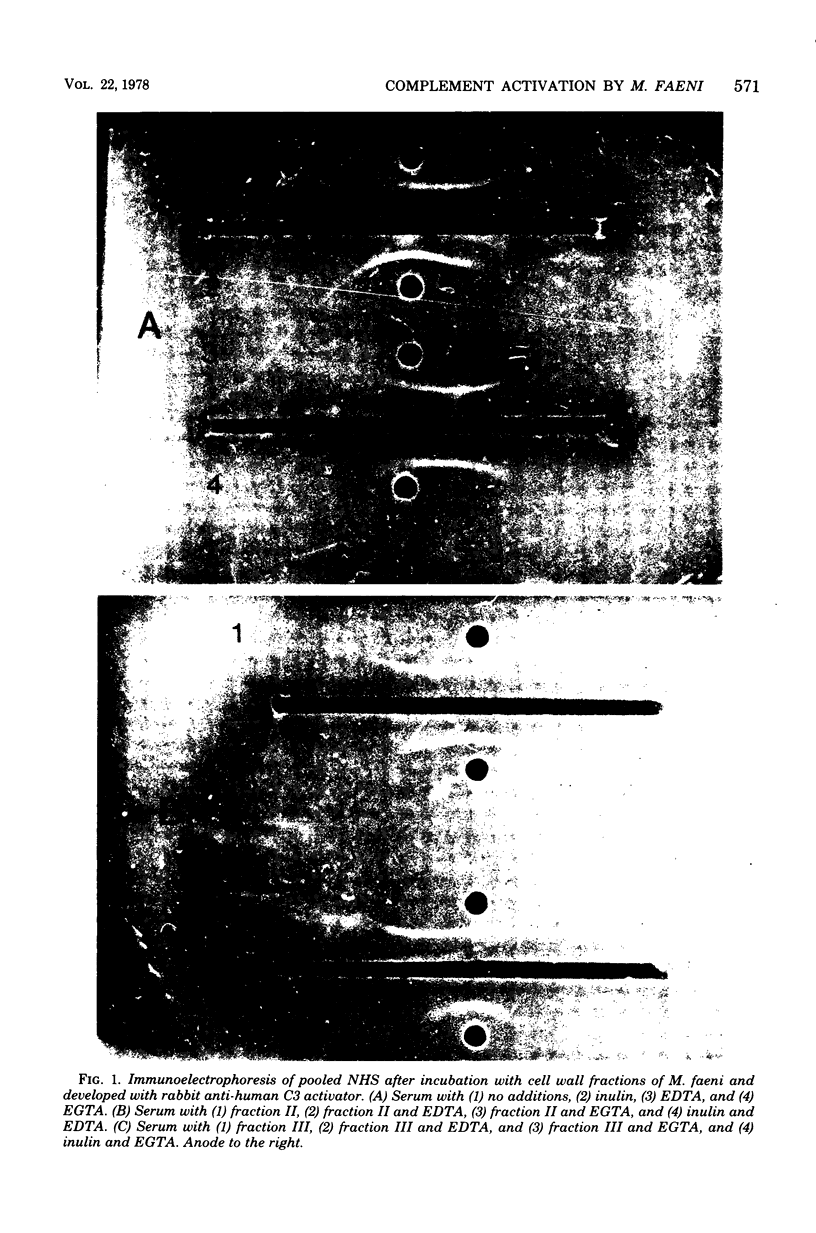
The ability of several cell wall fractions of Micropolyspora faeni, a thermophilic actinomycete associated with farmer's lung disease, to activate complement is reported. Cell walls, obtained by mechanical disruption, were purified by enzyme treatment and chemical extractions. Fractions containing the most purified cell walls were most active in consuming complement, as measured by reduction of hemolytic complement levels of normal human serum. Cell wall fractions activated the alternative complement pathway, as shown by monitoring the conversion of C3 proactivator (factor B) to C3 activator (activated factor B) in the presence of specific cation chelators. Selective degradation of cell walls by lysozyme resulted in a decreased ability to consume complement and implicated peptidoglycan as the major complement-reactive component. The role of this nonspecific complement activation in relation to farmer's lung disease is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulla E. M., Schwab J. H. Biological properties of streptococcal cell-wall particles. 3. Dermonecrotic reaction to cell-wall mucopeptides. J Bacteriol. 1966 Jan;91(1):374–383. doi: 10.1128/jb.91.1.374-383.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyave C. M., Vallota E. H., Müller-Eberhard H. J. Lysis of human erythrocytes due to activation of the alternate complement pathway by nephritic factor (C3NeF). J Immunol. 1974 Sep;113(3):764–768. [PubMed] [Google Scholar]
- Bice D. E., McCarron K., Hoffman E. O., Salvaggio J. Adjuvant properties of Micropolyspora faeni. Int Arch Allergy Appl Immunol. 1977;55(1-6):267–274. doi: 10.1159/000231935. [DOI] [PubMed] [Google Scholar]
- Burrell R., McCullough M. J. Production of thermophilic actinomycete-hay aerosols for use in experimental hypersensitivity pneumonitis. Appl Environ Microbiol. 1977 Dec;34(6):715–719. doi: 10.1128/aem.34.6.715-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. H. A quantitative study on the activation of the alternative pathway of complement by mouldy hay dust and thermophilic actinomycetes. Clin Allergy. 1976 Jan;6(1):19–25. doi: 10.1111/j.1365-2222.1976.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974 Nov;113(5):1583–1588. [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions: evidence for the selective release of lysosomal constituents. J Immunol. 1972 Feb;108(2):310–317. [PubMed] [Google Scholar]
- Lazáry S., Nicolet J., Rivera E., Wanner M. In vitro response of lymphocytes to Micropolyspora faeni extract in cattle. Res Vet Sci. 1975 Sep;19(2):195–200. [PubMed] [Google Scholar]
- Lopez M., Salvaggio J. Hypersensitivity pneumonitis: current concepts of etiology and pathogenesis. Annu Rev Med. 1976;27:453–463. doi: 10.1146/annurev.me.27.020176.002321. [DOI] [PubMed] [Google Scholar]
- Marx J. J., Flaherty D. K. Activation of the complement sequence by extracts of bacteria and fungi associated with hypersensitivity pneumonitis. J Allergy Clin Immunol. 1976 Apr;57(4):328–334. doi: 10.1016/0091-6749(76)90089-0. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- PEPYS J., JENKINS P. A., FESTENSTEIN G. N., GREGORY P. H., LACEY M. E., SKINNER F. A. FARMER'S LUNG. THERMOPHILIC ACTINOMYCETES AS A SOURCE OF "FARMER'S LUNG HAY" ANTIGEN. Lancet. 1963 Sep 21;2(7308):607–611. doi: 10.1016/s0140-6736(63)90398-2. [DOI] [PubMed] [Google Scholar]
- PEPYS J., JENKINS P. A. PRECIPITIN (F.L.H.) TEST IN FARMER'S LUNG. Thorax. 1965 Jan;20:21–35. doi: 10.1136/thx.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys J. Hypersensitivity diseases of the lungs due to fungi and organic dusts. Monogr Allergy. 1969;4:1–147. [PubMed] [Google Scholar]
- Richerson H. B., Seidenfeld J. J., Ratajczak H. V., Richards D. W. Chronic experimental interstitial pneumonitis in the rabbit. Am Rev Respir Dis. 1978 Jan;117(1):5–13. doi: 10.1164/arrd.1978.117.1.5. [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Zais D. P., Emanuel D. A. The frequency of precipitins to trichloroacetic acid-extractable antigens from thermophilic actinomycetes in farmer's lung patients and asymptomatic farmers. Am Rev Respir Dis. 1976 Jul;114(1):23–28. doi: 10.1164/arrd.1976.114.1.23. [DOI] [PubMed] [Google Scholar]
- Schatz M., Patterson R., Fink J. Immunopatholgenesis of hypersensitivity pneumonitis. J Allergy Clin Immunol. 1977 Jul;60(1):27–37. doi: 10.1016/0091-6749(77)90079-3. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H. Chemical structure of the peptidoglycan, its modifiability and relation to the biological activity. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):104–117. [PubMed] [Google Scholar]
- Smith S. M., Hill J. O., Snyder I. S., Burrell R. Mitogenicity of cell wall fractions of Micropolyspora faeni. Ann Allergy. 1978 Jan;40(1):12–14. [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Wilkie B., Pauli B., Gygax M. Hypersensitivity pneumonitis: experimental production in guinea pigs with antigens of Micropolyspora faeni. Pathol Microbiol (Basel) 1973;39(6):393–411. doi: 10.1159/000162686. [DOI] [PubMed] [Google Scholar]
- doPico G. A., Reddan W. G., Chmelik F., Peters M. E., Reed C. E., Rankin J. The value of precipitating antibodies in screening for hypersensitivity pneumonitis. Am Rev Respir Dis. 1976 Apr;113(4):451–455. doi: 10.1164/arrd.1976.113.4.451. [DOI] [PubMed] [Google Scholar]