Abstract
There has been a large increase in basic science activity in cell therapy and a growing portfolio of cell therapy trials. However, the number of industry products available for widespread clinical use does not match this magnitude of activity. We hypothesize that the paucity of engagement with the clinical community is a key contributor to the lack of commercially successful cell therapy products. To investigate this, we launched a pilot study to survey clinicians from five specialities and to determine what they believe to be the most significant barriers to cellular therapy clinical development and adoption. Our study shows that the main concerns among this group are cost-effectiveness, efficacy, reimbursement, and regulation. Addressing these concerns can best be achieved by ensuring that future clinical trials are conducted to adequately answer the questions of both regulators and the broader clinical community.
Keywords: Cell- and tissue-based therapy, translational medical research, regenerative medicine, tissue engineering, stem cells
Introduction
Cellular therapies offer significant potential to treat multiple diseases and injuries, many of which are poorly addressed by the current standards of care. These ground-breaking therapies have grown out of, and to some extent encompass, concepts from both tissue engineering (TE) and regenerative medicine (RM), first described in the 1980s1 and 1990s,2 respectively (see Table 1 for definitions). Despite significant developments in these areas, there are still relatively few commercially available products in widespread use that utilize the principles of these two fields, as seen in Table 2 (adapted from French et al.3).
Table 1.
Definition of key terms.
| Term | Definition |
|---|---|
| Tissue engineering | “An interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function.”38 |
| Regenerative medicine | A technique that “replaces or regenerates human cells, tissue or organs, to restore or establish normal function.”39 |
Table 2.
Cellular therapy products in use.
| Medical speciality | Company | Product | Condition |
|---|---|---|---|
| Cardiology | FCB Pharmicell | Heartcelligram | Acute myocardial infarction |
| Diabetes | Organogenesis | Dermagraft | Diabetic foot ulcers |
| Diabetes/Vascular Disease | Organogenesis | Apligraf | Venous leg and diabetic foot ulcers |
| Gastroenterology | Anterogen | Cupistem | Crohn’s disease |
| Maxillofacial Surgery | Organogenesis | Gintuit | Gingival repair |
| Orthopedics | Genzyme | Carticel | Articular cartilage repair |
| Orthopedics | Medipost | Cartistem | Articular cartilage repair |
| Orthopedics | NuVasive | Osteocel Plus | Skeletal defects |
| Orthopedics | Orthofix | Trinity Evolution | Musculoskeletal defects |
| Orthopedics | TiGenix | ChondroCelect | Articular cartilage repair |
| Plastics | Avita Medical | ReCell | Burns/scars |
| Plastics, Orthopedics, Ophthalmology | Japan Tissue Engineering | J-TEC Epidermis/Cartilage/Corneal Epithelium | Burns, cartilage repair, ocular repair |
| Urology | Dendreon | Provenge | Prostate cancer |
| Osiris | Prochymal | Graft-vs-host disease | |
| Genzyme | Epicel | Severe burns |
The most widely used products have been in the TE and bone marrow transplant areas, yet as outlined by Jaklenec et al.,4 the TE space has experienced a fitful tenure over the last 25 years. The failure of products to live up to the promise held during development has negatively impacted upon the willingness of clinicians to embrace these technologies, consequently resulting in a lack of long-term efficacy data.5 More recently though the sector has overcome some of these early disappointments facilitated by early evidence of clinical success, investor appetite, and expanded research funding.6–8
It is important to recognize that there is a lack of consistency in the use of terms such as “cell-based therapies,” “regenerative medicine,” “tissue engineering,” and “stem cell therapy,” with some treating the terms as distinct entities, while others use them synonymously.4 Within this article, the term “cellular” or “cell-based therapy” encompasses any treatment that involves the use of exogenous cells (autologous or allogenic) in an attempt to repair diseased or damaged tissue.
Cell-based therapy progress
The rapid growth of this sector is clearly demonstrated by the soaring number of clinical trials that seek to use cell-based therapies.9,10 Despite this, the number of cell-based therapies available in the marketplace has remained fairly stagnant.3
To better understand this disparity, we have sought to investigate the potential barriers to the clinical development and adoption of this class of novel therapeutics as perceived by the end users of such therapies, clinicians, via an initial pilot study. It is hoped that by successfully identifying these root causes, it will be possible to streamline and accelerate the process by which potentially useful cell-based therapies are developed and then brought into mainstream use. We therefore conducted a pilot study at a leading hospital in the United Kingdom as a necessary and informative basis for future investigations.
Materials and methods
To identify the main barriers to adoption from the point of view of clinicians, 50 specialists were approached at a leading academic teaching hospital group in the United Kingdom, evenly split into the following areas:
Cardiology
Neurology
Ophthalmology
Orthopedic Surgery
Plastic and Reconstructive Surgery
These specialities were carefully chosen as areas in which there has been research and development of cellular therapies or in which there is significant contemporary interest in basic stem cell science. Historically, Orthopedic Surgery has been the trailblazer since autologous chondrocyte implantation was first described 20 years ago.11 The other clinical specialities have been the focus of cell-based therapies in recent years with treatments designed for myocardial infarction,12 multiple sclerosis,13 and retinal photoreceptor loss.14
Participants were surveyed between July and August of 2013. Participants were asked about their experience using cellular therapeutics and then required to assess whether 12 areas were seen as a barrier to cellular treatment use. A copy of the questionnaire is provided in the Supplementary Material and the areas covered were as follows:
Safety
Efficacy
Clinical trial methodologies
Cost-effectiveness
Usability
Visibility
Patient characteristics
Patient attitudes and preferences
Infrastructure
Reimbursement
Community
Regulation
A Likert-style scale was used to assess responses. Each question was scored on a scale of 1 (No Barrier) to 5 (Significant Barrier). Responses from the questionnaire were averaged across all respondents and within each speciality and then ranked in order from the smallest perceived barrier to the largest.
The results were analyzed separately by speciality and also as a whole for the complete group. Analysis was carried out using Prism 6 (GraphPad Software Inc., San Diego, CA, USA).
Results
Responses were obtained from 50 individuals in total with 10 in each speciality group. The participants had a range of knowledge and experience with cell-based therapies as shown in Figure 1.
Figure 1.
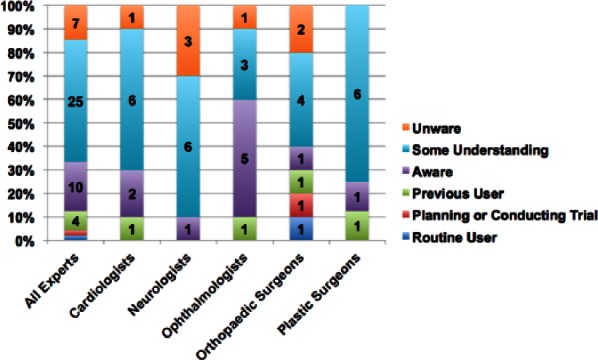
Survey participant knowledge and extent of familiarity with cell therapy products.
There was no significant difference in the experience spread within the individual specialities (Kruskal–Wallis = 7.7; p = 0.17). The majority of participants had some knowledge of the field of cellular therapies although very few had actually used them in a clinical setting.
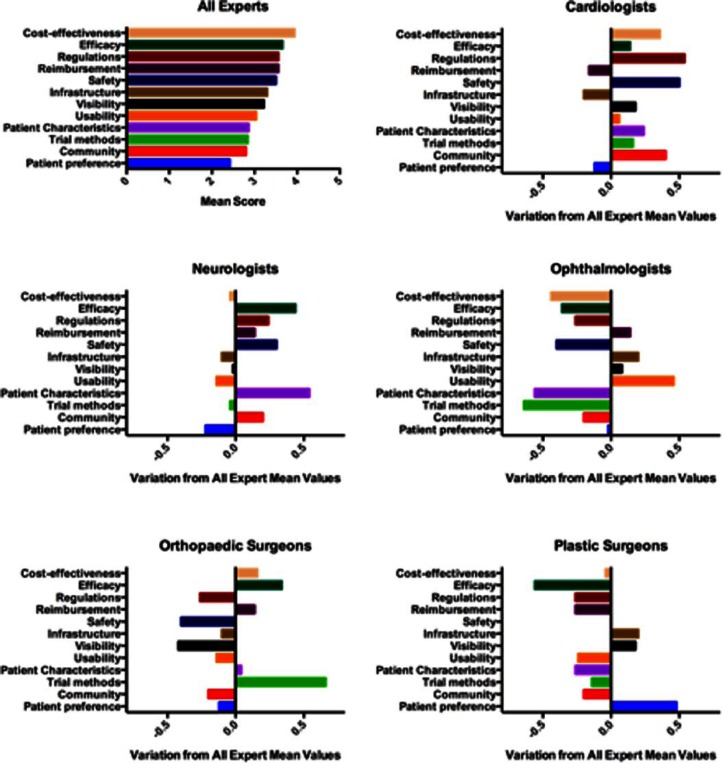
Figure 2 shows the mean level for perceived barrier for all respondents and the variation in the mean response for each speciality from the group mean. Higher numbers represent a larger perceived barrier to adoption. The data show that the most concerning barriers to clinicians were cost-effectiveness and efficacy, followed by regulation, reimbursement, and safety. Infrastructure also frequently occurred in the top three responses.
Figure 2.
Perceived barriers to clinical development and adoption as identified through pilot questionnaire. Responses are presented as the mean for the whole group and then variation in each speciality’s mean response from the group mean. A higher score represents a greater perceived barrier.
Figure 3 shows the average score for each question in each group. A heat map has been constructed with green being the smallest barrier and red the largest barrier to adoption to help visualize the spread of opinion across specialities. Interestingly, a number of notable concerns were identified in relation to clinical speciality, although due to the sample size of this pilot study, further investigation is required. Neurologists identified patient characteristics as a major barrier to cell therapy utilization in comparison to the other four clinical specialities (Figure 3). Ophthalmologists were concerned by usability; orthopedic surgeons identified clinical trial methods and plastic surgeons efficacy and patient preference.
Figure 3.
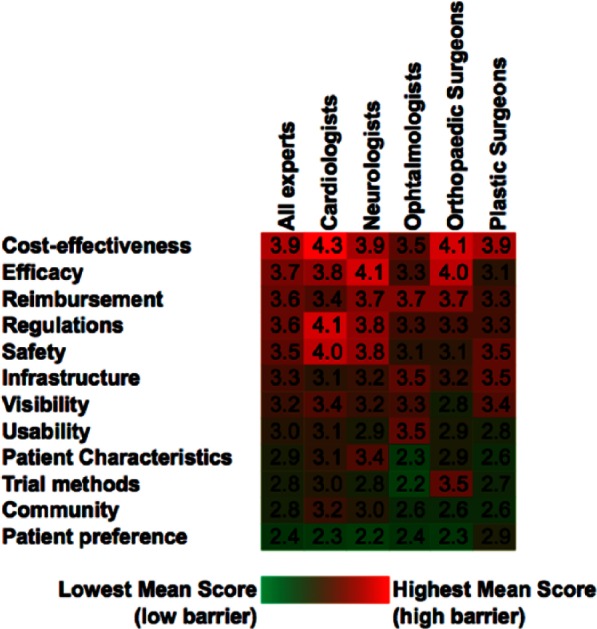
Heat map of mean response data to perceived barriers by clinical speciality. Data are presented as the mean response from the polled clinicians.
Discussion
The results from our pilot study show that across multiple specialities, there is a degree of consistency for which barriers to development and adoption are perceived as the largest for cell-based therapies, namely, efficacy and cost-effectiveness. The remaining barriers perceived to be significant do vary between groups with reimbursement, safety, regulations, and infrastructure all identified consistently within the highest ranking barriers, as shown in Figure 4.
Figure 4.
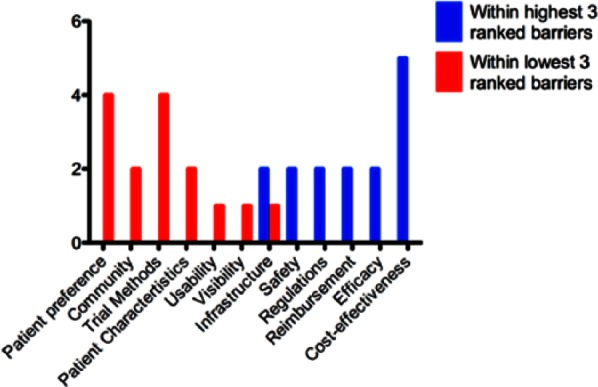
Frequency with which each barrier appears in highest or lowest three perceived barriers (ordered as per “All Experts” mean ranking).
It is no surprise that efficacy poses a considerable barrier in the opinion of those asked, followed closely by safety, as the primary aim of the clinician is to treat and hopefully cure the patient. Deficiencies in safety and efficacy results can to some extent be explained by the often limited understanding of the underlying mechanism of action.15 Additionally, heterogeneity in clinical trial structures, treatment protocols, and results presentation, combined with the limited body of historic patient outcome data, further impede clinical decision-making for when these treatments may be beneficial.16–19 Data limitations are also challenging from a health economics perspective.
In most cases, cellular therapies are significantly more expensive than the treatment that they seek to replace.20 They can, however, potentially offer a longer lasting solution and the opportunity to regenerate tissue, unlike traditional treatments.21 It is therefore vital, in both private and public provider systems, to ensure that adequate product cost-effectiveness is demonstrated to justify appropriate reimbursement codes for the entire treatment pathway, which in the case of autologous products includes medical procedures relating to tissue acquisition.22 As such, it is evident that many cell-based therapeutics are likely to exhibit different direct and indirect cost structures to established non-cell-based products and services,23 in part, due to the unique infrastructural demands concerning their delivery.
For all healthcare innovations, the requisite delivery infrastructure and appropriate regulation(s) are intimately linked;24 principally, due to the need to maintain product critical quality attributes (CQAs) throughout the entire biomanufacturing process from tissue acquisition to administration.25–27 Given the interdependence of cellular therapies on their bioprocessing environment,28 they are highly reliant upon the availability and compliance of Current Good Manufacturing Practices (cGMP) laboratories, internal and external to hospital settings, that must adhere to strict workflows for provision and manipulation of cells and tissues for the safe re-implantation to the patient.29 The lack of supporting data for point-of-care (POC) processing technologies which avoid the need for cGMP laboratories explains, to some extent, the lack of clinical confidence in these systems.30,31 Moving forward, these systems must therefore be designed such that ease of use and safety of the cellular material is paramount and the path to approval is clear.32
More generally, clinical trial methodologies were not felt to be a large barrier to adoption within our pilot study, which may be due to a perception of the rigor with which trials are conducted or a lack of concern regarding the methodology of clinical trials, yet recent late-stage failures intimate that greater caution may be required.33,34 Interestingly, orthopedics, a speciality in which cell therapies have been more frequently utilized, identified clinical trial methodologies as a barrier in comparison to the other clinical groups. Usability was of moderate concern to most groups which further emphasizes the need to develop technologies that are easily adopted for a wide variety of clinical settings, ideally with minimal training for support staff, such as operating department assistants. Finally, patient attitudes and preferences were frequently listed in the three smallest barriers to adoption, possibly due to a lack of patient awareness as to the available options and/or a clinician belief that it may not be worth discussing options with patients for which they have limited confidence or that are not readily available. It should be noted, however, that as this pilot study was performed in the United Kingdom, an emphasis with regard to patient attitudes and preferences in private payer systems, such as the United States, maybe expected to yield different results.
Additionally, the ranking distribution for different issues may be due to the variation in previous exposure to these treatments. Although there was no significant difference in the self-reported level of experience of cellular therapies between the groups, there is a great deal of variation in the extent to which trials of cell therapy treatments have penetrated the various specialities.35 Simply by examining the currently available cellular therapies as shown by the review undertaken by French et al.,3 very few of these treatments are available within normal clinical practice.
Limitations
The main limitation affecting this study comes from the use of a questionnaire and from the sample size of the pilot study. It is not possible with this design to ensure that there is exactly the same level of understanding of the issues being asked about between specialists. This variation in understanding will, however, better reflect the understanding of all clinicians rather than use subject experts only. It is also not possible to determine the exact nature of the reason for which experts attribute a particular score, so underlying motivations may be better elicited in future studies with the opportunity for qualitative responses. The restriction of this pilot study to a single hospital group will also skew the conclusions that we have attempted to draw from the results. Nonetheless, scores obtained were wide-ranging both between groups and individuals, hence the risk of having surveyed a biased subset of specialists was likely minimal.
The route ahead
While immense investment into the cell-based therapy market has occurred over the last decade,36,37 this increase has sadly not been matched by the widespread availability of treatments to patients. It is clear that while the scientific barriers to the creation and development of effective cellular therapies are gradually being overcome, another sizable potential barrier to broad adoption lies in the attitudes and decision-making processes of the clinical community to prescribe and recommend such innovative approaches.
Despite the diverse availability of cell-based therapies in different clinical specialities, it is clear from this pilot study that similar barriers to clinical development and adoption are common among all. The majority of these barriers related to clinician concern (cost-effectiveness, efficacy, and safety) can only be successfully tackled by conducting well-designed clinical trials that effectively address the following areas:
Appropriate length of follow-up to ensure that clinical benefits are maintained over a period that is clinically relevant. Significant benefits would also be gained from identifying short-term indicators correlated with long-term outcomes for the indication in question.
Use of a variety of clinical- and laboratory-based outcome measures to allow comparison between different trials.
Current standard of care, where possible, is included within the trials as a control arm.
Design therapies and trials to enable the regulatory issues of different markets to be addressed in an efficient manner.
Thoughtful clinical trial design, focused on the above criteria and involving the clinicians at an early stage, should allow not only effectiveness of a treatment to be confirmed but also safety and cost-effectiveness with relative ease. It should then be possible to address regulatory issues in such a way as to minimize delays in bringing these innovative treatments to market, and therein reaching the end goal of treating patients suffering with unmet medical needs.
It is our intention to progress the findings identified in this pilot study by carrying out a further round of questioning covering an international set of locations and widening the specialities to include others such as Ear, Nose, and Throat Surgery and Urology. The sample number of the clinicians surveyed will be significantly extended. This will, hopefully, allow us to capture a broader, and more representative view of the barriers that clinicians perceive to be preventing cellular therapy use. Findings will then be reviewed by an international expert panel to maximize the value of conclusions drawn from the data obtained.
Centre for the Advancement of Sustainable Medical Innovation Translational Stem Cell Consortium
Clinical perspective questionnaire
The Centre for the Advancement of Sustainable Medical Innovation (CASMI) Translational Stem Cell Consortium (CTSCC) is an international, multi-stakeholder collaboration aiming to provide a global nexus for the efficient and sustainable translation of fundamental stem cell science into tangible products and services. Due to the novel nature of cellular therapies, it is evident that the clinical community will play a critical role in their successful adoption. As such, it is imperative that we poll the opinions of surgical clinicians like yourself in order to best address the potential barriers that currently or may in the future be impediments to these therapies from your perspective.
Section 1: Respondent Background Information
Clinical Specialty: ____________________________
Job Title or Position: __________________________
Extent of Experience with Cellular Therapeutics: (Please indicate all that apply)
| Routine user of cellular therapies | Aware of available cellular therapies in your field; however, specific reason(s) unwilling to use them. (please state why below) |
| Currently holding or planning a cell therapy clinical trial | Some understanding the fundamental science underpinning cellular therapeutics |
| A previous user of cellular therapies; however, for any reason(s) have ceased using them | Unaware of landscape and possible cellular therapy options |
Please state the reasons that stopped you from using cellular therapy (if any):
Section 2: Assessment of Barriers to Clinical Adoption
Following an industry review, the following factors have been suggested as barriers to the adoption of cellular therapeutics. Below please indicate the extent to which you believe each case is a potential impediment to their clinical success, where 1 = No Barrier(s) and 5 = Significant Barrier(s)
Safety: Extent of available data concerning product safety including the stage of development of underlying science and/or characteristics of biomaterials utilized in tissue engineering.
Efficacy: Extent to which available data concerning product efficacy profiles, including the extent to which mechanisms of action, have been proposed and identified.
Clinical Trial Methodologies: Quality and rigor of clinical trial designs implemented.
Cost-Effectiveness: Availability of data to inform evaluations of the benefits of cellular therapeutics versus standard(s) of care.
Usability: Limitations in ease of use.
Visibility: Level of awareness of cellular therapeutics and their applications within the clinical community.
Patient Characteristics: Limitations in the number of patients with suitable prognosis to facilitate the administration of a cellular therapeutic:
Patient Attitudes & Preferences: Level of patient familiarity, resistance, or concern around cell therapies, such as concerns regarding “stem cell stigma.”
Infrastructure: Uncertainties in clinical delivery including available expertise, equipment, “chain(s) of custody” control, or others.
Reimbursement: Relative provision and/or knowledge of reimbursement pathways compared to routine therapies.
Community: Impact of peer opinion(s) on clinical decision-making.
Regulation: Level of certainty or ambiguity in the regulatory pathway including the regulatory classification of cellular therapeutics and/or responsibilities of clinical practitioners and fear of malpractice.
Supplementary Material
Acknowledgments
This work was carried out by the Centre for the Advancement of Sustainable Medical Innovation (CASMI) and we wish to express our sincere thanks to the following organizations that have contributed to the consortium as funding and event partners—without whom the consortium and the benefits it will bring to stem cell translation would be unacceptably constrained: GE Healthcare, CCRM, TAP Biosystems, Lonza, CIRM, SENS Research Foundation, UK Cell Therapy Catapult, and NIH Centre for Regenerative Medicine. We would also like to thank Mr Prasanna Sooriakumaran for his assistance with this study. Andrew Carr and David A Brindley are joint senior authors.
Footnotes
Declaration of conflicting interests: We would like to make the following declarations regarding conflicts of interest:
B.R. is a stockholder in Pathfinder Cell Therapy (MA, USA). R.W.B. is an independent director and/or stockholder in Celgene (NJ, USA). K.B. is an employee and/or stockholder in Sartorius Stedim (Göttingen, Germany). J.M.K. was supported by National Institutes of Health grant HL095722, Department of Defense grant W81XWH-13-1-0305, and by a Movember-Prostate Cancer Foundation Challenge. The content outlined herein represents the individual opinions of the authors and may not necessarily represent the viewpoints of their employers. D.A.B. gratefully acknowledges the support from the SENS Research Foundation (Mountain View, CA, USA). D.A.B. is a stockholder in Translation Ventures Ltd. (Charlbury, Oxfordshire, UK), a company that among other services provides cell therapy biomanufacturing, regulatory, and financial advice to clients in the cell therapy sector. D.A.B. is subject to the CFA Institute’s Codes, Standards, and Guidelines, and as such, this author must stress that this piece is provided for academic interest only and must not be construed in any way as an investment recommendation. Additionally, at the time of publication, D.A.B. and the organizations with which he is affiliated may or may not have agreed and/or pending funding commitments from the organizations named herein. D.A.B. has also conducted paid consultancy for Lonza Group and Sartorius Stedim within the past 7 years with a cumulative value of greater than US$10,000. All the other authors do not declare any additional conflicts of interest, as defined by the journal. However, the authors are happy to respond to direct requests for confirmation and stress that affiliations stated may or may not constitute a disclosure of employment and/or ownership of financial instruments in the named entity.
Funding: This work was supported by National Institutes of Health grant HL095722, Department of Defense grant W81XWH-13-1-0305, and by a Movember-Prostate Cancer Foundation Challenge Award to J.M.K.
References
- 1. Kemp P. History of regenerative medicine: looking backwards to move forwards. Regen Med 2006; 1(5): 653–669. [DOI] [PubMed] [Google Scholar]
- 2. Kaiser LR. The future of multihospital systems. Top Health Care Financ 1992; 18(4): 32–45. [PubMed] [Google Scholar]
- 3. French A, Buckler RL, Brindley DA. Commercialization of regenerative medicine: learning from spin-outs. Rejuvenation Res 2013; 16(2): 164–170. [DOI] [PubMed] [Google Scholar]
- 4. Jaklenec A, Stamp A, Deweerd E, et al. Progress in the tissue engineering and stem cell industry “Are we there yet?” Tissue Eng Part B Rev 2012; 18(3): 155–166. [DOI] [PubMed] [Google Scholar]
- 5. Hawkins RE, Gilham DE, Debets R, et al. Development of adoptive cell therapy for cancer: a clinical perspective. Hum Gene Ther 2010; 21(6): 665–672. [DOI] [PubMed] [Google Scholar]
- 6. Rao M. National Institutes of Health Center for Regenerative Medicine: putting science into practice. Stem Cells Dev 2013; 22(S1): 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson K, Foster EP. The Cell Therapy Catapult: growing a U.K. cell therapy industry generating health and wealth. Stem Cells Dev 2013; 22(S1): 35–39. [DOI] [PubMed] [Google Scholar]
- 8. Siva N. Pfizer’s $100 million stem cell stake. Nat Biotechnol 2009; 27(1): 10. [Google Scholar]
- 9. Culme-Seymour EJ, Davie NL, Brindley DA, et al. A decade of cell therapy clinical trials (2000–2010). Regen Med 2012; 7(4): 455–462. [DOI] [PubMed] [Google Scholar]
- 10. Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med 2014; 9: 27–39. [DOI] [PubMed] [Google Scholar]
- 11. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331(14): 889–895. [DOI] [PubMed] [Google Scholar]
- 12. Latham N, Ye B, Jackson R, et al. Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation 2013; 128(11 Suppl. 1): S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rice CM, Kemp K, Wilkins A, et al. Cell therapy for multiple sclerosis: an evolving concept with implications for other neurodegenerative diseases. Lancet 2013; 382(9899): 1204–1213. [DOI] [PubMed] [Google Scholar]
- 14. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006; 444(7116): 203–207. [DOI] [PubMed] [Google Scholar]
- 15. Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem 2012; 113(5): 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martell K, Trounson A, Baum E. Stem cell therapies in clinical trials: workshop on best practices and the need for harmonization. Cell Stem Cell 2010; 7: 451–454. [DOI] [PubMed] [Google Scholar]
- 17. Gómez-Barrena E, Rosset P, Müller I, et al. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med 2011; 15(6): 1266–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tibbetts MD, Samuel MA, Chang TS, et al. Stem cell therapy for retinal disease. Curr Opin Ophthalmol 2012; 23(3): 226–234. [DOI] [PubMed] [Google Scholar]
- 19. Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med 2006; 355(16): 1730–1735. [DOI] [PubMed] [Google Scholar]
- 20. Gerlier L, Lamotte M, Wille M, et al. The cost utility of autologous chondrocytes implantation using ChondroCelect® in symptomatic knee cartilage lesions in Belgium. Pharmacoeconomics 2010; 28(12): 1129–1146. [DOI] [PubMed] [Google Scholar]
- 21. Sergijenko A, Langford-Smith A, Liao AY, et al. Myeloid/Microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Mol Ther 2013; 21(10): 1938–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maziarz RT, Driscoll D. Hematopoietic stem cell transplantation and implications for cell therapy reimbursement. Cell Stem Cell 2011; 8(6): 609–612. [DOI] [PubMed] [Google Scholar]
- 23. L’Heureux N, Dusserre N, Marini A, et al. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med 2007; 4(7): 389–395. [DOI] [PubMed] [Google Scholar]
- 24. Fink DW. FDA regulation of stem cell-based products. Science 2009; 324(5935): 1662–1663. [DOI] [PubMed] [Google Scholar]
- 25. Rowley JA. Developing cell therapy biomanufacturing processes. Chem Eng Prog 2010: 106: 50–55. [Google Scholar]
- 26. Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell 2008; 3(4): 369–381. [DOI] [PubMed] [Google Scholar]
- 27. Ratcliffe E, Thomas RJ, Williams DJ. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Brit Med Bull 2011; 100(1): 137–155. [DOI] [PubMed] [Google Scholar]
- 28. Brindley D, Moorthy K, Lee J-H, et al. Bioprocess forces and their impact on cell behavior: implications for bone regeneration therapy. J Tissue Eng 2011; 2011: 620247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. Muscles Ligaments Tendons J 2012; 2(3): 243–247. [PMC free article] [PubMed] [Google Scholar]
- 30. Brindley DA, Wall IB, Bure KE. Automation of cell therapy biomanufacturing. Bioprocess Int 2013; 11(Suppl. 3): 18–25. [Google Scholar]
- 31. Scott C. Challenges in developing an infrastructure strategy. Bioprocess Int 2010; 8(6): S38–S40. [Google Scholar]
- 32. Bravery C. Regulating interface science healthcare products: myths and uncertainties. J R Soc Interface 2010; 7: S789–S795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brindley D, Mason C. News Commentary: human embryonic stem cell therapy in the post-Geron era. Regen Med 2012; 7(1): 17–18. [DOI] [PubMed] [Google Scholar]
- 34. Ilic D, Polak J. Stem cell based therapy—where are we going? Lancet 2012; 379(9819): 877–878. [DOI] [PubMed] [Google Scholar]
- 35. Martin I, Baldomero H, Bocelli-Tyndall C, et al. The survey on cellular and engineered tissue therapies in Europe in 2011. Tissue Eng Part A 2014; 20: 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brindley DA, French A, Suh J, et al. The implementation of novel collaborative structures for the identification and resolution of barriers to pluripotent stem cell translation. Stem Cells Dev 2013; 22(S1): 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mason C, Brindley DA, Culme-Seymour EJ, et al. Cell therapy industry: billion dollar global business with unlimited potential. Regen Med 2011; 6(3): 265–272. [DOI] [PubMed] [Google Scholar]
- 38. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926. [DOI] [PubMed] [Google Scholar]
- 39. Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med 2008; 3: 1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.