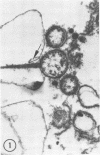
Abstract
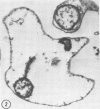
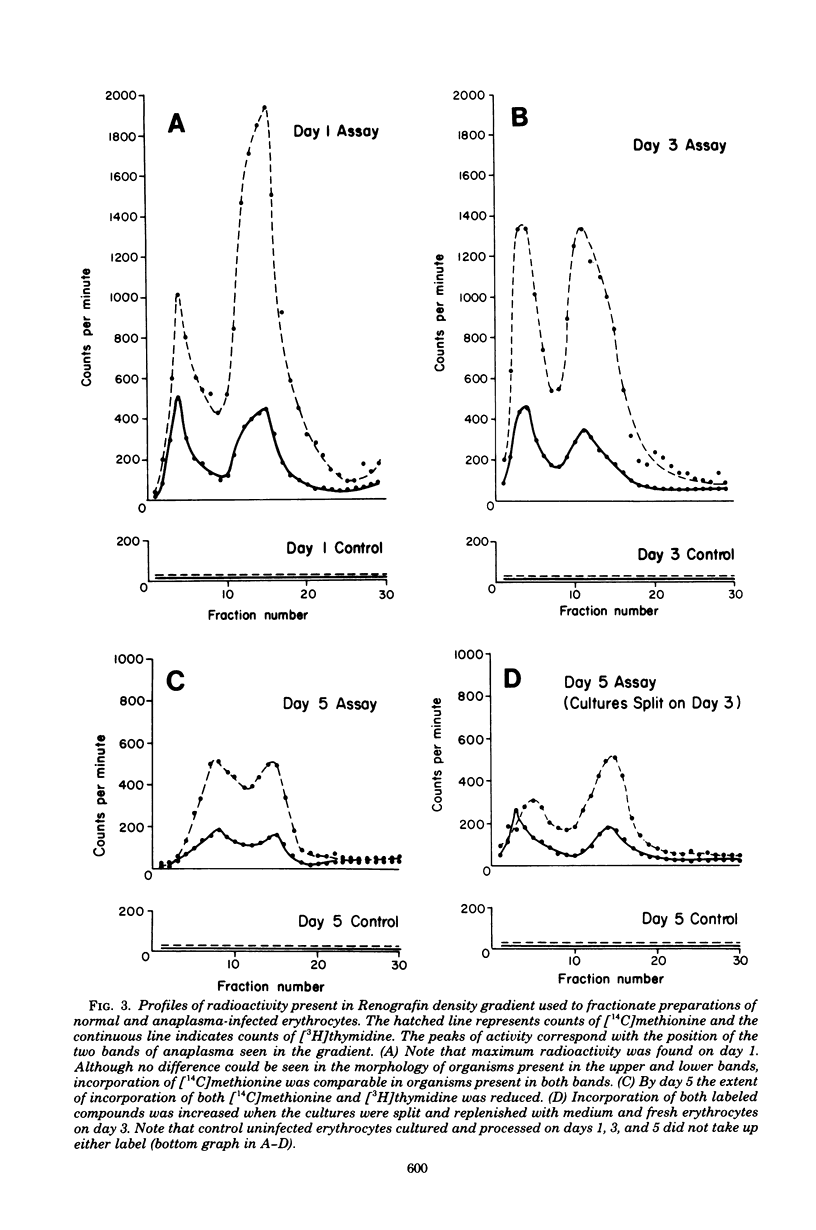
Bovine erythrocytes infected with Anaplasma marginale were cultured for 1 to 5 days in a CO2 incubation chamber, pulse-labeled with [3H]thymidine and [14C]methionine, lysed, and fractionated by differential centrifugation and continuous density gradient centrifugation in Renografin. Anaplasma and associated fragments of stroma formed two distinct bands in the dense region of the gradient. Electron microscopic examination of pelleted material from the bands from cells cultured for 1 day revealed the presence of organisms that were morphologically intact or in various states of degeneration. Examination of fractions from the gradient for incorporation of label revealed that analplasma present in erythrocytes can incorporate both [3H]thymidine and [14C]methionine. Subsequent experiments demonstrated that organisms cultured for 3 and 5 days incorporated the radiolabeled compounds also, but to a lesser extent. The experiments demonstrate that it is possible to culture analplasma in vitro for short periods of time and monitor their growth characteristics.
Full text
PDF

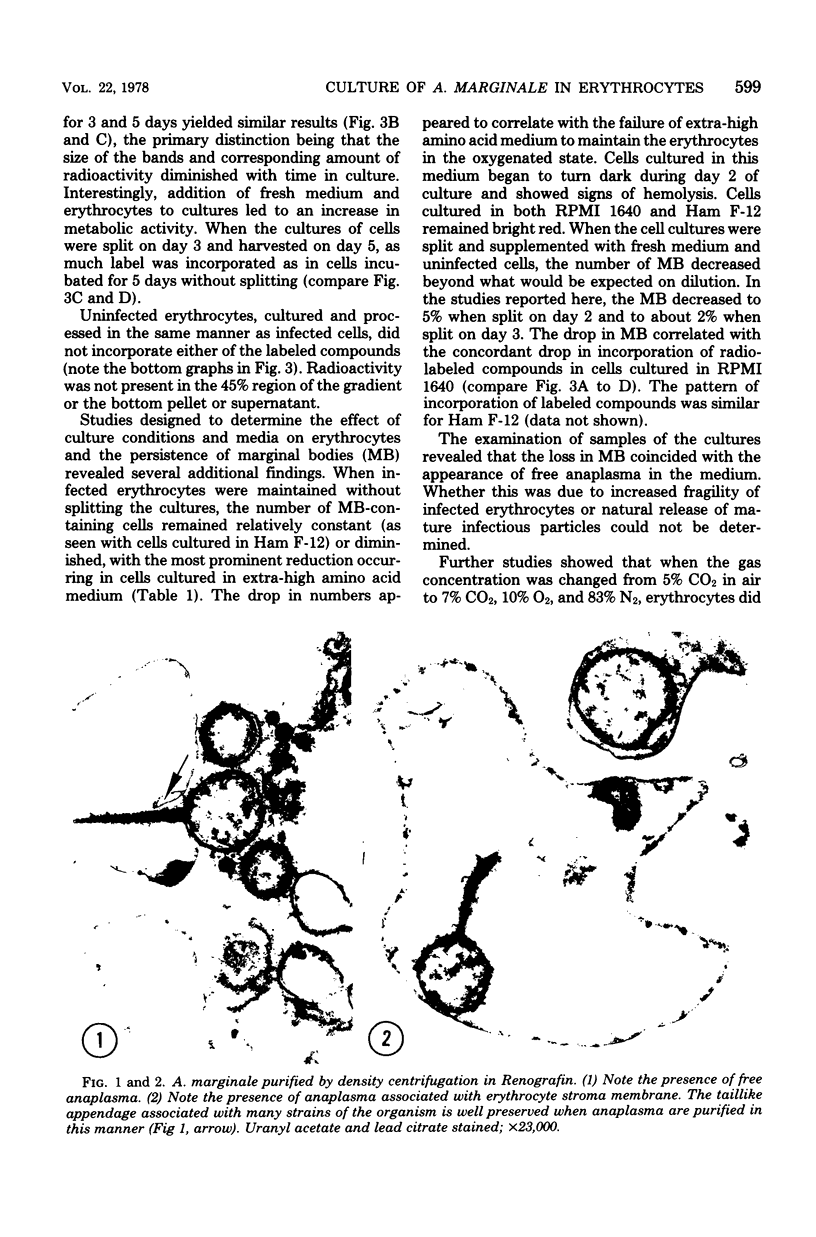


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen J. F., McNeal D. W. Anaplasma marginale infection in deer in the Sierra Nevada foothill area of California. Am J Vet Res. 1967 Mar;28(123):599–601. [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erp E., Fahrney D. Exit of Anaplasma marginale from bovine red blood cells. Am J Vet Res. 1975 May;36(5):707–709. [PubMed] [Google Scholar]
- Frank D. W., McGuire T. C., Gorham J. R., Davis W. C. Cultivation of two species of Neorickettsia in canine monocytes. J Infect Dis. 1974 Mar;129(3):257–262. doi: 10.1093/infdis/129.3.257. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo R. J. Propagation of Anaplasma marginale in bovine lymph node cell culture. Am J Vet Res. 1975 May;36(5):635–640. [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe D. L., Hepworth W. G. Anaplasmosis in big game animals: tests on wild populations in Wyoming. Am J Vet Res. 1965 Sep;26(114):1114–1120. [PubMed] [Google Scholar]
- Johns R. W., Dimopoullos G. T. In vitro uptake of 14C-labeled amino acids by preparations of partially purified Anaplasma marginale bodies. Infect Immun. 1974 Apr;9(4):645–647. doi: 10.1128/iai.9.4.645-647.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marble D. W., Hanks M. A. A tissue culture method for Anaplasma marginale. Cornell Vet. 1972 Apr;62(2):196–205. [PubMed] [Google Scholar]
- Mazzola V., Amerault T. E., Roby T. O. Survival of Anaplasma marginale in Aedes albopictus cells. Am J Vet Res. 1976 Aug;37(8):987–989. [PubMed] [Google Scholar]
- PILCHER K. S., WU W. G., MUTH O. H. Studies on the morphology and respiration of Anaplasma marginale. Am J Vet Res. 1961 Mar;22:298–307. [PubMed] [Google Scholar]
- Peck A. B., Bach F. H. A miniaturized mouse mixed leukocyte culture in serum-free and mouse serum supplemented media. J Immunol Methods. 1973 Oct;3(2):147–163. doi: 10.1016/0022-1759(73)90030-6. [DOI] [PubMed] [Google Scholar]
- Peterson K. J., Kistner T. P., Davis H. E. Epizootiologic studies of anaplasmosis in Oregon mule deer. J Wildl Dis. 1973 Oct;9(4):314–319. doi: 10.7589/0090-3558-9.4.314. [DOI] [PubMed] [Google Scholar]
- Renshaw H. W., Vaughn H. W., Magonigle R. A., Davis W. C., Stauber E. H., Frank F. W. Evaluation of free-roaming mule deer as carriers of anaplasmosis in an area of Idaho where bovine anaplasmosis is enzootic. J Am Vet Med Assoc. 1977 Feb 1;170(3):334–339. [PubMed] [Google Scholar]
- Vaughn H. W., Renshaw H. W., Frank F. W. Survey of anaplasmosis in elk of the Clearwater National Forest (Idaho). Am J Vet Res. 1976 May;37(5):615–617. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]