Abstract
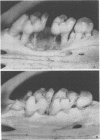
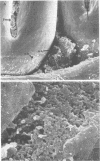
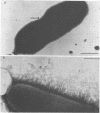
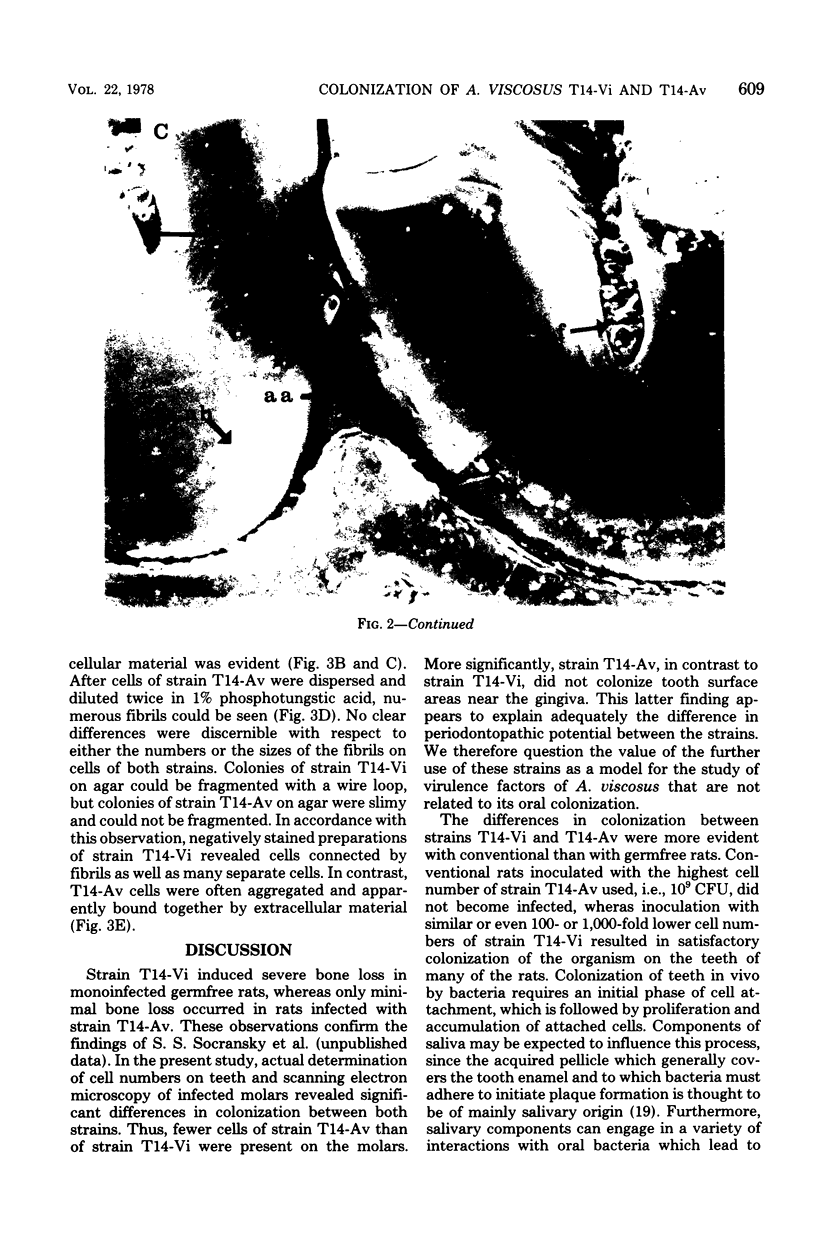
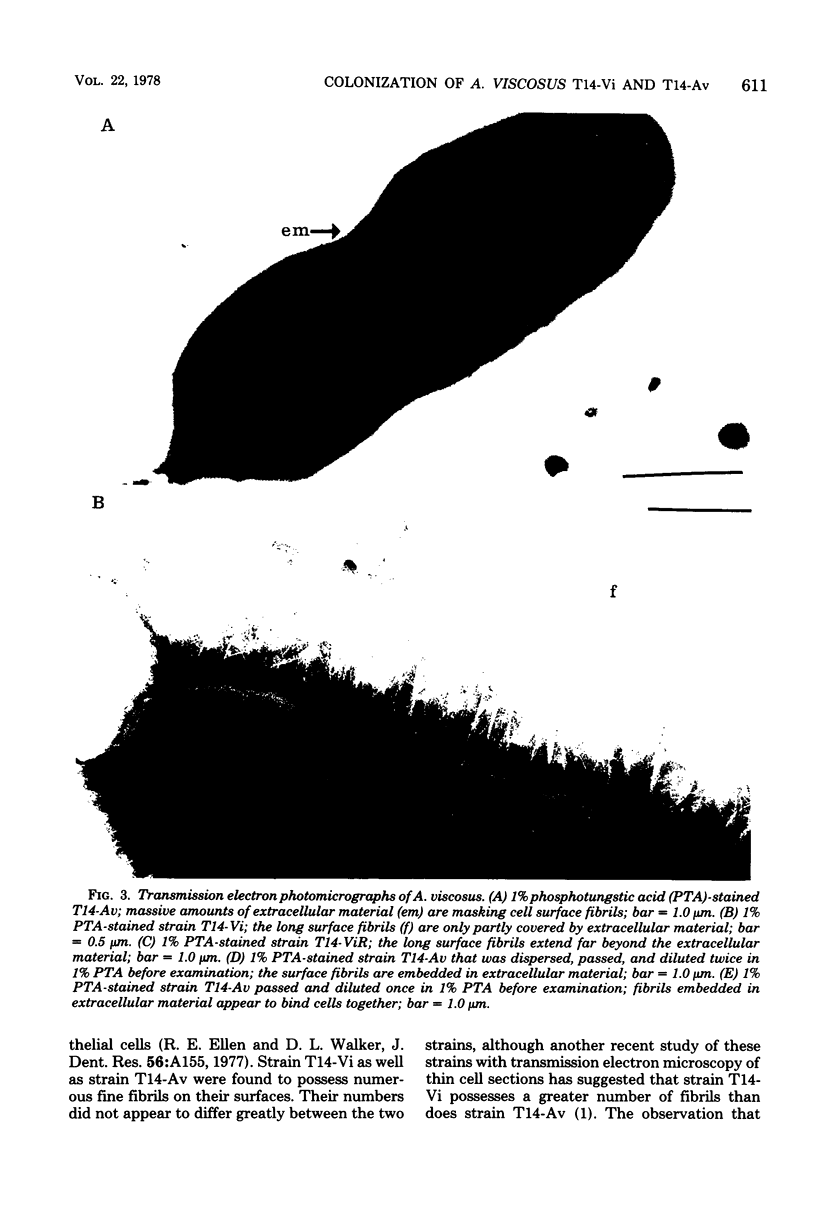
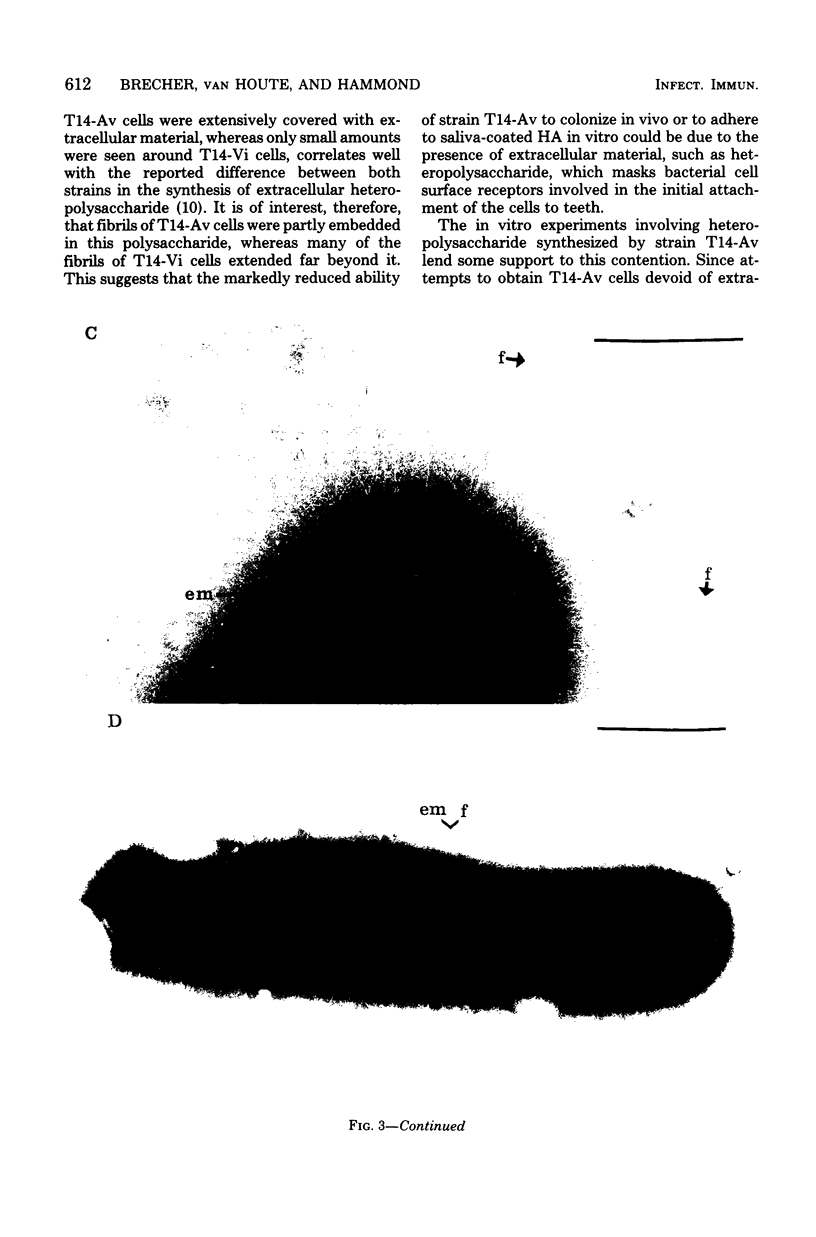
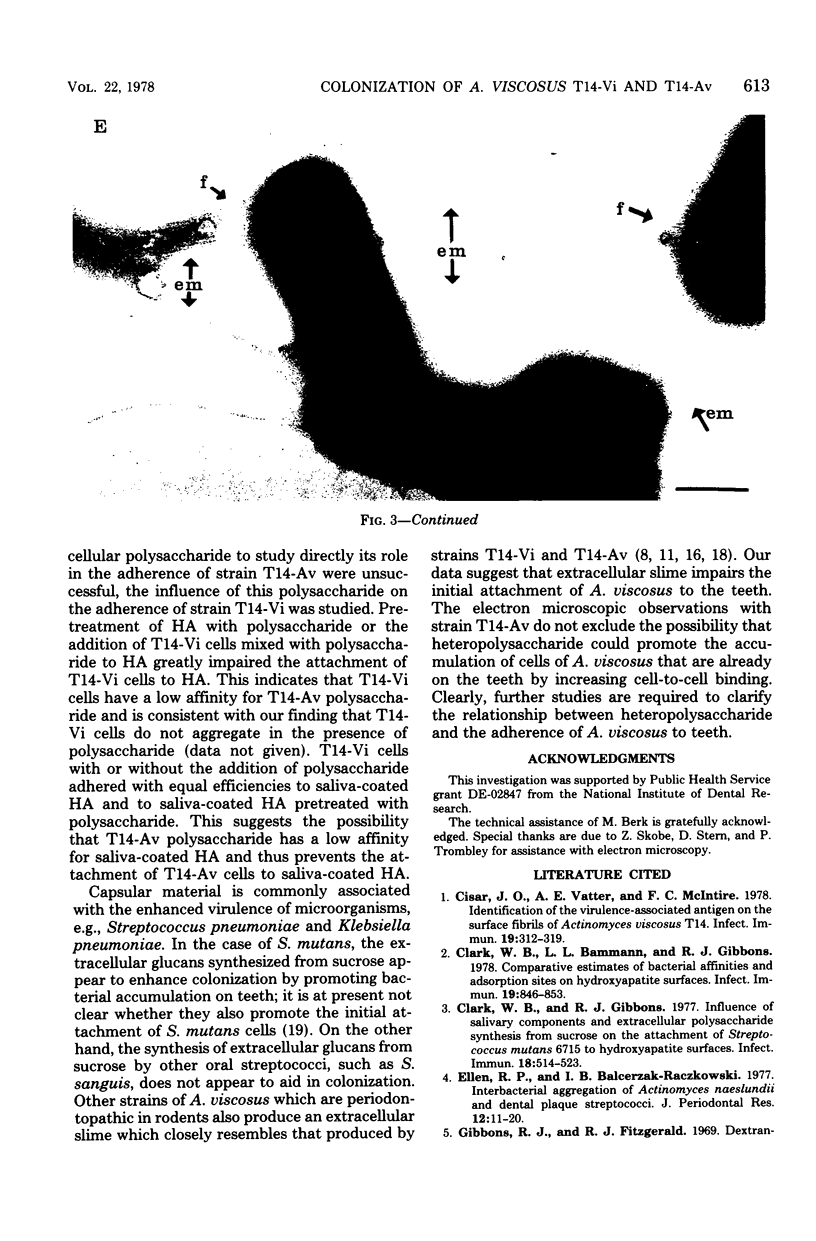
Germfree rats fed a high-sucrose diet were inoculated with Actinomyces viscosus strain T14-Vi (virulent) or T14-Av (avirulent). The mean recovery of strain T14-Vi from six extracted finely ground molars of rats sacrificed after 90 days was 1.1 × 108 colony-forming units (CFU). The mean recovery of strain T14-Av was 5.7 × 107 CFU, which was significantly less. Strain T14-Vi caused severe alveolar bone loss, but only minimal bone loss occurred in rats infected with strain T14-Av. Scanning electron microscopy of teeth of germfree rats revealed that strain T14-Vi colonized in the fissures as well as on tooth surface areas near the gingiva; strain T14-Av also colonized in fissures but was unable to colonize the teeth near the gingiva. In studies with conventional rats fed a high-sucrose diet, streptomycin-resistant strain T14-Vi colonized on the teeth of all rats inoculated with in the order of 108 or 107 CFU and on the teeth of about half of the rats inoculated with 106 or 105 CFU. In contrast, streptomycin-resistant strain T14-Av could not be detected on the teeth of any of the rats in groups similarly inoculated. In vitro “resting” cells of both strains suspended in conventional or germfree rat saliva survived to comparable degrees. [3H]thymidine-labeled T14-Vi cells adhered well to hydroxyapatite (HA) beads and to HA beads pretreated with saliva obtained from germfree or conventional rats. In contrast, T14-Av cells adhered less well than did T14-Vi cells to HA, whereas their adherence to saliva-coated HA was negligible. Transmission electron microscopy of negatively stained T14-Vi and T14-Av cells repeatedly passed in 1% phosphotungstic acid revealed fibrils on cells of both strains. T14-Av cells were covered by large amounts of extracellular material which was presumably heteropolysaccharide; little extracellular material was present on the surface of T14-Vi cells. T14-Vi cells had a relatively low affinity for the heteropolysaccharide synthesized by strain T14-Av. Other evidence also suggested that this polysaccharide had a relatively low affinity for saliva-coated HA. Collectively, the evidence indicates that the difference in periodontopathic potential between strains T14-Vi and T14-Av results from their different abilities to colonize teeth. This difference is probably due to the lower adherence of T14-Av cells to teeth rather than to their ability to grow in the mouth. The low affinity of T14-Av cells for tooth surfaces may be due, in part, to the presence of large amounts of cell-surface-associated polysaccharide.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., KAPSIMALIS B. ESTABLISHMENT OF HUMAN INDIGENOUS BACTERIA IN GERM-FREE MICE. J Bacteriol. 1964 Nov;88:1316–1323. doi: 10.1128/jb.88.5.1316-1323.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A. E., Jacius B. H. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol. 1974 Jan;19(1):71–79. doi: 10.1016/0003-9969(74)90228-3. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Reactions in the periodontium to continuous antigenic stimulation in sensitized gnotobiotic rats. Infect Immun. 1974 Sep;10(3):565–577. doi: 10.1128/iai.10.3.565-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- KEYES P. H., GOLD H. S. Periodontal lesions in the Syrian hamster. I. A method of evaluating alveolar bone resorption. Oral Surg Oral Med Oral Pathol. 1955 May;8(5):492–499. doi: 10.1016/0030-4220(55)90080-3. [DOI] [PubMed] [Google Scholar]
- LAUDIG L. W., ROSEN S., HOPPERT C. A., HUNT H. R. The use of pentamethylentetrazol to decrease mortality during collection. J Dent Res. 1960 Jul-Aug;39:862–862. doi: 10.1177/00220345600390041701. [DOI] [PubMed] [Google Scholar]
- Rosan B., Hammond B. F. Extracellular polysaccharides of Actinomyces viscosus. Infect Immun. 1974 Aug;10(2):304–308. doi: 10.1128/iai.10.2.304-308.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Nilsson U. R., McArthur W. P., Taichman N. S. Activation of the complement system by some gram-positive oral bacteria. Arch Oral Biol. 1977;22(5):309–312. doi: 10.1016/0003-9969(77)90027-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Edelstein S. Decreased oral colonization of Streptococcus mutans during aging of Sprague-Dawley rats. Infect Immun. 1977 Apr;16(1):203–212. doi: 10.1128/iai.16.1.203-212.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Jordan H. V., Skobe Z., Green D. B. Role of sucrose in colonization of Streptococcus mutans in conventional Sprague-Dawley rats. J Dent Res. 1976 Mar-Apr;55(2):202–215. doi: 10.1177/00220345760550020801. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S. A slime-producing microorganism in dental plaque of rats, selected by glucose feeding. Chemical composition of extracellular slime elaborated by Actinomyces viscosus, strain Nyl. Caries Res. 1974;8(3):193–210. doi: 10.1159/000260109. [DOI] [PubMed] [Google Scholar]