Abstract
Neuroimaging studies have found a correlation between activation in the anterior insula and love, and a correlation between activation in the posterior insula and lust. The present control-case study describes a neurological male patient, with a rare, circumscribed lesion in the anterior insula, whom we tested using a decision task that required he judge whether each of a series of attractive individuals could be the object of his love or lust. The patient, in contrast with neurologically typical participants matched on age, gender, and ethnicity, performed normally when making decisions about lust but showed a selective deficit when making decisions about love. These results provide the first clinical evidence indicating that the anterior insula may play an instrumental role in love but not lust more generally. These data support the notion of a posterior-to-anterior insular gradient, from sensorimotor to abstract representations, in the evaluation of anticipatory rewards in interpersonal relationships.
Keywords: anterior insula, stroke, neurology, pair-bonding, love, sexual desire, social neuroscience
1. Introduction
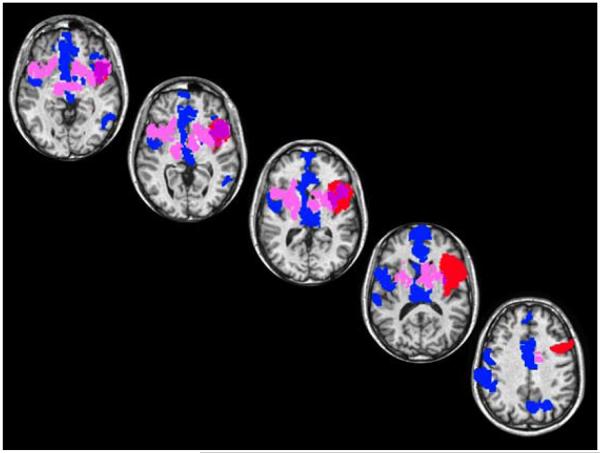
In a case report from 250 BCE, Greek physician and anatomist Erasistratos accurately diagnosed Antiochus’ ailment as lovesickness, marking the beginning of the first objective neurological case report of love. Nearly 2.5 millennia later, a growing number of neuroimaging studies in healthy volunteers point to a specific set of brain regions as activated when love is evoked but not when other biological drives are evoked, including sexual desire [1]. For instance, although both love and desire activate subcortical regions that are associated with euphoria, reward, and motivation, and cortical regions associated with self-representation and social cognition, a recent meta-analysis showed a selective brain matrix for desire (Figure 1, in blue) and one for love (Figure 1, in pink) with the posterior region of the insula being more activated by desire (but not love) whereas the anterior region of the insula is more activated by love (but not desire). This distinction has been interpreted to mean that desire is grounded in a relatively concrete representation of sensorimotor experiences, whereas love is a more abstract representation of those experiences in the context of a person’s current and prior experiences [1]. If this interpretation is correct, then a lesion in the anterior (or posterior) insula should be associated with a diminished capacity to ignite normal responses to love (or desire) in particular. We tested this hypothesis with a patient who had a circumscribed ischemic lesion in the anterior insula (Figure 1, in red), an area that overlaps the love (but not the desire) brain matrix (Figure 1, in purple).
Figure 1.
Transverse brain slices illustrating overlapping (in purple) brain regions between the patient’s brain lesion (in red), and a meta-analysis composite of brain regions that were uniquely activated by sexual desire (in blue) compared to love (in pink) in healthy subjects. The patient’s MRI image was placed in ICBM space and transformed into AfniTalairach space.
2. Materials and methods
2.1. Clinical case
This 48-year-old heterosexual male patient suffered from an ischemic brain lesion associated with headache, facial palsy, sialorrhea, dysphagia and temporary speech difficulties. At the moment of evaluation, the patient showed no symptoms and the neurological examination was normal. Detailed neuropsychological examination findings were also in the normal range. Addenbroke’s Cognitive Examination-Revised version score was 96/100 and INECO Frontal Screening (measure of frontal functioning) score was 27/30. Affective state and mood evaluation were also in the normal range (anxiety state (STAIT-S) = 27 points (50th percentile); anxiety trait = 37 points (85th percentile); and Beck’s Depression Scale = 9 = No depression). The patient performed accurately in multimodal emotion recognition, and social cognition tasks of reading the mind in the eyes and empathy for pain task.
2.2. Non-lesion control group
Seven healthy individuals matched with the patient on gender, nationality, ethnicity, and age served as a non-lesion control group (mean age 46.88 years ± 3.01).
2.3. Assessment of love and desire
The patient (and the control group) performed a neuropsychological computer task in which he/they viewed a series of 40 photographs (see further details below). Each of these 40 photographs was presented twice in blocks of 20 randomly ordered photographs. Based on a counterbalanced randomized repeated measures design (ABBA), the patient (and the control group) first viewed a random set of 20 of the photographs and categorized each as relevant to (e.g., evocative of) feelings of love (yes/no), then viewed a random order of 40 photographs (in two blocks of 20) and categorized each as relevant to sexual desire (yes/no), and then viewed the remaining 20 of the photographs and categorized each as relevant to love (yes/no).
2.4. Stimuli and procedure
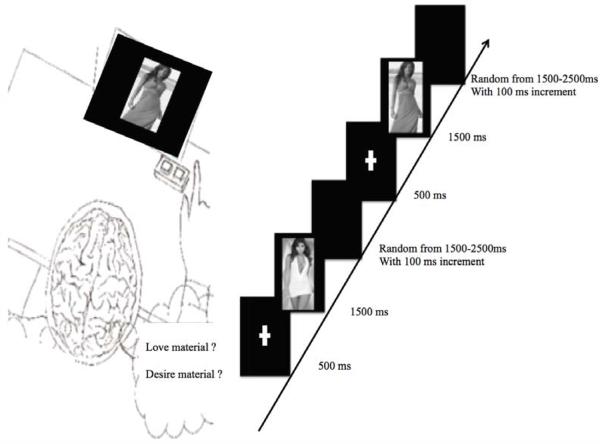
The photographs depicted attractive females in appealing short or long dresses. Photographs represented whole-body models of women aged 18-30. To control for visual features between stimuli, all pictures had the same brightness and size (200 × 500 pixels), and all contained black and white stimuli with matched facial orientation, facial expression, and matched gaze orientation (i.e., gaze directed towards the camera). Each trial began with central presentations of a 500 ms-fixation cross followed by a 1500 ms-target stimulus. A random 1500-2500 inter-stimulus interval separated each trial (Figure 2).
Figure 2.
Experimental design. Example of stimulus sequence presented to the male patient. The visual stimulus on each trial was composed of a sequence of three frames. First a central fixation cross was presented for 500 ms, followed by a target stimulus for 1500 ms and then a blank screen with a black background was randomly presented for 1500 to 2500 ms with 100 ms increment.
2.5. Measures and statistical analyses
The percentage of yes responses and their associated response latencies (ms) served as the primary behavioral data. Analyses were performed using the Crawford & Garthwaite’s Revised Standardized Difference Test, a statistical test developed for single-case research in neurology and neuropsychology [2].
3. Results
The anamnesis indicated that the patient was unaware of any differences in his feelings of love or desire, whereas behavioral testing revealed a selective deficit for love (but not sexual desire). Specifically, although the patient and the control group did not differ in the percentage of the photographs categorized as relevant to feelings of love (patient’s mean = 35.0%, control group’s mean = 42.8%) and desire (patient’s mean = 57.5%, control group’s mean = 60.6%), t(6) = 0.033; n.s.; effect size = 0.038), the patient took significantly longer time than the control group to categorize a photograph as relevant to feelings of love (patient’s mean reaction times: 1279.07 ms ± 398.61; control group reaction times: 1020.3 ms ± 130.4), in contrast to categorizing a photograph as relevant to feelings of mean reaction times: 926.46 ms ± 210.26, control sexual desire (patient’s group’s: 958.6 ms ± 142.4; t(6) = 2.537, p = .04; effect size using Bayesian methods for the difference between case and controls = 3.332).
4. Discussion
The current findings provide the first evidence indicating that the anterior insula may play an instrumental role in the realization of feelings of love, and not sexual desire. This reinforces a recent fMRI meta-analysis indicating that the anterior insula is activated when feelings of love are evoked, whereas the posterior insula is activated when feelings of sexual desire are evoked [1, 3-12]. The current results also indicate that the anterior insula may not be necessary for, but simply contributory to, feelings of love. Specifically, we found that the patient was similar to healthy controls in the percentage of the stimuli he categorized as relevant to feelings of love and to feelings of sexual desire, but he was slower than the controls when making judgments about feelings of love (but not desire). Feature encoding in sensory brain areas is transformed into more abstract and experience-dependent representations, a categorization process that animal research indicates is dependent on parietal neurons [1]; the parietal region was intact in the current insula patient. Love differs from sexual desire in that the former represents a more abstract, integrative representation that underlies more general affective and motivational responses. The anterior insula is not the only area involved in the so-called love brain network, but the current findings build on prior research to suggest it is a critical neural nexus for abstract and experience-dependent emotional processing [1]. Specifically, the focused interference of the anterior insula damage in the speed of judgments of love (but not desire) suggests that the anterior insula may play a role in accessing information about abstract forms of human emotions such as love. This is consistent with a growing body of the work placing the anterior insula within a larger neural network dedicated to internal state evaluation, valence processing and executive control due to its connections with areas involving (but not restricted to) the temporal pole, parietal regions, the caudal orbitofrontal gyrus and receiving projections from the posterior parts of the insula, the pericallosal anterior cingulate, and the medial temporal lobes [4, 8, 11].
The current work makes it possible to disentangle love from other biological drives and to approach feelings of love not only by the analysis of subjective self-report questionnaires but by investigating the neurological pattern of activity in the healthy brain and by examining subtle differences in the behavioral response to stimuli evocative of feelings of love versus closely related emotions (e.g., desire) in patients following brain damage.
5. Acknowledgments
This work was supported by the Swiss National Science Foundation (Grant #PP00_1_128599/1 to SC), the University Funds Maurice Chalumeau of the University of Geneva in Switzerland (to SC), the Mind Science Foundation (Grant # TSA2010-2 to SC), the NCRR NIHCOBRE Grant E15524 (to the Sensory Neuroscience Research Center of West Virginia University), INECO Foundation (to FM, LS, BC, AI), CONICET (to BC, LS, AI) and FONDECYT (#1130920 to AI). The authors thank Robin Weiss for the 3D video of the patient’s lesion.
6. References
- 1.Cacioppo S, Bianchi-Demicheli F, Frum C, Pfaus JG, Lewis JW. The Journal of Sexual Medicine. 2012;9(4):1048–54. doi: 10.1111/j.1743-6109.2012.02651.x. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JR, Garthwaite PH. Neuropsychology. 2005;19:318–331. doi: 10.1037/0894-4105.19.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Berntson GG, Norman GJ, Bechara A, Bruss J, Tranel D, Cacioppo JT. Psychological science. 2011;22(1):80–6. doi: 10.1177/0956797610391097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig AD. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 5.Ibañez A, Gleichgerrcht E, Manes F. Brain structure function. 2010;214(5-6):397–410. doi: 10.1007/s00429-010-0256-y. Springer Berlin/Heidelberg. [DOI] [PubMed] [Google Scholar]
- 6.Isnard J, Guenot M, Sindou M, Mauguiere F. Epilepsia. 2004;45(9):1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- 7.Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovero KL, Simmons AN, Aron JL, Paulus MP. NeuroImage. 2009;45(3):976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manes F, Paradiso S, Robinson RG. The Journal of nervous and mental disease. 1999;187(12):707–712. doi: 10.1097/00005053-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mavridis I, Boviatsis E, Anagnostopoulou S. Surgical and radiologic anatomy SRA. 2011;33(4):319–328. doi: 10.1007/s00276-010-0699-0. [DOI] [PubMed] [Google Scholar]
- 11.Wager TD, Barrett LF. Emotion. 2004;129:2865. [Google Scholar]
- 12.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]