Abstract
Aim
Although motor impairment is frequently observed in children with autism spectrum disorders (ASD), the manner in which these impairments aggregate in families affected by autism is unknown. We used a standardized measure of motor proficiency to objectively examine quantitative variation in motor proficiency in sibling pairs concordant and discordant for ASD.
Methods
Motor impairment of sibling pairs from 67 ASD-affected families comprising 29 concordant pairings and 48 discordant pairings were assessed using the Bruininks Oseretsky Test of Motor Proficiency, 2nd Edition, a standardized measure of motor proficiency.
Results
Motor skills were substantially impaired among ASD-affected children and highly correlated with autistic severity and IQ, whereas motor skills in unaffected siblings were essentially normal. Total motor composite scores of at least one standard deviation below the general population mean were seen in 83% of the affected group compared with 6% in the unaffected siblings.
Interpretation
Findings indicate that motor impairment constitutes a core characteristic of ASD (not necessarily an ASD endophenotype), which has distinct implications for taxonomy, diagnosis, and approaches to intervention.
Keywords: sibling studies, body coordination, manual dexterity, fine manual control, strength, agility, motor coordination, pervasive developmental disorder, endophenotype
Deficits in a wide range of motor skills, including fine and gross motor coordination, strength, agility, praxis, performance of skilled gestures, and imitation, and the subtle neurological signs of overflow, dysrhythmia, motor impersistence, and muscle tone, have been reported in studies of children with autism spectrum disorders (ASD). The variety of methods for ascertaining motor impairment in these studies have generally converged on the notion that 80–90% of children with ASD show some degree of motor abnormality (David et al., 2009; Dzuik, et al., 2007; Ghaziuddin and Butler, 1998; Ming et al., 2007). Minshew et al., (1997) found motor function to be one of the discriminating domains differentiating individuals with ASD from unaffected controls. A recent robust meta-analysis concluded that ASD is associated with significant and widespread alterations in motor performance, suggesting a tentative argument that motor deficits are a potential core symptom of ASD (Fournier et al. 2010). We previously examined the relationship between the degree of motor impairment and the degree of autistic severity (Hilton et al., 2007), finding a high correlation in children with Asperger’s disorder, but the relationship between these two constructs has not been examined across the autism spectrum, or among unaffected siblings of ASD probands.
Motor impairment has significant functional implications for individuals with ASD because it can lead to decreased abilities to perform many activities of daily living, including getting dressed, handwriting, and participating in athletic and recreational activities. Difficulty with motor skills can limit social opportunities in children with ASD (Church et al., 1999). In other groups of children, motor problems have been observed to be related to isolation, anxiety, and emotional and social problems for children and their families (Chen and Cohn, 2003; Piek et al., 2010). Motor disturbances have been implicated as earlier indicators than behavioral impairment in the evolution of symptoms of autism in young children (Teitelbaum et al., 1998).
Previous research has found that motor impairment is related to cognitive abilities. Bruininks and Bruininks (2005) found differences of about two standard deviations between motor scores of individuals with mild to moderate cognitive impairment and their typically developing peers. IQ scores were correlated with motor proficiency in children with ASD in another study (Ghaziuddin and Butler, 1998), and gross motor and fine motor skills in children at 9 months of age were both significant predictors of cognitive ability at 5 years in a large British cohort (Schoon et al., 2010). Papadopoulous et al. (in press) found greater motor impairment among participants who had autism with lower IQ (low functioning autism) than among those with higher IQ (high functioning autism and Asperger’s disorder). Previous studies have linked motor, social, and communicative impairments to differences in fronto-striatal (basal-ganglia) and cerebellar brain regions in children with ASD (Rinehart et al., 2006; Qui et al., 2010). This suggests common neural mechanisms influencing outcomes in each of these neurodevelopmental domains.
A greater understanding of endophenotypes (traits that are associated with a diagnosis, are heritable, and manifest in family members with or without the diagnosis) may continue to refine underlying contributors to syndromes of neurodevelopmental impairment, particularly quantitative impairments such as those that characterize the autistic syndromes (Constantino, 2010). An aggregation of subclinical autistic social impairment traits has been found in unaffected family members of children with ASD, suggesting that such impairment constitutes an autism endophenotype (Constantino et al., 2010). Other studies have identified face processing (Webb et al., 2010) and immunological functions (Saresella et al., 2009) as autism endophenotypes.
Recently, Lichtenstein et al. (2010) found that 40% of twin pairs with developmental coordination disorder had genetic influences in common with ASD in a large population study suggesting a genetic relationship between motor impairment and autism. Reiersen and colleagues (2008) observed in a general population sample that children with this combination of motor coordination problems and ADHD symptomatology (but not either condition alone) were more likely to show clinical-level autistic social impairment, reminiscent of the Deficits in Attention, Motor Proficiency and Perception (DAMP) syndrome described in previous reports. Because previous studies have found children with autism to have a high incidence of motor proficiency abnormalities, we conducted this initial exploration of how such abnormalities aggregate in ASD-affected families, beginning with the examination of siblings. To our knowledge, no previous observational studies of motor impairment in autism have examined unaffected siblings or explored the relationship between degree of motor impairment and valid quantitative ratings of autistic severity. Questions addressed in our study were: a) Is motor impairment present in unaffected siblings of children with ASD? and b) What is the relationship between degree of motor impairment and autistic severity in children with ASD? We used a standardized measure of motor proficiency to objectively examine quantitative variation in motor proficiency in sibling pairs concordant (both with a diagnosis) and discordant (only one with a diagnosis) for ASD.
Method
Participants
Participants were 144 children from 67 ASD-affected families from a larger longitudinal sibling study. The larger study includes 295 families with ASD and 49 with other mental health conditions, who were recruited from the psychiatry practice of the senior author (JC) and through various public recruitment efforts over the previous 6 years, the period for which the larger study was funded. Having African American ethnicity and one or more full male siblings were priority characteristics for recruitment of the families for the parent study. For this study, we contacted families from most recently enrolled to more distantly enrolled, oversampling for concordant affected sib pairs to approximate a 2:1 ratio of discordant to concordant sibling pairs to conduct the comparisons planned for this study. Among the 67 enrolled families, each had one index child with an ASD diagnosis between the ages of 4 and 21 years and at least one additional full biological sibling in the same age range. Severity gradations were addressed in this study via quantitative measurements of severity rather than inference from diagnostic distinctions within ASD. In multiplex families, the older ASD-affected child was identified as the index case (proband). In ten families, both concordant and discordant sibling pairs were available, so the index case was compared with each. Among the siblings, 29 (including 6 identical twins) were co-affected with ASD and 48 were unaffected. See Table 1 for characteristics of the participants. Data from the Autism Diagnostic Interview – Revised (ADI-r; Rutter et al., 2003) and the Social Responsiveness Scale (SRS; Constantino and Gruber, 2005), were systematically collected and final diagnostic determination was made by an experienced clinician according to DSM-IV criteria after review of assessments and either direct or videotaped observation of the participant. Among the ASD-affected children, comorbidities included attention deficit hyperactivity disorder (ADHD; 22 participants), bipolar disorder (4 participants), epilepsy (4 participants), language delay or disorder (5 participants), thyroid disorder (1 participant), Tourette’s disorder (2 participants), Arnold Chiari malformation (1 participant), 5p 13.2 duplication (1 participant), and XXY chromosome (1 participant). Among the unaffected children, diagnoses included ADHD (4 participants), developmental delay (2 participants), epilepsy (1 participant), and language delay (1 participant). These determinations were made by the children’s clinicians and were extracted from medical and school records that were submitted by families. The research was approved by the Washington University Human Research Protection Office. All participants and their parents gave informed consent to their participation in the research and for publication of results.
Table 1.
Characteristics of sibling pairs
| Characteristic | Index cases n = 67 | Concordant siblings n = 29 | Discordant siblings n = 48 |
| Age range (mean) | 4 years 7 months to 16 years 7 months (9 years 8 months) | 4 yrs, 2 months to 17 years 8 months (9 years 11 months) | 4 years 4 months to 20 years 6 months (9 years 11 months) |
| Gender | |||
| Male | 57 | 28 | 26 |
| Female | 10 | 1 | 22 |
| Ethnicity | |||
| Caucasian | 46 | 26 | 29 |
| African American | 20 | 3 | 18 |
| Asian | 1 | 0 | 1 |
Measures
The Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT2; Bruininks and Bruininks, 2005), the Developmental Coordination Disorder Questionnaire 2007 (DCDQ’07; Wilson et al., 2007), and the Social Responsiveness Scale (SRS, Constantino and Gruber, 2005) were used to evaluate each child in the study. The BOT2 is the latest version of an individually administered standardized measure of motor proficiency (videotaped in this study) that is widely used in studies of children with disabilities (Miles et al., 1988). The BOT2 was recently revised from its original version to improve the functional relevance of the test content, expand coverage of fine and gross motor skills, and expand the inclusive age range. As of this writing, we are aware of no previously published study that has reported scores for children with ASD using this version of the instrument. In contrast to other motor assessments, it provides scores across a full range of aptitude, measuring variation from well below to well above average performance. It generates gender-specific composite subscale scores for: fine manual control, manual coordination, body coordination, and strength and agility, and a full motor composite score and has been normed for children between ages 4 and 21 years (Bruininks and Bruininks, 2005).
All examiners achieved inter-rater item level reliability of >90% before scoring the assessments. When children with ASD had difficulty following directions, testers would demonstrate the test items and would put the children in the start position. Various incentives were used to foster compliance, including involvement of parents or siblings to encourage performance of tasks, use of food-related rewards, singing the participant’s favorite song while the participant performed the task, and allowing breaks to perform preferred activities, such as ball throwing or application of deep pressure (e.g. like a shoulder massage). Testers substantiated observed inability or lack of attempts to perform the test items by checking with siblings and parents when uncertain whether a true effort had been made by the participants.
The DCDQ’07 (Wilson et al., 2007) is the latest version of a brief parent-report questionnaire that ascertains components of motor impairment that predict Developmental Coordination Disorder (Martin et al., 2006) and is standardized for children from 5 to 15 years of age. Correlations between DCDQ’07 scores and other measures of motor proficiency and visual motor integration (Movement Assessment Battery for Children; r = .55; Henderson and Sugden, 1992) and the Beery Test of Visual-Motor Integration (r = .42; Beery, 1997) have supported the validity of the instrument, but its correlation with the BOT2 has not previously been tested.
The SRS (Constantino and Gruber, 2005), a quantitative trait measure of autistic social impairment, was completed by a parent for each child. It capitalizes on observations of children in their naturalistic social settings and generates a total score for autistic social impairment, empirically validated via factor, cluster, and latent class analysis (Constantino et al., 2004). Higher scores indicate greater social impairment severity. The SRS shows non-significant correlations with IQ and substantial agreement with the ADI-r (Constantino et al., 2004). Parent-report scores on the SRS are highly heritable (Constantino and Todd, 2005), continuously distributed in clinical and non-clinical populations, and distinguish children with ASD from those with other psychiatric conditions (Constantino et al., 2007). SRS T scores were available for 139 of the 145 participants in this group.
IQ data from assessments done in the past 2 years were obtained for 33 (31 ASD-affected and 2 unaffected) of the research participants, 12 from school reports provided by parents, 16 from the Wechsler Abbreviated Scale of Intelligence (Wechsler. 1999), and 5 from either the Differential Abilities Scale, 2nd Edition (Elliot, 2007) or the Mullen Scales of Early Learning (Mullen, 1995). IQ data not provided by parents were obtained from testing in another study conducted by this research group (Constantino et al., unpublished data).
Statistical analysis
Separate comparisons of mean scores for motor proficiency between index cases, affected siblings, and unaffected siblings were conducted for the BOT2 subscale and motor composite scores and the total score derived from the DCDQ’07. The extent to which diagnosis, age, gender, IQ, and ethnicity contributed to variation in motor proficiency scores was then examined using linear regression methods.
Sibling correlations for motor proficiency, separating identical twins, concordant ASD-affected sibling pairs, and sibling pairs discordant for ASD were then examined. Finally, the correlation between the degree of autistic severity, as measured by the SRS t-scores, and motor proficiency, both within the ASD-affected group and in the sample as a whole, was analysed. T-scores are standardized scores with a mean of 50 and standard deviation of 10. For the SRS, T-scores were calculated separately for males and females, so can be similarly interpreted for both genders (Constantino and Gruber, 2005).
Results
Comparison of motor scores between siblings
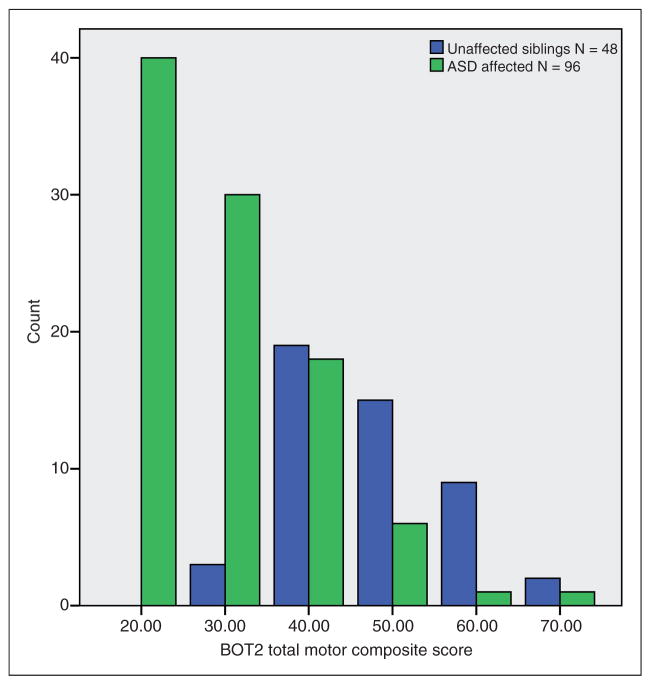
Motor scores for the BOT2 subscales, total motor composite, and the DCDQ’07 total score (which were continuously distributed in our ASD participants) were poorer for children with ASD than for their unaffected siblings. The motor scores of the latter were essentially normal, as shown in Table 2. Scores of index cases were not significantly different from those of affected siblings. Total motor composite scores of at least one standard deviation below the general population mean were seen in 83% of the affected group compared to 6% in the unaffected siblings and scores of at least two standard deviations below were observed in 43% of the group compared with none in the unaffected siblings. No significant differences were found between scores of African American and white participants. Figure 1 depicts unimodal distributions for both affected (skewed pathologically) and unaffected children. The discrepancy in the respective distributions was highly statistically significant (t = 10.1, df = 142, p < .0001), presented here for total scores and summarized for each subdomain of motor impairment in Table 2.
Table 2.
Standardized gender-specific scores from Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT2) and mean total scores from the Developmental Coordination Disorder Questionnaire 2007 (DCDQ’07)
| Motor score | Participant category Index case (n = 67) Affected sibling (n = 29) Unaffected sibling (n = 48) |
Mean* | SD | p |
|---|---|---|---|---|
| BOT2 Fine Manual Control | Index case | 36.1 | 11.2 | |
| Affected sibling | 35.9 | 10.9 | ns | |
| Unaffected sibling | 50.2 | 9.2 | < .001 | |
| BOT2 Manual Coordination | Index case | 33.7 | 9.7 | |
| Affected sibling | 36.3 | 11.5 | ns | |
| Unaffected sibling | 50.7 | 9.4 | < .001 | |
| BOT2 Body Coordination | Index case | 35.6 | 12.3 | |
| Affected sibling | 36.3 | 12.7 | ns | |
| Unaffected sibling | 52.6 | 9.3 | < .001 | |
| BOT2 Strength and Agility | Index case | 35.5 | 10.8 | |
| Affected sibling | 37.8 | 14.0 | ns | |
| Unaffected sibling | 54.6 | 8.1 | < .001 | |
| BOT2 Total Motor Composite | Index case | 33.0 | 10.2 | |
| Affected sibling | 34.3 | 13.4 | ns | |
| Unaffected sibling | 52.6 | 9.4 | < .001 | |
| DCDQ’07 Total Score | Index case (n = 33) | 42.4 | 13.1 | |
| Affected sibling (n = 14) | 38.6 | 13.2 | ns | |
| Unaffected sibling (n = 19) | 66.0 | 7.4 | < .001 |
BOT2 Normative Mean = 50; DCDQ’07 total possible = 75; p values compare index case scores with specified sibling groups using Student’s t test.
Figure 1.
BOT2 Total Motor Composite Score Distribution, Normative mean = 50.
Motor scores were compared between the children with ASD and comorbid ADHD (n = 22) and those without ADHD (n = 74) and were found to be significantly better in those who had both diagnoses (t = −3.1, df = 94, p = .003). To examine whether this might be due to a medication effect, motor scores from those with both diagnoses who were medicated (n = 11) were compared with those who were not medicated and no significant difference was found.
Sibling correlations for the BOT2 total motor composite scores between identical twins concordant for ASD, non-identical siblings concordant for ASD, and non-identical siblings discordant for ASD were compared. Correlations were very strong for identical twins, moderate for non-identical concordant siblings, and non-significant for non-identical discordant siblings, consistent with the pattern expected for an inherited trait associated with ASD. See Table 3 for details by motor area. A small subset of non-identical discordant siblings were from multiplex families and were examined separately (n = 9 pairs). In this group, standard score means were all in the normal range for the unaffected siblings, but we did observe substantial sibling correlations for motor abilities for all BOT2 motor standard score categories except for strength and agility, on the order of r = .66 to .71.
Table 3.
Correlations between siblings’ BOT2 standardized scores
| Motor subscale | Identical twins concordant for ASD n = 6 pairs | Non-identical siblings concordant for ASD n = 21 pairs | Non-identical siblings discordant for ASD n = 48 pairs |
|---|---|---|---|
| Fine Manual Control | .684 | .284 | .042 |
| Manual Coordination | .927** | .443* | .088 |
| Body Coordination | .713 | .480** | .083 |
| Strength and Agility | .894* | .550** | .101 |
| Total Motor Composite | .864* | .437* | .037 |
p < .05,
p < .01.
A regression analysis revealed that social responsiveness (SRS total score) was a significant predictor of the BOT2 (n = 138; F(4,133) = 25.34, p < .000001, r2 = .433 for total score), when controlling for age, gender, and ethnicity. In the subgroup for which IQ was available, IQ scores were moderately correlated with BOT2 standard scores (n = 41; r = .48 to .62). In that group, social responsiveness and IQ were both significant predictors of BOT2 total motor composite scores (F(5,35) = 7.25, pSRS = .011, pIQ = .001, r2= .509), when controlling for age, gender, and ethnicity.
Correlation between motor and severity scores
Among the ASD-affected children, the BOT2 total motor proficiency score was inversely correlated with degree of social impairment (n = 92; r = −.389; p = .0001). Correlations between SRS and BOT2 subscales ranged from −.26 to −.43 for the ASD-affected group. For the entire sample, correlations of SRS with each motor area of the BOT2 ranged from −.56 to −.65 (n = 138), all significant at p < .0001.
Comparison between DCDQ’07 and BOT2
DCDQ’07 data were available from 66 children in the standardized age range (5 to 15 years). Correlations between BOT2 Total Motor Composite score and DCDQ’07 subscale scores ranged from .71 to .75; the correlation between the DCDQ’07 Total Score and the BOT2 Total Motor Composite was .79. These moderately strong relationships suggest that the utility of the DCDQ’07 as a proxy for measurement of motor impairment when direct individually administered measures are not available or feasible.
Discussion
The observations (a) that motor proficiency shows a substantially impaired distribution in children with ASD, (b) that the degree of motor impairment is correlated with the degree of social impairment in ASD, and (c) that motor proficiency is not impaired in unaffected siblings suggest that motor impairment constitutes a core feature of the autistic syndrome, rather than an ASD endophenotype. The association between scores for autistic severity and those for motor impairment hold true for all BOT2 subscale scores, including fine manual control, manual coordination, body coordination, and strength and agility.
The higher degree of correlation between scores from concordant identical twins in comparison than between those of non-identical concordant siblings suggests the importance of the genetic contribution to motor impairment which further substantiates its candidacy as a core feature of a substantially inherited syndrome. Linear regression analyses further supported the association between social responsiveness, IQ, and motor impairment when controlling for age, gender, and ethnicity.
Moderately strong correlations between BOT2 and DCDQ’07 scores suggest that use of the latter may facilitate assessment of motor impairment in children with ASD in larger studies of children with ASD, and in the clinic where time may not allow for BOT2 administration. It also supports the potential for measuring motor impairment as standard practice for clinicians, similar to measuring the degree of social impairment in ASD, to contribute to a comprehensive evaluation of the impairments inherent in a given clinical presentation of an ASD.
The finding that children with ASD and comorbid ADHD had significantly better motor scores than those without ADHD was not expected, because high motor impairment alone in the general population does not predict ASD (Reiersen et al., 2008). This paradox suggests the importance of further examination of this relationship through future research in a larger sample that has well characterized ADHD and motor impairment.
This study was limited by a relatively low number of unaffected children from multiple-incidence families. It would be important to examine whether subtle motor deficits, which this study may have been inadequately powered to detect, might be appreciable in a larger sample of unaffected multiplex ASD siblings, as has been observed for sub-clinical deficits in social behavior, language, and repetitive behavior (Constantino et al., 2006, Virkud et al., 2009, Constantino et al., 2010). This study is also limited by the reduced availability of current IQ data for the participants. Motivation to perform motor tasks is often compromised in children with ASD and, although many efforts were made to increase compliance and to support their participation in assessment, it is possible that the actual motor abilities of these participants with ASD may not have consistently been demonstrated by use of these observational methods. Nevertheless, the high degree of correlation between parent reports of the children’s capabilities and what we directly observed strongly supports the validity of the observational assessments.
The current observations support the possibility that motor impairment constitutes a core component of the autistic phenotype and warrants consideration for inclusion in diagnostic paradigms for the autistic syndrome. Although the motor impairments observed in ASD are not necessarily unique to ASD (i.e. similar in quality to those that occur, for example, in Developmental Coordination Disorder), they nevertheless occur extremely commonly in ASD, and correlate in severity with symptoms in the other DSM-IV criterion domains that characterize ASD. Furthermore, previously published associations between motor coordination and social cognition raise the possibility that motor impairments provide a window of observation on fundamental high-conserved neural mechanisms whose disruptions may have a role in the ontogeny of higher level deficits.
Variation in motor proficiency in children with ASD can be feasibly and objectively measured (either by an individually administered standardized measure or parent report) and may serve as a robust index of neurodevelopmental dysfunction with which to explore associations with genetic variation (e.g. linkage and association studies) and patterns of brain activity (e.g. via neuroimaging research). It is also possible that interventions that prove successful for the enhancement of motor proficiency may serve as models for therapeutic approaches directed toward improving the complex social-cognitive impairments of children with ASD.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Contributor Information
Claudia List Hilton, Washington University in St Louis, USA.
Yi Zhang, Washington University in St Louis, USA.
Megan R. Whilte, Washington University in St Louis, USA
Cheryl L. Klohr, Washington University in St Louis, USA
John Constantino, Washington University in St Louis, USA.
References
- Beery KE. The Beery-Buktenica Developmental Test of Visual-Motor Integration. 4. Cleveland: Modern Curriculum Press; 1997. [Google Scholar]
- Bruininks RH, Bruininks BD. Bruininks-Oseretsky Test of Motor Proficiency. 2. Minneaopolis: Pearson; 2005. [Google Scholar]
- Chen H, Cohn E. Social participation for children with developmental coordination disorder: Conceptual, evaluation and intervention considerations. Physical and Occupational Therapy in Pediatrics. 2003;23(4):61–78. [PubMed] [Google Scholar]
- Church C, Alisanski S, Amanullah S. The social, behavioral, and academic experiences of children with Asperger syndrome. Focus on Autism and Other Developmental Disabilities. 1999;15(1):12–20. [Google Scholar]
- Constantino JN, Hilton CL, Abbacchi A, Zhang Y. IQ data from children with autism and their siblings. (unpublished data) [Google Scholar]
- Constantino J, Gruber C. Social Responsiveness Scale (SRS) Manual. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanant N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M. Autistic Social Impairment in the Siblings of Children with Pervasive Developmental Disorders. American Journal of Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Constantino JN, LaVesser PD, Zhang Y, Abbacchi AM, Gray T, Todd R. Rapid quantitative assessment of autistic social impairment by classroom teachers. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi A, Law P. Sibling recurrence and the genetic epidemiology of autism. American Journal of Psychiatry. 2010;167:1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FJ, Baranek GT, Giuliani CA, Mercer VS, Poe MD, Thorpe DE. A pilot study: Coordination of precision grip in children and adolescents with high functioning autism. Pediatric Physical Therapy. 2009;21:205–211. doi: 10.1097/PEP.0b013e3181a3afc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzuik MA, Gidley-Larson JC, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social and communicative deficits. Developmental Medicine and Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential Abilities Scale. 2. San Antonio: Pearson; 2007. [Google Scholar]
- Fournier KA, Hass CJ, Sagar KN, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;10:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in autism and AS: A further report. Journal of Intellectual Disability Research. 1998;42(pt1):43–48. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden D. The Movement Assessment Battery for Children. London: The Psychological Corporation; 1992. [Google Scholar]
- Hilton CL, Wente L, LaVesser P, Ito M, Reed C, Herzberg G. Relationship between motor skill impairment and severity in children with Asperger syndrome. Research in Autism Spectrum Disorders. 2007;1:339–349. [Google Scholar]
- Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. American Journal of Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- Martin NC, Piek JP, Hay D. DCD and ADHD: a genetic study of their shared aetiology. Human Movement Science. 2006;25(1):110–124. doi: 10.1016/j.humov.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Miles B, Nierengarten M, Nearing R. A review of the eleven most often-cited assessment instruments used in adapted physical education. Clinical Kinesiology. 1988;42:33–41. [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain and Development. 2007;29:563–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines: American Guidance Service Inc; 1995. [Google Scholar]
- Papadopoulous N, Rinehart N, Tonge N, Bradshaw J, Saunders K, Murphy A. Motor proficiency and emotional-behavioural disturbance in autism and Asperger’s disorder: Another piece of the neurological puzzle? Autism: International Journal of Research and Practice. doi: 10.1177/1362361311418692. (in press) [DOI] [PubMed] [Google Scholar]
- Piek JP, Barrett NC, Smith LM, Rigoli D, Gasson N. Do motor skills in infancy and early childhood predict anxious and depressive symptomatology at school age? Human Movement Science. 2010;29:777–786. doi: 10.1016/j.humov.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Qui A, Adler M, Crocetti D, Miller M, Mostofsky S. Basal ganglia shapes predict social, communication and motor dysfunctions in boys with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(6):539–551. doi: 10.1016/j.jaac.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Todd RD. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(6):662–672. doi: 10.1097/CHI.0b013e31816bff88. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Iansek B, McGinley J, Brereton AV, Enticot PG, et al. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Developmental Medicine and Child Neurology. 2006;48:819–824. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R. Autism Diagnostic Interview - Revised Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Saresella M, Marventano I, Guerini FR, Mancuso R, Ceresa L, Zanzottera M, et al. An autistic endophenotype results in complex immune dysfunction in healthy siblings of autistic children. Biological Psychiatry. 2009;66(10):978–984. doi: 10.1016/j.biopsych.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Schoon I, Cheng H, Jones E. Resilience in children’s development. In: Hansen K, Joshi H, Dex S, editors. Children of the 21st Century. Bristol: Policy Press; 2010. pp. 235–248. [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences: Psychology. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics Part B. 2009;150B:328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Namkung J, Toth K, Greenson J, et al. Toddlers with elevated autism symptoms show slowed habituation to faces. Child Neuropsychology. 2010;16(3):255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Pearson; 1999. [Google Scholar]
- Wilson BN, Kaplan BJ, Crawford SG, Roberts G. The Developmental Coordination Disorder Questionnaire 2007. Calgary: Alberta Children’s Hospital; 2007. [Google Scholar]