Abstract
Endogenous opioid and cannabinoid systems are thought to act synergistically regulating antinociceptive and reward mechanisms. To further understand the human implications of the interaction between these two systems, we investigated the role of the common, functional missense variant Pro129Thr of the gene coding fatty acid amide hydrolase (FAAH), the major degrading enzyme of endocannabinoids, on psychophysical and neurotransmitter (dopaminergic, opioid) responses to pain and placebo-induced analgesia in humans. FAAH Pro129/Pro129 homozygotes, who constitute nearly half of the population, reported higher placebo-analgesia and more positive affective states immediately and 24 hours after placebo administration; no effects on pain report in the absence of placebo were observed. Pro129/Pro129 homozygotes also showed greater placebo-induced μ-opioid, but not D2/3 dopaminergic, enhancements in neurotransmission in regions known involved in placebo effects. These results show that a common genetic variation affecting the function of the cannabinoid system is serving as a probe to demonstrate the involvement of cannabinoid and opioid transmitters on the formation of placebo effects.
Introduction
Both opioid and non-opioid mechanisms are involved in placebo analgesia. While the involvement of the former has been extensively described 1, 2, non-opioid mechanisms of placebo analgesia are increasingly recognized. Such a candidate neurotransmitter system is the endocannabinoid (eCB) system, comprised of cannabinoid CB1 and CB2 receptors and their endogenous ligands, including N-arachidonoyl ethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG) 3. eCBs are thought to be involved in analgesia 4 and reward/reinforcement 5 mechanisms, both of which are thought to be engaged during the development of placebo effects 6. Recent work has shown that in the context of a conditioning paradigm, the cannabinoid receptor 1 (CBR1) antagonist SR 141716A (Rimonabant) blocked non-opioid, ketorolac-conditioned placebo analgesia, but not opioid placebo responses after morphine conditioning 7. The involvement of the eCB system on non-conditioned, expectation-related placebo effects has not been explored.
CBR1 and μ-opioid receptors (MORs) are colocalized in brain structures involved in nociceptive control 8, and they functionally interact. These interactions between endogenous opioids and eCBs have been demonstrated following genetic deletion of μ-opioid or CBR1 receptors 9. In animal models, thermal nociception is synergistically reduced by the combination of Δ9-tetrahydrocannabinol (Δ9-THC) with morphine 10. The replacement of Δ9-THC with AEA in combination with the fatty acid amide hydrolase (FAAH) inhibitor URB597 elicits a similar antinociceptive potentiation, which is also reversed by MOR but not δ-opioid receptor (DOR) antagonists 11. Moreover, synergistic responses to opioids combined with exo- or eCBs occur in tissues and pathways in which their physiological roles in pain, reward and mood regulation have been well established 10, 11.
Differently from endogenous opioids, eCBs are rapidly synthesized on demand by neurons in response to depolarization and consequent Ca2+ influx. After release, they are removed from the extracellular space and degraded by FAAH. Mice deficient in FAAH have higher steady state concentrations of brain AEA, indicating that FAAH regulates eCB tone 12. In humans, a single-nucleotide polymorphism in the FAAH gene, C385A (rs324420), encodes a Pro129Thr missense substitution. The Thr129 allele decreases the activity of FAAH and thereby leads to higher endocannabinoid levels 13. Both alleles are abundant (Pro: Thr = 0.8: 0.2) and thus this polymorphism represents a naturally occurring probe to examine the role of eCBs in human samples. To examine the contribution of eCBs to the regulation of pain and placebo analgesia, we studied the effect of the Pro129Thr functional variant of FAAH during a sustained pain challenge with and without the administration of placebo with potential analgesic properties. We used positron emission tomography (PET) scanning and selective radiotracers labeling μ-opioid and D2/3 receptors to trace the effects of endocannabinoid function in placebo response to two of the major neurotransmitter systems that have been implicated in responses to pain and placebos14, 15. We initially hypothesized that Thr385, by protecting AEA from degradation and allowing AEA to accumulate, would synergize with opioid, but not dopamine responses to pain and placebo administration, as evidence shows that the eCB system is not involved in cocaine reinforcement 16, 17.
Materials and Methods
1. Subjects
Forty-two healthy subjects (23 females, 19 males, mean age ± s.d: 26 ± 4), were recruited via advertisement. Participants were right handed, with no personal history of major medical illnesses or psychiatric disorders. Volunteers were not taking psychotropic medications. At the time of recruitment, participants were verbally informed about the details of the study and provided written consent. Results on 20 of the 42 subjects in the current sample were part of a previous manuscript 14. All procedures were approved by the Institutional Review Board and the Radioactive Drug Research Committee at the University of Michigan.
2. Genotyping
FAAH Pro129Thr (C385A, rs324420) was genotyped using the Illumina Golden Gate platform (San Diego, California), employing the Addictions Array content of 130 genes (1350 SNPs) and 186 Ancestry Informative Markers (AIMs), to calculate ethnic factor scores 18. Genotyping accuracy was confirmed by replicate genotyping of 10% of the total sample with a completion rate of >93% (mean 99.4%, median 100%). Other non-functional FAAH SNPs in the Addictions Array [rs6703669 (C/T), rs3766246 (C/T) and rs2295633 (C/T)]) were not part of this investigation, which was focused on the known functional polymorphism rs324420 (Pro129Thr)13.
Of the total sample, 23 subjects carried at least one Thr129 allele and 19 were homozygotes for the Pro129 allele. The FAAH C385A genotype distribution was in Hardy-Weinberg equilibrium (χ2=0.26, p=0.6), and there were no significant differences between the two genotype groups with respect to sex, age or the European and African ethnic factor score (Table 1). Nevertheless, population stratification was evaluated as a potential confounder using European and African ethnic factor scores derive from the AIMs against psychophysical and neuroimaging data and no confounding was present in C385A genotypes due to ethnic differences.
Table 1.
FAAH Pro129Thr demographics and effects on reported pain ratings and its recall after 24 hours, Δ in pain ratings after placebo administrations and its recall after 24 hours and levels of expectations of analgesic efficacy.
| FAAH Pro129Thr | Pro129/Pro129 (n= 19) | Thr129 carriers (n= 23) | ||
|---|---|---|---|---|
| mean ± s.e. | mean ± s.e. | F/χ2 | p | |
| Demographics | ||||
| Age | 25 ± 1 | 26 ± 1 | .1 | .7 |
| Sex (M/F) | 9/10 | 10/13 | .06 | .8 |
| European AIM | .7 ± .09 | .6 ± .08 | .12 | .7 |
| African AIM | .16 ± .07 | .14 ± .06 | .05 | .8 |
| Pain | ||||
| Avg. VAS (every 15 s) | 28 ± 2.7 | 33 ± 2.5 | 2.28 | .1 |
| Avg. VAS/Vol (mL) | 11 ± 2 | 16 ± 2 | 3 | .1 |
| MPQ Total | 24 ± 2 | 20 ± 2 | 1.5 | .2 |
| MPQ Sensory | 15 ± 7 | 13 ± 6 | 1.01 | .3 |
| MPQ Affect | 5.5 ± .7 | 3.5 ± .7 | 3.1 | .08 |
| Memory of pain after 24 hours | ||||
| Memory MPQ Total | 21 ± 2.3 | 20 ± 2.1 | .39 | .5 |
| Memory MPQ Sensory | 14 ± 1.5 | 14 ± 1.3 | .05 | .8 |
| Memory MPQ Pain Affect | 3.8 ± .6 | 3.1 ± .6 | 2.14 | .1 |
| Changes in affective state after pain (changes from baseline) | ||||
| Δ PANAS Positve | − 5.3 ± 1.8 | − 3 ± 1.4 | 1.5 | .23 |
| Δ PANAS Negative | − 1.1 ± .8 | − 1.4 ± .6 | .02 | 0.9 |
| Δ POMS TMD | − 5 ± 3 | − 4 ± 2 | .55 | .8 |
| Changes in pain ratings after placebo | ||||
| Δ Avg. VAS | − 8 ± 3 | − 6 ± 3 | .2 | .6 |
| Avg. VAS/Vol (mL) | 4 ± 2 | 2.7 ± 1.7 | .5 | .5 |
| Δ MPQ Total | − 5 ± 2 | .3 ± 1.7 | 7.9 | .008 |
| Δ MPQ Sensory | − 2.4 ± 1.3 | .04 ± 1.2 | 4.1 | .048 |
| Δ MPQ Pain Affect | − 2 ± .6 | − .08 ± .5 | 11.6 | .002 |
| Changes in the memory of pain ratings 24 hours after placebo | ||||
| Δ Memory MPQ Total | − 4 ± 2 | − .1 ± 1.7 | 4.1 | .048 |
| Δ Memory MPQ Sensory | − 2 ± 1.3 | − 1.18 ± 1.1 | .11 | .74 |
| Δ Memory MPQ Affect | − 1.11 ± .6 | .7 ± .5 | 10.5 | .003 |
| Changes in the affective state after placebo | ||||
| Δ PANAS Positve | − .26 ± .7 | − 2.2 ± .6 | 4.4 | .04 |
| Δ PANAS Negative | − .4 ± .6 | − .5 ± .5 | .2 | .6 |
| Δ POMS TMD | − 4.1 ± 1.4 | .5 ± 1.3 | 4.6 | .038 |
| Expectations of analgesia | 45.5 ± 6 | 48.3 ± 6 | .18 | .67 |
Abbreviations: M/F: Male/Female. VAS: Visual Analog Scale; Δ MPQ: McGill Pain Questionnaire; PANAS: Positive and Negative Affect Scale; POMS TMD: Profile of Mood State, Total Moods Disturbance score.
3. Experimental design
Four 90-minute PET studies were acquired per subject (2 [11C]carfentanil and 2 [11C]raclopride) 14. Subjects were placed in the scanner gantry with needles placed in both masseter muscles approximately 30 minutes before radiotracer administration. Each scanning session consisted of a control condition (0.9% isotonic saline, 5-25 min after start of scanning) and a painful condition (5% hypertonic saline, 45-65 min after start of scanning), infused in the left masseter muscle with and without placebo administration. Volunteers were informed that we were studying the effects of an intervention thought to reduce pain by the activation of internal pain regulatory mechanisms. The placebo condition consisted of the introduction of 1 mL of 0.9% isotonic saline into 1 of the intravenous ports every 4 minutes starting 2 minutes before the pain anticipation and the pain challenges in view of the volunteer, and lasting for 15 seconds (sec) each time. An individual infusion profile was developed to induce the same level of pain intensity across individuals, targeting 40 VAS units, and repeated across the 4 PET scans 19. Momentary pain intensity was recorded every 15 sec for the duration of the pain challenge, as was the infusion volume of hypertonic saline required for pain maintenance. The ratio of the two then represents a measure of sustained pain sensitivity over 20 min. The order of each pair of scans was randomized and counterbalanced across subjects.
In order to evaluate the sensory and affective component of pain, subjects completed the Positive and Negative Affect Scale (PANAS) 20 and the Profile of Mood States (Total Mood Disturbance score) (POMS-TMD) 21 before and after each of the challenges; a visual analog scale (VAS) of pain intensity every 15 seconds during the pain challenge (both during control and pain periods), and the McGill Pain Questionnaire 22 (ΔMPQ) at the completion of the pain challenge. Changes in the each measure in the absence and presence of placebo were used for the assessment of placebo responses.
Levels of expectancy and subjectively assessed placebo effectiveness were also measured with the questions: ‘From 0 to 100 how effective do you think the treatment will be?’, and ‘From 0 to 100 how effective was the treatment?’.
The recall of analgesic effects was obtained 24 hours after each scan by administering the MPQ during a phone interview. A subtraction of the MPQ scores obtained 24 hours after each scan (pain and pain with placebo scans) was used as a measure of placebo effect recall.
4. Neuroimaging Methods
Participants were positioned in the PET scanner gantry, and 2 intravenous (antecubital) lines were placed. A light forehead restraint was used to eliminate intrascan head movement. As previously described (1), four, 90 min PET scanning sessions, two with [11C]carfentanil and two with [11C]raclopride, with and without placebo administration, were completed (Siemens HR+, Knoxville, Tennessee). Images were acquired in 3-dimensional mode (reconstructed full-width/half-maximum resolution, approximately 5.5 mm in plane and 5.0 mm axially), with the septa retracted and scatter correction. Tracer administrations were separated by at least 2 hours to allow for radiotracer decay. [11C]carfentanil and [11C]raclopride studies were randomized and counterbalanced in order. [11C]carfentanil was synthesized at high specific activity by the reaction of [11C]methyl iodide and a normethyl precursor as previously described 23. [11C]raclopride was synthesized at high specific activity by the reaction of o-desmethyl raclopride with [11C]methyl triflate. 15 ± 1 mCi were administered in each scan, with cold mass limits of 0.028 ± 0.013 μg/kg for carfentanil and 0.20 ± 0.15 μg/kg of raclopride. These doses ensured that the compounds were administered in tracer quantities, that is, subpharmacological doses occupying less than 1% of the available receptors. Fifty percent of the radiotracer doses were administered as an initial bolus and the remaining 50% by continuous infusion for the remainder of the study to more rapidly achieve steady-state levels. For each study, 21 sets of dynamic scans were acquired with an increasing duration (four 30-second frames, three 1-minute frames, two 2.5-minute frames, eight 5-minute frames, and four 10-minute frames).
Images were reconstructed using iterative algorithms (brain mode; Fourier rebinning algorithm with ordered-subsets expectation maximization, 4 iterations, and 16 subsets; no smoothing) into a 128×128-pixel matrix in a 28.8-cm-diameter field of view. Attenuation correction was performed through a 6-minute transmission scan (Ge68 source) obtained before the PET study and with iterative reconstruction of the blank/transmission data, followed by segmentation of the attenuation image. Small head motions during PET were corrected by an automated computer algorithm for each subject before analysis, and the images were coregistered with the same software (32). Time points were then decay corrected during reconstruction of the PET data. Image data were then transformed on a voxel-by-voxel basis into 2 sets of parametric maps, a tracer transport measure (K1 ratio) and a receptor-related measure (non-displaceable binding potential, BPND, or receptor availability in vivo. To avoid the need for arterial blood sampling, these measures were calculated using a modified Logan graphical analysis 24, and the occipital cortex (an area devoid of μ-opioid receptors) or the cerebellum (an area with low D2/3 receptor concentrations) as reference regions. Using the bolus-continuous infusion protocol described above, the slope of the Logan plot becomes linear 5~7 min post-tracer administration and is proportional to the receptor concentration divided by its affinity for the radiotracer [BPND + 1, or (f2Bmax/Kd) +1]. Bmax is the receptor concentration and Kd, the receptor-ligand dissociation constant. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value. Reductions in the in vivo availability of receptors, the BPND measure, after an acute challenge (i.e., placebo administration during experimental pain) are thought to reflect processes, such as competition between radiotracer and endogenous ligand, associated with neurotransmitter release 25.
Anatomic MRI studies were acquired on a 3-T scanner (General Electric, Milwaukee, Wisconsin). Acquisition sequences were axial spoiled gradient recall inverse recovery prepared magnetic resonance [echo time, 3.4 milliseconds; repetition time, 10.5 milliseconds; inversion time, 200 milliseconds; flip angle, 25°; number of excitations, 1; using 124 contiguous images, 1.5-mm thickness]. The K1 and BPND images for each experimental period and the anatomical MRI were coregistered to each other and to the Montreal Neurological Institute (MNI) stereotactic atlas orientation.
5. Data analysis
To examine the effects of the FAAH Pro129Thr polymorphism on placebo-induced μ-opioid system activation (reductions in the receptor availability measure, nondisplaceable binding potential, BPND from the pain to pain + placebo conditions) we applied a mixed model analysis of variance on a voxel-by-voxel basis using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, University College, London, England), with each genetic variant as the between subject factor and the change in BPND as the dependent variable. Sex and age were included in the analysis as nuisance covariates. No global normalization was applied to the data, and therefore the calculations presented are based on absolute Bmax/Kd estimates. A cortical mask that excluded the cerebellum was used in the analysis. Only voxels with specific μ-opioid receptor binding were including in the analyses (BPND > 0.1) to reduce statistical noise. A p<0.05 false discovery rate (FDR)-corrected was considered significant. These data were extracted for quantification of regional changes in BPND, graphing, correlational analyses and the identification of potential outliers. Further statistical analyses were performed with commercially available statistical software (SPSS for Macintosh, version 17; SPSS Inc, Chicago, Illinois). ANCOVA models were performed using the Pro129Thr variant of the FAAH as the between-subject factors and differences in pain and affective ratings from pain to the pain + placebo condition as the within subject factor, with European AIM and the average VAS during pain as covariates of no interest. Planned ANCOVA and correlational analysis were consider significant at a p<0.05.
Results
FAAH Pro129Thr psychophysical effects
The FAAH Pro129Thr polymorphism had an isolated effect on placebo response, independent of other aspects of pain. This molecularly functional polymorphism did not predict higher pain ratings either at the time of the study or 24 hours later. Also, it did not predict pain sensitivity (VAS/mL of algesic input), or changes in affective state scores during pain (Table 1). However, Pro129/Pro129 homozygotes showed significantly greater psychophysical placebo responses: changes (Δ) in MPQ Total (F=7.9, p=.008), ΔMPQ Sensory (F=4.1, p=.048) and ΔMPQ Pain Affect (F=11.6, p=.002). Twenty-four hours after placebo administration, the Pro129/Pro129 genotype also predicted increases in the scoring of the placebo analgesic experience during its recall: ΔMPQ Total24h (F= 4.1, p=.048) and ΔMPQ Pain Affect24h (F=10.5, p=.003). Pro129/Pro129 homozygotes additionally reported a more positive internal affective state during the placebo condition as compared to Thr129 carriers: ΔPANAS positive (F=4.4, p=.04) and less negative affect: ΔPOMS (F=4.6, p=0.038). No effects of genotype were observed for the initial ratings of expectancy of placebo analgesia.
FAAH Pro129Thr effects on pain and placebo-induced μ-opioid and D2/3 DA neurotransmission
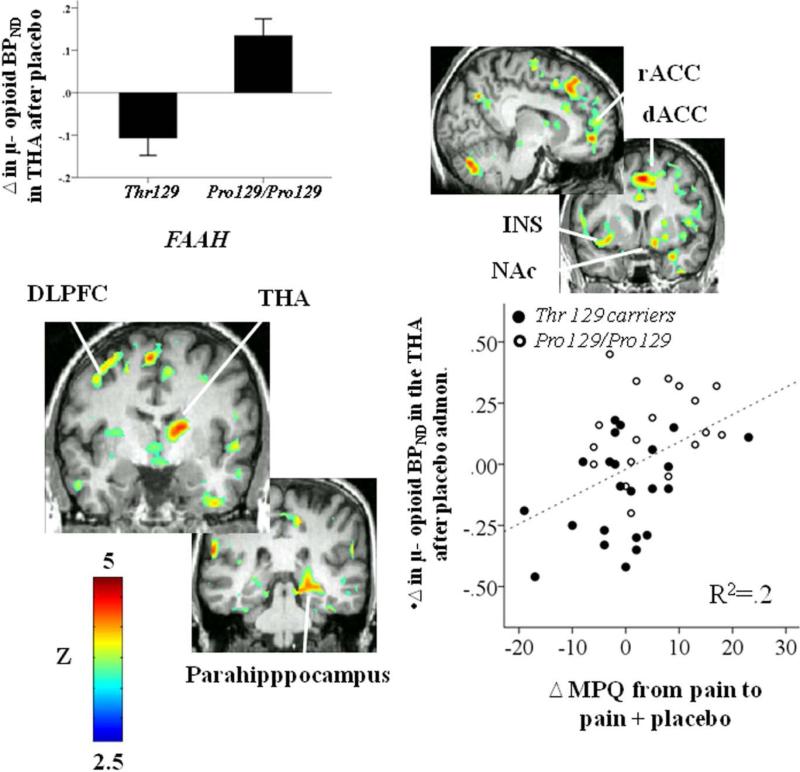
Analyses of the molecular imaging data showed that FAAH genotype had a selective effect on opioid-mediated placebo analgesia, but not on pain-induced opioid system responses or dopaminergic function. We studied the relationship of FAAH genotype variation to μ-opioid and DA D2/3 receptor BPND measures both at baseline and following activation of these neurotransmitter systems during the pain challenge, using a t-test and mixed model analysis of variance, respectively, applied on a voxel-by-voxel basis. No effects of Pro129Thr were observed for baseline μ-opioid and DA D2/3 receptor BPND, or for pain-induced activation of μ-opioid or DA D2/3 neurotransmission, as might be expected based on the lack of genotype effects on subjective psychophysical responses to the pain challenge. We then tested for an effect of FAAH genotype variation on placebo-induced μ-opioid and DA D2/3 system activation. FAAH Pro129/Pro129 homozygotes showed greater endogenous opioid system activation during placebo administration (for all regions, p <0.05 after FDR correction for multiple comparisons) in areas of the prefrontal cortex, including the dorsolateral prefrontal cortex (DLFPC), the dorsal and ventromedial prefrontal cortex (d/v MPFC), the lateral and medial orbitofrontal cortex (l/m OFC), the inferior frontal gyrus (IFG), the dorsal, rostral and subgenual anterior cingulate cortex (d/r/sg ACC), the anterior and posterior insula (a/p INS) and the hippocampus and parahippocampal gyrus. Subcortically, regions where an effect of FAAH genotype variation was observed included the nucleus accumbens (NAc), extending posteriorly to the mammillary region (MR), the dorsal and ventral putamen (d/v PUT) and the anterior and posterior thalamus (a/p THA) (Fig. 1, Table 2). No significant effect of FAAH genotype variation was found for the opposite contrast. No effects of FAAH genotype variation on placebo-induced DA D2/3 system activation were obtained.
Fig. 1.
Figures represent voxel-by-voxel brain effects of FAAH Pro129Thr (Pro129/Pro129 > Thr 129 carriers) on Δ μ-opioid BPND after placebo administration during pain. Upper left: regional effects of FAAH Pro129Thr (Pro129/Pro129 > Thr 129 carriers) on Δ μ-opioid BPND in the thalamus (THA) after placebo administration during pain. Lower right: Pearson correlation between Δ μ-opioid BPND after placebo administration in the thalamus and Δ in pain ratings after placebo administration. Abbreviations: DLPFC: dorsolateral prefrontal cortex; dACC: dorsal anterior cingulate cortex; NAc: nucleus accumbens; THA: thalamus; MPQ: McGill Pain Questionnaire.
Table 2.
FAAH Pro129Thr effect on Δ BPND during pain after placebo administration.
| Ha | A carriers (mean ±s.e.)b | CC (mean±s.e.)b | x,y,z {mm}c | Cluster sized | Ze | p(FDR-cor) | |||
|---|---|---|---|---|---|---|---|---|---|
| DLPFC | R | −.06 ± .03 | .08 ± .03 | 37 | 13 | 52 | 317 | 4.31 | .005 |
| L | −.12 ± .03 | .04 ± .04 | −30 | 21 | 50 | 308 | 4.25 | .005 | |
| dMPFC | L | −.09 ± .03 | .08 ± .03 | −19 | −44 | −49 | 160 | 4.55 | .004 |
| vMPFC | R | −.07 ± .02 | .04 ± .02 | 4 | 55 | 24 | 560 | 3.96 | .007 |
| mOFC | R | −.09 ± .02 | .06 ± .03 | 19 | 36 | 26 | 1963 | 4.63 | .004 |
| L | −.06 ± .02 | .06 ± .03 | −23 | 57 | 20 | 572 | 3.96 | .007 | |
| lOFC | L | −.08 ± .02 | .07 ± .03 | −43 | 40 | −11 | 1367 | 4.51 | .005 |
| IFG | L | −.10 ± .03 | .06 ± .03 | −46 | 29 | 12 | 521 | 3.74 | .009 |
| rACC | L | −.08 ± .03 | .06 ± .03 | −10 | 47 | −6 | 521 | 4.23 | .005 |
| sgACC | R | −.07 ± .04 | .10 ± .05 | 1 | 19 | −13 | 22 | 3.35 | .014 |
| dACC | R | −.12 ± .02 | .07 ± .03 | 1 | 7 | 49 | 2703 | 4.62 | .004 |
| L | −.08 ± .02 | .09 ± .03 | −7 | 26 | 48 | 1328 | 4.60 | .004 | |
| aINS | L | −.10 ± .03 | .11 ± .03 | −36 | 13 | −14 | 1672 | 5.28 | .004 |
| R | −.08 ± .02 | .08 ± .03 | 46 | 11 | 6 | 1610 | 3.90 | .008 | |
| pINS | L | −.08 ± .02 | .05 ± .03 | −41 | −15 | 15 | 300 | 3.96 | .007 |
| Hipp | R | −.06 ± .02 | .08 ± .02 | 35 | −18 | −9 | 124 | 3.74 | .009 |
| Phipp | R | −.09 ± .02 | .06 ± .02 | 21 | −47 | −2 | 4621 | 4.88 | .004 |
| L | −.06 ± .03 | .07 ± .03 | −32 | −37 | −8 | 159 | 3.67 | .010 | |
| NAc/MR | R | −.13 ± .05 | .19 ± .08 | 11 | 4 | −14 | 547 | 4.63 | .004 |
| dPUT | R | −.13 ± .04 | .06 ± .03 | 21 | 13 | 6 | 315 | 4.02 | .007 |
| vPUT | R | −.15 ± .05 | .10 ± .07 | 23 | 8 | −7 | 86 | 3.61 | .011 |
| aTHA | R | −.11 ± .04 | .13 ± .04 | 17 | −8 | 15 | 1117 | 4.96 | .004 |
| pTHA | L | −.06 ± .03 | .11 ± .04 | −20 | −22 | −2 | 82 | 3.36 | .014 |
Abbreviations: DLPFC: dorsolateral prefrontal cortex; dMPFC and vMPFC: dorsal and ventro medial prefrontal cortex; lOFC and mOFC: lateral and medial orbitofrontal cortex; IFG: Inferior frontal gyrus; sgACC, rACC, dACC: subgenual, rostral and dorsal anterior cingulate cortex; aINS and pINS: anterior and posterior insula; Hipp: hippocampus; PHipp: parahippocampus; NAc/MR: nucleus accumbens/mammilary region; dPUT and vPUT: dorsal and anterior putamen; aTHA and pTHA: anterior and posterior thalamus.
H: hemisphere
Δ BPND after placebo administration
Montreal Neurological Institute (MNI) coordinates of peak voxel
Cluster size in mm3
Two-sided voxel-level Z score at peak voxel
The effects of FAAH on placebo-induced regional activation of μ-opioid neurotransmission were significantly correlated with psychophysical responses to placebo, as measured by changes in the MPQ Total, Sensory or Pain Affect score after placebo administration. As shown in Table 3, correlations ranged from 0.30-0.42 for MPQ Total, MPQ Sensory and MPQ Pain Affect in regions known to be relevant to the regulation of sensory and affective responses to pain and placebo responses 1, 15. Placebo-induced activation of μ-opioid neurotransmission was also correlated with an enhancement of the recall of placebo effects that were significant for MPQ Pain Affect scores 24 hours after the pain challenge in multiple cortical brain regions (r values = 0.31 – 0.43; Table 3). Increases in PANAS positive affect scores after placebo administration were positively correlated with placebo-induced activation of μ-opioid neurotransmission in the dorsal PUT (r=.41; p=.006) and the posterior THA (r=.34; p=.02).
Table 3.
Correlational analysis between regional changes in μ-opioid BPND and pain reports after placebo administration.
| DLPFC | mOFC | lOFC | IFG | rACC | sgACC | pINS | PHipp | NAc | dPUT | vPUT | aTHA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | R | L | R | L | L | L | L | R | L | R | R | R | R | R | |
| Δ MPQ | r | .19 | .24 | .42 | .39 | .16 | .30 | .37 | .45 | .18 | .42 | .38 | .35 | .36 | .40 |
| Total | Sig. | .2 | .1 | .005* | .01* | .3 | .01* | .01* | .01* | .3 | .005* | .01* | .02* | .02* | .01* |
| Δ MPQ Pain | r | .30 | .30 | .41 | .39 | .25 | .21 | .29 | .31 | .32 | .33 | .40 | .31 | .34 | .34 |
| Affect | Sig. | .057 | .04* | .007* | .01* | .1 | .2 | .065 | .04* | .03* | .03* | .008* | .04* | .02* | .02* |
| Δ MPQ | r | .15 | .22 | .33 | .34 | .10 | .30 | .41 | .40 | .09 | .41 | .33 | .34 | .35 | .38 |
| Sensory | Sig. | .3 | .2 | .03* | .02* | .5 | .01* | .006* | .007* | .6 | .006* | .03* | .03* | .02* | .01* |
| Δ MPQ Pain | r | .40 | .28 | .33 | .38 | .37 | .22 | .31 | .12 | .18 | .23 | .27 | .24 | .24 | .24 |
| Affect at 24h | Sig. | .006* | .080 | .042 | .017* | .02* | .2 | .052 | .5 | .3 | .2 | .094 | .1 | .1 | .1 |
Abbreviations: DLPFC: dorsolateral prefrontal cortex; mOFC and lOFC: medial and lateral orbitofrontal cortex; IFG: Inferior frontal gyrus; sgACC and rACC: subgenual and rostral anterior cingulate cortex; aINS and pINS: anterior and posterior insula; PHipp: Parahippocampus; NAc: nucleus accumbens; dPUT and vPUT: dorsal and anterior putamen; aTHA: anterior thalamus.
Discussion
Accumulating evidence in animal models shows that CBR1s are involved in mediating the reinforcing properties of exogenous opioids 9, 26. Here we have extended these results by investigating the role of the FAAH Pro129 allele, known to chronically increase AEA brain levels, on psychophysical and neurochemical (endogenous opioid and DA) responses to pain and the administration of a placebo with potential analgesic effects. We found that functional FAAH genotype variation selectively influenced psychophysical placebo responses and placebo-induced activation of μ-opioid receptor mediated neurotransmission. Mu-opioid receptor mediated neurotransmission was activated by placebo administration in a network of regions previously involved in placebo-induced analgesia 1, 14, such as the prefrontal cortex, rostral, dorsal and subgenual ACC, insula, thalamus and NAc. In addition, activation of this neurotransmistter system was observed in areas associated with reward-motivated learning and memory processing 27, including the recall of analgesic placebo effects 28, such as the mammillary region, the anterior thalamic nuclei, the cingulate cortex and hippo/parahippocampal gyrus. Endogenous opioid release in these regions was indeed significantly correlated with decreases in pain ratings and the recall of the pain experience after placebo administration, as well as increases in positive affect.
FAAH Thr129 carriers, despite their chronic greater tonic eCB concentrations, showed lower psychophysical placebo responses and regional μ-opioid activation during placebo administration, compared with Pro129/Pro129 homozygotes. These results are in apparent contradiction with animal models where transgenic mice lacking FAAH have shown increased CBR1-dependent analgesia 29. One logical explanation would be the development of tolerance to chronic eCB exposure in humans. Δ9-THC tolerance has been associated with cellular events similar to morphine tolerance, which include desensitization 30 and down-regulation of CBR1s 31. Cross-tolerance mechanisms are also plausible. Accumulating evidence also shows that CBR1s are involved in mediating the reinforcing properties of opioids. For example, genetic deletion of CBR1s in mice greatly reduces both opioid self-administration 9 and opioid induced conditioned place preference 32. Similarly, administration of the CBR1 antagonist Rimonabant attenuates both morphine-induced conditioned place preference 33 and heroin self-administration in rodents 34. Consistent with our results, substantial evidence suggests that CBR1 modulates opioid but not psychostimulant reward; CBR1 knockout mice display significantly attenuated heroin-induced conditioned place-preference and heroin self-administration but not cocaine place conditioning or self-administration 16, 17. Desensitization and/or down-regulation of CBR1s in FAAH Thr129 carriers could occur after chronic exposure to elevated levels of eCBs, and therefore provide a mechanism for the reduction of endogenous opioid system function. Additionally, it is becoming increasingly clear that the eCB system is affected in varying ways for a given stressful or pathological stimulus depending on the regional localization of the effects 35. These findings suggest that while FAAH may represent an attractive therapeutic target for treating pain and or other neurological disorders, as suggested by preclinical data 36, further studies will need to carefully consider the consequences of chronic stimulation of the eCB system.
One single study has elegantly investigated the role of the endocannabinoid system in placebo responses 7. In this study Rimonavant had no effect on opioid-preconditioned placebo analgesia but it completely blocked placebo analgesia induced by non-opiod preconditioning. The lack of effect of the cannabinoid system in opioid mediated placebo analgesia was not replicated in the present study using a sustained pain model and no preconditioning procedures, suggesting that conditioned and unconditioned placebo analgesic responses may involve different mechanisms. Here we show a selective effect of an abundant, functional FAAH polymorphism on psychophysical and specific neurotransmitter responses (endogenous opioid, but not dopaminergic) to placebo administration, without significant effects on responses to a pain challenge. These effects were also observed for the formation of the memory of placebo analgesic effects, relevant for the maintenance of placebo effects over time, a phenomenon repeatedly observed in clinical trials and thought to involve the progressive engagement of conditioning mechanisms 2. These data are therefore consistent with an interaction between eCB and μ-opioid neurotransmission in the formation of placebo responses. This information is important to understand variability in responses to placebo and active drugs in states that present high rates of biological placebo responses, such as experimental and clinical pain or Major Depression. It may also provide new insights into the neurobiology of placebo effects in conditions such as substance use disorders, where opioid and cannabioid interactions play a critical role 34, 37, 38. In this case, an interaction between this polymorphism and the effects of drugs of abuse on eCB and endogenous opioid systems might explain inter-individual variability in placebo and therapeutic effects. From the perspective of clinical trials, the examination of FAAH Pro129Thr as a marker for patient stratification appears warranted, in particular for pathological states that are potentially influenced by eCB and endogenous opioid systems, and for studies of analgesic effects of drugs that impact on eCB or opioid neurotransmission.
Acknowledgments
The authors wish to acknowledge the nuclear medicine technologists of the PET Center at the University of Michigan for the assistance in PET data acquisition and reconstruction.
Funding: Work was supported by R01 DA 022520, R01 DA027494 and the Phil F. Jenkins Foundation (JKZ). MMJ was supported by the Spanish Ministry of Education (MMJ: AP2008-03742)
Footnotes
Author contributions: J.K.Z., C.S.S. and D.G. were responsible for the study design and procured the study funding; J.K.Z. and C.S.S. collected data; M.P., M.M.J. and C.H. analyzed the data; M.P. and J.K.Z. wrote the manuscript.
Competing interests: The authors declare that they have no competing or conflicting interests.
References and Notes
- 1.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan NM, Mechoulam R. The chemistry of endocannabinoids. Journal of endocrinological investigation. 2006;29(3 Suppl):3–14. [PubMed] [Google Scholar]
- 4.Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chemistry and physics of lipids. 2002;121(1-2):173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 5.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiology of disease. 1998;5(6 Pt B):502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 6.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136(1-2):211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17(10):1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 8.Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12(17):3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 9.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283(5400):401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 10.Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21(2):143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- 11.Haller VL, Stevens DL, Welch SP. Modulation of opioids via protection of anandamide degradation by fatty acid amide hydrolase. European journal of pharmacology. 2008;600(1-3):50–58. doi: 10.1016/j.ejphar.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 13.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13(18):2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 14.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of general psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 15.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 16.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7(10):1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 17.Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15(1):31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, et al. Addictions biology:aplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stohler CS, Kowalski CJ. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79(2-3):165–173. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 21.Pollock V, Cho DW, Reker D, Volavka J. Profile of Mood States: the factors and their physiological correlates. The Journal of nervous and mental disease. 1979;167(10):612–614. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34(1):50–59. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nuclear medicine and biology. 2001;28(6):733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 24.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12(11):4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 27.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Pecina M, Stohler CS, Zubieta JK. Role of mu-opioid system in the formation of memory of placebo responses. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim LJ, Selley DE, Xiao R, Childers SR. Differences in G-protein activation by mu- and delta-opioid, and cannabinoid, receptors in rat striatum. European journal of pharmacology. 1996;307(1):97–105. doi: 10.1016/0014-2999(96)00211-7. [DOI] [PubMed] [Google Scholar]
- 31.Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxaz inyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303(1):36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 32.Rice OV, Gordon N, Gifford AN. Conditioned place preference to morphine in cannabinoid CB1 receptor knockout mice. Brain Res. 2002;945(1):135–138. doi: 10.1016/s0006-8993(02)02890-1. [DOI] [PubMed] [Google Scholar]
- 33.Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, et al. Reduction of opioid dependence by the CB(1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132(8):1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21(14):5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nature reviews Drug discovery. 2008;7(5):438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 36.Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334(1):182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci. 2007;25(7):2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- 38.Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, et al. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav. 2005;81(2):343–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]