ABSTRACT
The yeast Saccharomyces cerevisiae is a widely used cell factory for the production of fuels, chemicals, and pharmaceuticals. The use of this cell factory for cost-efficient production of novel fuels and chemicals requires high yields and low by-product production. Many industrially interesting chemicals are biosynthesized from acetyl coenzyme A (acetyl-CoA), which serves as a central precursor metabolite in yeast. To ensure high yields in production of these chemicals, it is necessary to engineer the central carbon metabolism so that ethanol production is minimized (or eliminated) and acetyl-CoA can be formed from glucose in high yield. Here the perspective of generating yeast platform strains that have such properties is discussed in the context of a major breakthrough with expression of a functional pyruvate dehydrogenase complex in the cytosol.
COMMENTARY
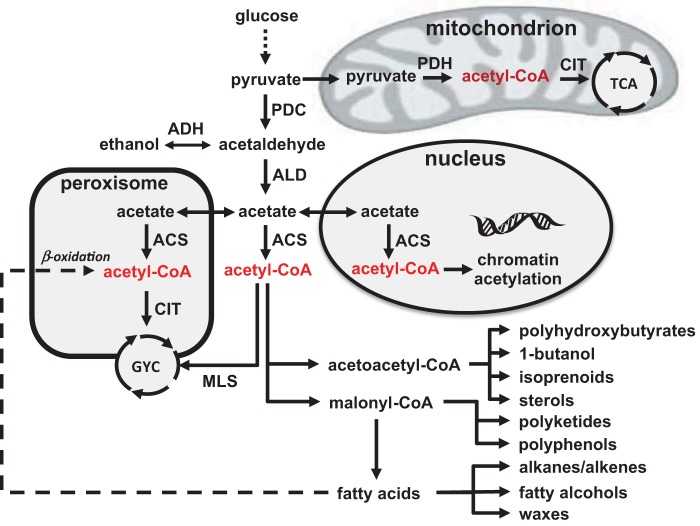
There are many prospects for using microorganisms for sustainable production of fuels and chemicals, and even though the 2010 market for renewable chemicals was only about 1% of the total chemical market, i.e., about $30 billion out of a total of $3,000 billion, it has the potential to grow to $80 billion by 2020. To support this expansion in production, it is necessary to develop efficient cell factories through metabolic engineering (1), and here the yeast Saccharomyces cerevisiae is among the preferred cell factories in industry (2). In order for a novel bioprocess to be economically viable, the cell factory typically has to meet ambitious metrics in terms of titer, rate, and yield (TRY). Meeting the TRY metrics generally requires not only optimization of the pathway leading to the desired product but also engineering of the intermediary metabolism of the cell factory. This is generally challenging, as this has evolved to operate within a certain spectrum of fluxes, and redirection of the carbon fluxes to a precursor metabolite for the product of interest may therefore require either deregulation of the central carbon metabolism or insertion of alternative routes that bypass inherent regulation, e.g., through the use of tools from synthetic biology (3). If fluxes in the central carbon metabolism can be redirected toward a precursor metabolite, then such a strain can often serve as a platform strain for production of a range of different products (4). Acetyl coenzyme A (acetyl-CoA) is a particularly important precursor, as a range of different valuable chemical products can be derived from it (Fig. 1) as listed below.
FIG 1 .
Overview of acetyl-CoA metabolism in yeast. Glucose is converted to pyruvate in glycolysis and can then enter the mitochondria for respiration. Here pyruvate is converted to acetyl-CoA by the pyruvate dehydrogenase complex (PDH), and acetyl-CoA is further oxidized by the TCA cycle with citrate synthase (CIT) catalyzing the first reaction. In the cytosol, pyruvate can be converted to acetaldehyde by pyruvate decarboxylase (PDC), and acetaldehyde can be further converted to either ethanol by alcohol dehydrogenase (ADH) or to acetate by aldehyde dehydrogenase (ALD). Acetate can enter both the nucleus and peroxisome, and in both compartments, as well as in the cytosol, it can be converted to acetyl-CoA by acetyl-CoA synthetase (ACS). In the nucleus, acetyl-CoA is used for histone acetylation, whereas in the peroxisome, it can enter the glyoxylate cycle through reaction with CIT. Acetyl-CoA of the cytosol can also enter the glyoxylate cycle (GYC) through reaction with malate synthase (MLS). The net result of the glyoxylate cycle is formation of 1 mol of malate from 2 mol of acetyl-CoA, and malate can be transported to the mitochondria for oxidation. This route is essential for growth on acetate or ethanol. Cytosolic acetyl-CoA is used for biosynthesis of lipids, i.e., either ergosterol via acetoacetyl-CoA or fatty acids via malonyl-CoA. Many valuable biotechnological products can be derived from these two intermediates.
Polyhydroxybutyrates that are biopolymers with a range of different applications.
1-Butanol that can be used as a biofuel and a chemical building block.
Isoprenoids that form a very broad class of chemicals that can be used as biofuels, e.g., farnesene, pharmaceuticals, e.g., the antimalarial drug artemisinic acid, perfumes, and fine fragrances, e.g., santalene, and nutraceutical ingredients, e.g., β-carotene and lycopene.
Sterols such as ergosterol that can be used as dietary supplements.
Polyketides that can be used as pharmaceuticals, e.g., cholesterol-lowering agents and anticancer drugs.
Polyphenols, another very broad class of compounds, that can be used as antioxidants and nutraceutical ingredients, e.g., resveratrol and a range of flavonoids.
Alkanes/alkenes that can be used as advanced biofuels, e.g., diesel for trucks and airplanes.
Fatty alcohols that can be used as biofuels and chemical building blocks.
Waxes that can be used in detergents, as lubricants, and in cosmetics.
When heterologous pathways have to be implemented in yeast for production of these different products, it is generally preferable to reconstruct these pathways so that they function in the cytosol, either because there may be a need for already existing enzymes present in this compartment or because it is preferable to limit the transport of the end product out of the cell to pass through only a single membrane structure. As illustrated in Fig. 1 however, acetyl-CoA metabolism in yeast is quite complex, as acetyl-CoA is being synthesized in four different compartments (5). Furthermore, production in the cytosol goes via acetaldehyde, which is also the intermediate for conversion of pyruvate to ethanol. Also, the cytosolic route to acetyl-CoA is energetically expensive (2 ATP equivalents), and this may have impacts on the yield of product on glucose. As an additional complication, there is glucose repression of the tricarboxylic acid (TCA) cycle activity and the respiratory system, and when there is excess glucose, the majority of carbon is therefore converted into ethanol, which is obviously an undesired by-product for production of the many different compounds listed above. It may seem an obvious strategy to simply delete alcohol dehydrogenase activity (ADH) to prevent conversion of acetaldehyde to ethanol, but yeast contains a very large number of ADH enzymes, and many of the specific product pathways may also rely on ADH activity, e.g., butanol biosynthesis. A further complication in redirection of flux toward cytosolic acetyl-CoA is that acetyl-CoA synthetase (ACS) activity is highly regulated. Yeast contains two ACS-encoding genes, ACS1 and ACS2, and the locations of their encoded proteins depend on the carbon source, but both can be active in the cytosol (6). Acs1 is repressed by glucose, whereas Acs2 is expressed during growth on glucose and serves an important role in ensuring provision of cytosolic acetyl-CoA for production of fatty acids and sterols. In bacteria, ACS is regulated posttranscriptionally by acetylation, but a similar regulation has not yet been identified in yeast. This knowledge led to the use of a deregulated variant from Salmonella enterica, combined with overexpression of aldehyde dehydrogenase (ALD) (more specifically ALD6), as a successful strategy for increasing the flux toward sesquiterpenes derived from acetyl-CoA (7). This strategy was further complemented by overexpression of ADH2 that catalyzes the conversion of ethanol to acetaldehyde, deletion of either CIT2 or MLS1 that prevents acetyl-CoA from entering the glyoxylate cycle, and overexpression of ERG10 that catalyzes the conversion of acetyl-CoA to acetoacetyl-CoA for improving sesquiterpene production (8). The latter strategy was also shown to be efficient for improving the production of other products such as 1-butanol (9), polyhydroxybutyrates (10), 3-hydroxypropionic acid (11), and fatty acyl ethyl esters (12), and this hereby illustrated the value of establishing a platform strain for improved production of acetyl-CoA.
Even though these strategies were successful in improving flux toward acetyl-CoA-derived products, they still did not overcome the problem of ethanol being a major by-product. As mentioned above, deletion of ADH is not a feasible strategy, and this therefore led to a strategy of deleting pyruvate decarboxylase (PDC). Yeast has three PDC-encoding genes, PDC1, PDC5, and PDC6, and by deleting all three genes, the capacity to produce ethanol is completely lost (13). However, the yeast cannot rely on respiratory metabolism on excess glucose due to repression of the TCA cycle and respiratory metabolism, but the group headed by Jack Pronk at the Delft University of Technology (TU), Delft, The Netherlands, who has pioneered mapping of pyruvate metabolism in yeast, solved this by evolving the PDC-deficient strain to grow in excess glucose (13). In a later study, the mechanisms were identified to be associated with an internal deletion of MTH1, which is involved in transcriptional regulation of hexose transporters, pointing to a mechanism of attenuated glucose influx possibly resulting in decreased repression of respiration in the evolved strain (14). This strain represents a very important platform strain for expressing alternative pathways for conversion of pyruvate to acetyl-CoA, as it does not produce ethanol. However, the strain does not efficiently convert pyruvate to acetyl-CoA in the cytosol, and in order to solve this problem, the Delft group has now reported in mBio the expression of a functional cytosolic pyruvate dehydrogenase complex (PDH) in yeast (15). This study represents a major scientific and technological breakthrough in metabolic engineering of yeast for production of fuels, chemicals, and pharmaceuticals.
PDH is a ubiquitous protein complex found in all domains of life. As illustrated in Fig. 1, it catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA with the concurrent transfer of electrons to NAD+, resulting in the formation of NADH. PDH consists of three catalytic subunits, pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3), which together form multimeric complexes of various sizes. In eukaryotes and some bacteria, E1 consists of two proteins, E1α and E1β. Due to its role in catalyzing several reactions and its size, PDH represents a molecular machine that in some organisms exceeds the size of a ribosome. PDH requires three cofactors: thiamine pyrophosphate, which is bound to E1 and involved in pyruvate decarboxylation; lipoic acid, which is covalently bound to E2 and contains two sulfur groups forming a disulfide bond that serves as initial electron acceptors in the catalytic process; and FAD, which is bound to E3 and serves as an internal elector acceptor in the regeneration of oxidized lipoic acid. In the last step of the catalytic cycle, the electrons are transferred from FADH2 to NAD+. The PDH complex has a complex architecture involving up to 60 copies per subunit in eukaryotes and Gram-positive bacteria, whereas the PDH complex of Gram-negative bacteria is smaller.
Due to the complexity of PDH, many previous attempts to express this enzyme complex in the cytosol of yeast have failed. A key to the success of the Delft study was likely the expression of the PDH complex from Enterococcus faecalis, which is insensitive to high NADH/NAD+ ratios and therefore may operate better in the reduced environment of the yeast cytosol. Furthermore, using genome annotation and similarity with genes encoding ligases involved in protein lipoylation, the group identified two putative protein-encoding genes in E. faecalis, namely, lplA and lplA2. Using codon-optimized genes for the E1α, E1β, E2, and E3 subunits of the E. faecalis PDH together with codon-optimized lplA and lplA2, a functional PDH complex was assembled. Whereas the thiamine and FAD cofactors are present in the cytosol of yeast, lipoic acid is not. This cofactor is synthesized in the mitochondria, and it is not believed to traverse the mitochondrial membranes. The study also clearly demonstrated that functional PDH activity in the cytosol required the addition of lipoic acid to the medium. The cytosolic localization of the enzyme was demonstrated by subcellular fractionation followed by gel filtration and mass spectrometry. Furthermore, enzyme activity confirmed activity of the enzyme complex, and this was found to be higher than the endogenous mitochondrial PDH complex of yeast.
As discussed in the paper, PDH expression has been claimed in an earlier study and here a PDC deletion strain was used as background strain (16). In this study, the authors expressed the PDH subunits from Escherichia coli or yeast in the cytosol and demonstrated improved production of 1-butanol derived from cytosolic acetyl-CoA. However, the authors did not present any data on localization and enzyme activity, and it is questionable whether the PDH was functional, since they did not coexpress ligases for activation of the E2 subunit with lipoic acid nor did they include lipoic acid in the medium.
In the study of the Delft group, the PDH complex was not expressed in a PDC deletion strain, but in a strain lacking ACS activity (deletion of both ACS1 and ACS2). However, expression of the E. faecalis PDH complex in a strain lacking PDC will clearly allow the strain to efficiently convert glucose into cytosolic acetyl-CoA and hereby lay the basis for efficient production of the whole range of products illustrated in Fig. 1. The work therefore represents an important landmark in metabolic engineering of yeast.
ACKNOWLEDGMENTS
I thank all my Ph.D. students, postdocs, and collaborators working with me on acetyl-CoA metabolism in yeast for excellent collaboration and fruitful discussions in connection with this commentary, in particular Verena Siewers, Yun Chen, Anastasia Krivoruchko, Yiming Zhang, Zongjie Dai, and Yongjin Zhou.
The views expressed in this Commentary do not necessarily reflect the views of this journal or of ASM.
Footnotes
Citation Nielsen J. 2014. Synthetic biology for engineering acetyl coenzyme A metabolism in yeast. mBio 5(6):e02153-14. doi:10.1128/mBio.02153-14.
REFERENCES
- 1. Chen Y, Nielsen J. 2013. Advances in metabolic pathway and strain engineering paving the way for sustainable production of chemical building blocks. Curr. Opin. Biotechnol. 24:965–972. 10.1016/j.copbio.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen J, Jewett MC. 2008. Impact of systems biology on metabolic engineering of Saccharomyces cerevisiae. FEMS Yeast Res. 8:122–131. 10.1111/j.1567-1364.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen J, Fussenegger M, Keasling J, Lee SY, Liao JC, Prather K, Palsson B. 2014. Engineering synergy in biotechnology. Nat. Chem. Biol. 10:319–322. 10.1038/nchembio.1519. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen J, Larsson C, van Maris A, Pronk J. 2013. Metabolic engineering of yeast for production of fuels and chemicals. Curr. Opin. Biotechnol. 24:398–404. 10.1016/j.copbio.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 5. Pronk JT, Steensma HY, van Dijken JP. 1996. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12:1607–1633. . [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Siewers V, Nielsen J. 2012. Profiling of cytosolic and peroxisomal acetyl-CoA metabolism in Saccharomyces cerevisiae. PLoS One 7:e42475. 10.1371/journal.pone.0042475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiba Y, Paradise EM, Kirby J, Ro DK, Keasling JD. 2007. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids. Metab. Eng. 9:160–168. 10.1016/j.ymben.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8. Chen Y, Daviet M, Schalk M, Siewers V, Nielsen J. 2013. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab. Eng. 15:48–54. 10.1016/j.ymben.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9. Krivoruchko A, Serrano-Amatriain C, Chen Y, Siewers V, Nielsen J. 2013. Improving biobutanol production in engineered Saccharomyces cerevisiae by manipulation of acetyl-CoA metabolism. J. Ind. Microbiol. Biotechnol. 40:1051–1056. 10.1007/s10295-013-1296-0. [DOI] [PubMed] [Google Scholar]
- 10. Kocharin K, Chen Y, Siewers V, Nielsen J. 2012. Engineering of acetyl-CoA metabolism for the improved production of polyhydroxybutyrate in Saccharomyces cerevisiae. AMB Express 2:52. 10.1186/2191-0855-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Bao J, Kim IK, Siewers V, Nielsen J. 2014. Coupled incremental precursor and co-factor supply improves 3-hydroxypropionic acid production in Saccharomyces cerevisiae. Metab. Eng. 22:104–109. 10.1016/j.ymben.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 12. de Jong BW, Shi S, Siewers V, Nielsen J. 2014. Improved production of fatty acid ethyl esters in Saccharomyces cerevisiae through up-regulation of the ethanol degradation pathway and expression of the heterologous phosphoketolase pathway. Microb. Cell Fact. 13:39. 10.1186/1475-2859-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Maris AJA, Geertman JMA, Vermeulen A, Groothuizen MK, Winkler AA, Piper MDW, van Dijken JP, Pronk JT. 2004. Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate hyperproducing yeast. Appl. Environ. Microbiol. 70:159–166. 10.1128/AEM.70.1.159-166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oud B, Flores CL, Gancedo C, Zhang X, Trueheart J, Daran JM, Pronk JT, van Maris AJA. 2012. An internal deletion in MTH1 enables growth on glucose of pyruvate-decarboxylase negative, non-fermentative Saccharomyces cerevisiae. Microb. Cell Fact. 11:131. 10.1186/1475-2859-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kozak BU, van Rossum HM, Luttik MAH, Akeroyd M, Benjamin KR, Wu L, de Vries S, Daran J-M, Pronk JT, van Maris AJA. 2014. Engineering acetyl coenzyme A supply: functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. mBio 5(5):e01696-14. 10.1128/mBio.01696-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lian J, Si T, Nair NU, Zhao H. 2014. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab. Eng. 24:139–149. 10.1016/j.ymben.2014.05.010. [DOI] [PubMed] [Google Scholar]