Abstract
This paper reviews research relevant to a proposed conceptual model of exercise adherence that integrates the dual mode model and hedonic theory. Exercise intensity is posited to influence affective response to exercise via interoceptive (e.g., ventilatory drive) and cognitive (e.g., perceived autonomy) pathways; affective response to exercise is posited to influence exercise adherence via anticipated affective response to future exercise. The potential for self-paced exercise to enhance exercise adherence is examined in the context of the proposed model and suggestions are given for future research. Further evidence in support of self-paced exercise could have implications for exercise prescription, especially among overweight, sedentary adults, who are most in need of interventions that enhance adherence to exercise programs.
Given the numerous health benefits of physical activity participation (Knowler et al., 2002; McTiernan et al., 2003; Slattery, 2004; Thompson et al., 2003; Vuori, 2001), and the dismal rates of activity among Americans (Macera et al., 2005), further understanding of physical activity mechanisms to aid in the design of effective physical activity interventions is crucial (U.S. Department of Health and Human Services, 2000). Studies attempting to identify determinants of physical activity have focused on cognitive (McAuley & Blissmer, 2000), social (Brassington, Atienza, Perczek, DiLorenzo, & King, 2002), and more recently, environmental factors (Trost, Owen, Bauman, Sallis, & Brown, 2002), consistent with prevailing theoretical models (Ajzen, 1991; Bandura, 1986; King, Stokols, Talen, Brassington, & Killingsworth, 2002; Prochaska & DiClemente, 1984; Rogers, 1983). These studies have consistently explained a modest percent of the variance in physical activity behavior (Baranowski, Anderson, & Carmack, 1998). Therefore, further understanding is necessary to produce far-reaching, meaningful, and sustainable physical activity change.
In comparison to cognitive, social, and environmental factors, affective processes as determinants of adoption and maintenance of physical activity behavior have received considerably less attention (for some notable exceptions see Kiviniemi, Voss-Humke, & Seifert, 2007; D. M. Williams et al., 2008). Recent conceptual and methodological advances in laboratory-based studies examining acute affective responses to brief exercise stimuli (Ekkekakis, 2003; Ekkekakis, Hall, & Petruzzello, 2005; Ekkekakis & Petruzzello, 2000, 2002) have led to a growing literature examining the first link in a potential causal chain from exercise intensity to affective response to exercise adherence (Ekkekakis, Backhouse, Gray, & Lind, 2008; Ekkekakis & Lind, 2006). Additionally, some researchers have suggested that prescribing self-paced exercise may have considerable promise for increasing adherence to exercise programs (Ekkekakis & Lind, 2006; Parfitt, Rose, & Burgess, 2006). For example, Ekkakakis and Lind (2006) “theorize that a causal chain exists, linking (a) the intensity of physical activity (not only its level but also whether it is imposed or self-selected), (b) affective responses (pleasure vs displeasure) and perceived exertion, and, finally, (c) adherence” (p. 653). The purpose of this paper is to critically examine existing research relevant to a proposed model of exercise intensity, affective response, and adherence, which represents an integration of the dual mode model and hedonic theory. The review focuses on self-paced exercise as a potential tool for enhancing affective response to exercise and exercise adherence. Preceding the review of self-paced exercise is a brief discussion of recent conceptual and methodological advances in research on affective response to exercise, as well as brief overviews of the dual mode model and hedonic theory. The paper concludes with a discussion of public health implications and suggestions for future research.
Recent Conceptual and Methodological Advances
Ekkekakis and colleagues (Ekkekakis, 2003; Ekkekakis et al., 2005; Ekkekakis & Petruzzello, 2000, 2002) have provided a number of critical insights over the past several years that have laid the groundwork for examination of the proposed exercise-affect-adherence relationship.1 Perhaps most important to their framework, Ekekkakis and Petruzzello (2000; 2002) advocate for a dimensional approach to affect assessment instead of a categorical approach. Specifically, the circumplex model is recommended as a conceptual framework for measuring “basic” or “core” affect along two dimensions: pleasure/displeasure and high/low arousal (Ekkekakis & Petruzzello, 2002). For at least two critical reasons, this approach is more conducive to testing hypotheses concerning the exercise-affect-adherence relationship than a categorical approach, in which the presence or absence and magnitude of more distinct affective states (e.g., invigoration, tranquility, distress, exhaustion) are assessed. First, a dimensional approach can theoretically capture the full range of basic affective response to exercise (Ekkekakis & Petruzzello, 2000, 2002). Conversely, a categorical approach, while ideal for learning about specific affective states, may omit measurement of one or more distinct affective states that may be crucial to prediction of exercise adherence. Second, dimensional approaches tend to involve single item assessments of basic affective dimensions that are more easily administered multiple times prior to, during, and following an exercise bout, while multi-item categorical measures of distinct affective states are more difficult to administer during exercise (Ekkekakis & Petruzzello, 2002). Indeed, measurement of affective response only prior to and following exercise has led to the conclusion that acute bouts of physical activity uniformly improve affective states (Yeung, 1996). However, according to learning theory, more immediate responses to exercise behavior should be more predictive of future exercise behavior than affective experiences occurring after the exercise (e.g., Neef, Shade, & Miller, 1994), which could instead be conceptualized as affective response to completion of the exercise bout (Hall, Ekkekakis, & Petruzzello, 2002; Wininger, 2007).
The conceptual and methodological positions outlined above have not come without controversy (Gauvin & Rejeski, 2001). Undoubtedly, there is merit to alternative approaches. Particularly, as indicated by Ekkekakis and Petruzzello (2000, p. 79; 2002, p. 46), the use of categorical approaches to measure distinct affective states is preferable to dimensional approaches if the purpose is to describe and understand specific feelings or emotions in response to exercise stimuli. For example, a categorical approach may be more useful in determining the best exercise stimulus for alleviating symptoms of clinical depression and/or anxiety. Moreover, the use of categorical and dimensional approaches should not be seen as mutually exclusive. Tellegen, Watson and Clark (1999) have argued that affective experience is arranged hierarchically, with each basic affective experience being further understood by more distinct, categorical affective experiences. Indeed, recent studies have incorporated this hierarchical approach in examining affective response to exercise (Focht, Knapp, Gavin, Raedeke, & Hickner, 2007; Raedeke, Focht, & Scales, 2007). Nonetheless, if the purpose, as it is here, is to understand the exercise-affect-adherence link, then, for the reasons outlined above, a dimensional approach has clear advantages. Thus, this review focuses on studies that have employed a dimensional approach to affective assessment. Moreover, the review focuses on affective valence (i.e., pleasure/displeasure) responses to exercise because of the theorized link between affective valence and exercise adherence (e.g., Kahneman, 1999; Young, 1952).
The Dual-Mode Model
In addition to formulating a conceptual and methodological approach to affect assessment, Ekkekakis and colleagues (Ekkekakis, 2003, 2005; Ekkekakis & Acevedo, 2006; Ekkekakis et al., 2005) have posited the dual-mode model for understanding affective response to exercise of different intensities.1 An underlying premise of the model is that when examining the exercise-affect-adherence relationship, intensity is best defined with respect to the ventilatory threshold (VT; Svedahl & MacIntosh, 2003) instead of percentage of maximum heart rate or maximum oxygen consumption (e.g., American College of Sports Medicine, 2005). Consistent with a review of studies (Ekkekakis, 2003), the dual-mode model posits that exercise performed below the VT, at the VT, and above the VT elicits shifts in affective valence that are consistently positive, highly variable, and consistently negative, respectively (Ekkekakis, 2003, 2005; Ekkekakis & Acevedo, 2006). The “dual-mode” in the dual mode model refers to cognitive and interoceptive influences on affective response to exercise of different intensities. According to the model, interoceptive factors have increasing influence on affective responses as the intensity of exercise exceeds the VT and physiological homeostasis is threatened, thus accounting for the consistent decline in affective valence during exercise above the VT. During exercise at or approaching the VT, however, cognitive variables, such as goals, perceived efficacy, and expectations are more important, which accounts for the variability observed in this intensity range. Finally, the model stipulates that cognitive factors have less importance during exercise below the VT than during exercise at the VT, with interoceptive factors having almost no influence, as this intensity is no threat to homeostasis and is experienced by most people as pleasant (Ekkekakis, 2003, 2005; Ekkekakis & Acevedo, 2006; Ekkekakis et al., 2005).
It should be noted that most of the research on the dual mode model has involved healthy, relatively active adults (Ekkekakis et al., 2005); thus, some of the posited relationships are yet to be adequately tested among people of various ages, levels of exercise experience, and health status. For example, the dual mode model stipulates less variability—though still “low to moderate” variability—in affective responses to low intensity exercise (relative to moderate intensity exercise). Thus, for individuals who are sedentary and lack experience with exercise, negative cognitive states could lead to displeasure even in response to low intensity exercise. Indeed, at least one study conducted among previously sedentary participants suggests that variability in affective response to exercise may occur at intensities below the VT, as well as intensities at the VT (Welch, Hulley, Ferguson, & Beauchamp, 2007). Additional research is needed to examine the relationships posited in the dual-mode model among unfit, sedentary, and clinical populations.
Hedonic Theory
The dual mode model provides a framework for understanding affective responses to exercise of varying intensities; hedonic theory provides a framework for understanding how affective response to exercise relates to exercise adherence. Hedonic theory can be traced at least as far back as Bentham (1789/2007), who stated: “Nature has placed mankind under the governance of two sovereign masters, pain and pleasure … They govern us in all we do, in all we say, in all we think …” (p. 1). This idea that people seek to enhance or prolong pleasure and avoid or minimize pain is often referred to as “the hedonic principle” (e.g., Higgins, 1997). While the hedonic principle has had wide-ranging influence on psychological (and economic) theorizing and research, it has progressed along multiple divergent paths and lacks a single, unifying model, perhaps because of its overwhelming pervasiveness. In this paper, hedonic theory comprises all theoretical models that explain behavior as a function of its affective consequences or anticipation of its affective consequences. The remainder of this section provides a brief overview of some of the various manifestations of hedonic theory that are most relevant to the presently proposed model.
The hedonic principle was represented in psychological theory as early as Thorndike's (1911) dictum that behaviors are “stamped in when pleasure results from the act and stamped out when it doesn't” (p. 147; see also Freud, 1920/1952). While affective processes were not prominent in prevailing theories of behavior inspired by Thorndike's work (e.g., Skinner, 1950), Young (1959) saw affective processes as the intervening variable between stimulus and response (p. 104), and as an alternative to Hull's (1952) drive reduction in explaining reinforcement and punishment (Young, 1952, p. 261). More recently, Kahneman (1999), in his “hedonic psychology,” has adopted Bentham's (1789/2007) general idea that the perceived utility of a behavior (or experience) is defined by one's affective response to the behavior, and will determine whether he/she will repeat the behavior. However, Kahneman and colleagues' research has shown that people's retrospective, global evaluation of a behavior differs from the aggregate of instantaneous, affective experiences that occur during the target behavior (Kahneman, Wakker, & Sarin, 1997). Specifically, when formulating a retrospective evaluation of a behavior (or experience) people tend to place greater emphasis on the most intense (i.e., peak) and most recent (i.e., ending) instantaneous affective experiences that occurred during the target behavior (Frederickson & Kahneman, 1993; Kahneman, Fredrickson, Schreiber, & Redelmeier, 1993; Redelmeier & Kahneman, 1996; Varey & Kahneman, 1992), rather than considering the aggregate of all affective experiences. On the other hand, when anticipating affective response to a behavior or experience, people tend to focus on only the most salient aspects (Kahneman, Krueger, Schkade, Schwarz, & Stone, 2006; Schkade & Kahneman, 1998), as well as the initial contrast between their current affective state and the anticipated affective state (Kahneman & Tversky, 1979).
Other psychological models have also sought to understand the influence of anticipated affective responses on behavior. For example, according to response expectancy theory (Kirsch, 1990) and expected pleasure theory (Mellers, Schwartz, & Ritov, 1999), behaviors are determined by the expectation of affective response to the behavior and its associated outcomes. Additionally, both models offer hypotheses that link expected affective response to actual affective response. In response expectancy theory, anticipated affective response to a behavior may influence one's actual affective response when performing the behavior (Kirsch, 1997). In expected pleasure theory, affective response to a behavioral outcome is partially dependent on the subjective probability of that outcome and on the subjective value of outcomes not received (i.e., counterfactuals; Mellers, Schwartz, Ho, & Ritov, 1997; see also Bell, 1982, 1985 and Loomes & Sugden, 1982, 1986). Thus, both models emphasize the importance of expected pleasure versus displeasure in determining behavior, but each model offers more sophisticated, potentially complimentary hypotheses involving how expected affective response relates to actual affective response.
Numerous additional psychological models, either explicitly or implicitly, are based on hedonic theory or seek to expand upon it (e.g., Bindra, 1974; Higgins, 1997; Kavanagh, Andrade, & May, 2005; Lowenstein & Prelec, 1993; Wegener & Petty, 1994). In terms of health behavior research, hedonic theory has been used extensively in studies of obesity and eating (e.g., Cota, Tschop, Horvath, & Levine, 2006; Finlayson, King, & Blundell, 2007; Garg, Wansink, & Inman, 2007; Mela, 2006; Urala & Lahteenmaki, 2006), smoking (Blendy, Strasser, & Walters, 2005; Cook, Spring, & McChargue, 2004; Dawkins, Acaster, & Powell, 2007; Kenny & Markou, 2005; J. D. Robinson, Cinciripini, & Tiffany, 2007; Shiffman, Ferguson, & Gwaltney, 2006), and substance abuse (Berridge, 2007; Everitt & Robbins, 2005; Le Foll, Goldberg, & Sokoloff, 2005; Stevens, Peschk, & Schwarz, 2007). However, hedonic theory has rarely been applied to exercise behavior (see below for notable exceptions) perhaps because it is only recently that variability in acute affective responses to exercise has been systematically examined as a potential determinant of exercise adherence.
Given the lack of research applying hedonic theory to exercise promotion and adherence, the field is ripe for testing the multitude of hypotheses that can be generated from the hedonic models briefly mentioned above. In the following sections a proposed model of exercise, affect, and adherence is presented that integrates the dual mode model and hedonic theory. The model is discussed in the context of a review of literature that, consistent with the proposed model, illustrates the potential of self-paced exercise to increase adherence to exercise programs.
Self-paced Exercise and Affective Response
The hedonic principle has been demonstrated previously in the context of exercise by Cabanac and LeBlanc (1983), who systematically varied either treadmill intensity or ambient temperature and gave participants control over the other variable. They found that participants made adjustments to either treadmill intensity or ambient temperature in order to maximize pleasure and reduce displeasure (Cabanac & Leblanc, 1983). Consistent with these findings and with hedonic theory, research has shown that when people are asked to self-select their exercise intensity, they choose an intensity that results in a positive affective response (Ekkekakis et al., 2008; Ekkekakis & Petruzzello, 2000).
If people tend to maximize pleasure and minimize displeasure when self-selecting exercise intensity, then, intuitively, self-selected intensity exercise should elicit an affective response that is at least as positive as exercise performed at an imposed intensity. A number of recent studies have tested this idea. For example, Ekkekakis and Lind (2006) asked 16 overweight (mean age = 43.00 ± 5.40y; mean BMI = 31.06 ± 4.91y) and 9 normal weight (mean age = 43.67 ± 4.24y; mean BMI = 22.34 ± 1.82 kg/m2) healthy, inactive women to complete one walking bout at a self-selected pace, and one at a prescribed intensity, set at a 10% faster pace than the self-selected pace. Affective valence stayed the same across time among all participants in the self-paced condition and among normal weight participants in the prescribed intensity condition, but declined significantly (i.e., became less positive) from baseline to 20 minutes among overweight participants in the prescribed intensity condition. While this study showed a more positive affective response to self-paced versus prescribed intensity exercise among overweight women, an additional question of interest is whether the findings can be attributed to the self-selection of exercise intensity or to the fact that the self-paced exercise was at a lower intensity.
A study by Parfitt, Rose, and Burgess (2006) helps to address this issue. The authors used a within subjects design to compare the effects of exercise above the lactate threshold (LT), below the LT, and self-selected intensity exercise on affective valence among 12 sedentary men (mean age = 36.5 ±10.4y; mean BMI = 28.5 ± 4.7 kg/m2). The above LT condition resulted in a consistent decline in affective valence from before exercise to during exercise (83% of participants declined, 17% of participants remained unchanged), and there was considerable variability (58% improved, 25% declined, 17% unchanged) in the below LT condition. However, there was a consistent positive shift in the self-paced condition (93% improved, 7% unchanged) even though mean blood lactate level in the self-paced condition was between that of the below lactate and above lactate conditions, suggesting that the more positive affective response to self-paced exercise cannot be attributed solely to lower intensity of self-paced exercise. It should be noted that the LT—while representing a somewhat different physiological mechanism than the VT—occurs at virtually the same time (Svedahl & Mac Intosh, 2003). However, in the study by Parfitt and colleagues (2006) above-LT and below-LT were defined by lactate concentration levels of 4 mmol/L and 2 mmol/L, respectively, instead of directly assessing each participant's LT.
In a follow-up study conducted among 19 sedentary women (mean age = 39.37 ± 10.29y; mean BMI = 25.5 ± 3.6 kg/m2), Rose and Parfitt (2007) added an at-LT exercise session to the protocol from the previous study and determined LT on an individual basis. Mean blood lactate level in the self-paced intensity condition was between blood lactate level in the below LT and at-LT conditions, and lower than blood lactate level in the above LT condition. As with the previous study (Parfitt et al., 2006), participants in the above LT condition showed consistent declines in affective valence from pre-exercise to during exercise (79% declined, 11% improved, 10% were unchanged or fluctuated throughout the exercise bout). There was also considerable variability in shifts in affective valence in both below LT (42% improved, 32% declined, 26% unchanged or fluctuated) and at-LT (21% improved, 42% declined, 37% unchanged or fluctuated) conditions. In the self-paced condition affective response was more consistently positive, although there was still considerable variability (47% improved, 26% declined, 26% unchanged or fluctuated).
Taken together, affective response to self-paced exercise appears to be more positive than response to prescribed intensity exercise, even when the self-paced exercise is above or at similar intensities to prescribed intensity exercise (Parfitt et al., 2006; Rose & Parfitt, 2007). Nonetheless, there was considerable variability in affective response to self-paced exercise in one study, with less than 50% of women having a positive affective response to self-paced exercise (Rose & Parfitt, 2007). Qualitative data from this study may provide some explanation for the variability, as at least some of the participants' self-selected intensities may have been influenced by perceived experimenter expectations (Rose & Parfitt, 2007). Finally, the findings of Ekkekakis and Lind (2006) indicate that the difference in affective response to self-paced versus prescribed intensity exercise may be more pronounced among overweight adults.
Potential Interoceptive Pathways between Self-paced Exercise and Affective Response
Why might self-paced exercise elicit a more positive affective response than prescribed intensity exercise? According to the dual-mode model, both interoceptive and cognitive factors influence affective response to exercise, with interoceptive factors having a greater impact as exercise exceeds the VT (Ekkekakis, 2003; Ekkekakis et al., 2005). Indeed, studies have shown that affective valence consistently becomes less positive/more negative as the VT is exceeded (Ekkekakis, Hall, & Petruzzello, 2004; Hall et al., 2002; Welch et al., 2007), presumably for evolutionarily adaptive reasons (Ekkekakis, 2003), as exercise above the VT cannot be maintained for a prolonged period of time (Wasserman, 1987).
Unfortunately, traditional exercise prescriptions may result in exercise that exceeds the VT. The American College of Sports Medicine and American Heart Association (Haskell et al., 2007) recommend exercise of at least moderate intensity, which is defined generally as 3–6 metabolic equivalents (e.g., a 3–4 mph walk), or more specifically, as 64–76% of maximum heart rate, 40-60% heart rate reserve (HRR), or 40-60% of maximal oxygen uptake (American College of Sports Medicine, 2005). The latter prescription can only be obtained through maximal fitness testing; however, even this “gold standard” intensity prescription does not necessarily map on to metabolic processes (Ekkekakis et al., 2004; Welch et al., 2007). Indeed, among healthy adults, VTs may range from 35–80% of maximal oxygen consumption (Palange, Ward, & Whipp, 2006). Among overweight, previously sedentary individuals, the population most likely to be targeted in exercise promotion programs, VTs may be even more likely to fall outside the range of standard exercise prescriptions. This inconsistency between VT and traditional methods of prescribing moderate intensity exercise may be even greater when less individualized prescriptions are used, such as percent of age-predicted maximal heart rate, or 3–4 mph walking. Moreover, even if one's VT does fall within the range of prescribed moderate intensity, the breadth of typically prescribed ranges (e.g., 40-60% VO2 max) increases the likelihood that one will be at, above, or below their VT at different intensities within the prescribed intensity range.
In contrast to prescribed exercise intensity, self-paced exercise, according to hedonic theory, should not exceed the VT, so as to avoid the decline in affective valence associated with this higher intensity exercise. Indeed, a number of studies have shown that when allowed to self-select exercise intensity, people tend to select an intensity that approaches, but does not exceed the VT (Ekkekakis, Lind, & Joens-Matre, 2006; Lind, Joens-Matre, & Ekkekakis, 2005; Parfitt et al., 2006; Rose & Parfitt, 2007). Thus, exercise that is self-paced seems less likely to exceed the VT than exercise intensity prescribed by traditional methods (e.g., 40-60% VO2 max; 64–76% maximal heart rate), and thus is less likely to result in a decline in affective valence.
There are, however, some caveats with respect to variability in self-selected intensity. For example, Focht & Hausenblas (2003) showed that previously low-active, female college students participating in a study on social physique anxiety showed a (non-significant) trend toward higher intensity exercise in a crowded university fitness center compared to a private laboratory setting. This study, one of few examining self-selected intensity in naturalistic settings, suggests that environmental factors may influence the self-selection of exercise intensity. Moreover, in one laboratory study of previously sedentary women, mean intensity for self-selected exercise approached the VT; however, there was considerable variability, with a range of 62–160% of oxygen consumption at the VT (Ekkekakis et al., 2006). Thus, while Ekkekakis and colleagues have argued for self-selection of exercise intensity, at least for overweight and chronically sedentary populations (Ekkekakis & Lind, 2006), they also warn that individual differences and inexperience (as well as environmental conditions) may lead to exercise that exceeds the VT (Ekkekakis et al., 2006). An intuitively appealing alternative is to ask participants to self-select their intensity, but to avoid exercise that elicits a decline in affective valence, thus increasing the likelihood that the individual will approach, but not exceed the VT (Ekkekakis et al., 2004).
Potential Cognitive Pathways between Self-paced Exercise and Affective Response
As discussed in the preceding section, exercise that exceeds the VT generally results in a decline in affective valence (Ekkekakis & Lind, 2006; Parfitt et al., 2006; Rose & Parfitt, 2007), thus potentially explaining why self-paced exercise elicits more positive affective responses than higher intensity exercise (see also Kilpatrick, Kraemer, Bartholomew, Acevedo, & Jarreau, 2007; Parfitt, Eston, & Connolly, 1996; Parfitt, Markland, & Holmes, 1994). Recall, however, that self-paced exercise also elicited a more positive affective response than exercise at lower or similar intensities (Parfitt et al., 2006; Rose & Parfitt, 2007). What might account for these findings?
According to the dual mode model, the variability in affective response to exercise below the VT can be attributed to cognitive factors. A discussion of potential cognitive pathways from self-paced exercise to basic affective response is almost completely speculative given the near absence of research in this area. Nonetheless, a number of authors have pointed out that the influence of choice of exercise intensity on cognitive factors is consistent with self-determination theory (Ekkekakis & Lind, 2006; Parfitt et al., 2006; Parfitt, Rose, & Markland, 2000; Rose & Parfitt, 2007), which posits that increased choice over behavior leads to enhanced perceptions of competence and autonomy (Deci & Ryan, 1985; Ryan & Deci, 2000). Indeed, previous research has shown that giving participants choice over their behavior leads to greater feelings of autonomy or self-determination, which in turn enhance behavior or increase persistence at a behavioral task (Moller, Deci, & Ryan, 2006; Thogersen-Ntoumani & Ntoumanis, 2006; G. C. Williams, Freedman, & Deci, 1998; G. C. Williams, Grow, Freedman, Ryan, & Deci, 1996; G. C. Williams, Rodin, Ryan, Grolnick, & Deci, 1998; Zuckerman, Porac, Lathin, Smith, & Deci, 1978). Specific to exercise behavior, research has shown that ability to choose one's mode of exercise is related to more positive affective response to the exercise than when the mode of exercise is imposed (Daley & Maynard, 2003; Parfitt & Gledhill, 2004).
Additionally, consistent with response expectancy theory, anticipated affective response to exercise may directly influence actual affective response. Peoples' expectations about how they will respond to alcohol, drugs, and chemotherapy have been shown to predict actual responses to alcohol consumption (Goldman, Darkes, & Del Boca, 1999), drug use (Vogel-Sprott & Fillmore, 1999), and anticipatory nausea among chemotherapy patients (Montgomery & Bovbjerg, 2001), respectively. Similarly, Desharnais and colleagues (1993) found that those who were led to believe that a physical activity program would improve their mood showed greater improvements in self-esteem than those who were told that the program would yield only physiological benefits.
In summary, based on self-determination theory and response expectancy theory, both perceived autonomy and anticipated affective response are potential cognitive pathways that could, in addition to interoceptive factors, mediate the impact of self-paced exercise on affective response to exercise. Again, readers should be cautioned, as there are currently very little data that directly support this argument.
Affective Response and Exercise Adherence
As discussed earlier, hedonic theory (Kahneman, 1999; Young, 1952) posits that human behavior is shaped by a tendency to maximize pleasure and minimize displeasure. Moreover, Kahneman, Fredrickson, Schreiber, and Redelmeier (1993) have shown that affective responses to a behavior may influence decisions regarding whether or not to repeat the behavior. This link between affective response to behavior and behavioral maintenance has recently been hypothesized in the context of exercise (Cabanac, 2006; Ekkekakis et al., 2005). However, only a few studies have empirically examined the link between affective response to exercise and future physical activity.
One set of studies conducted by Annesi (Annesi, 2002a, 2002b, 2005) and Berger and Owen (Berger & Owen, 1992) measured participants' affect prior to and following exercise participation biweekly during a 14- to 15-week period. Differences in pre-post scores were aggregated over time, and used to predict attendance at the exercise facility during the program period. Two additional studies measured affective states before and after a baseline exercise session to predict exercise participation during the subsequent 10 weeks (Klonoff, Annechild, & Landrine, 1994) or 6 months (Carels, Berger, & Darby, 2006). Findings from these studies have been mixed, with some showing support for the hypothesized relationships (Berger & Owen, 1992), others showing no support (Annesi, 2002a, 2002b; Klonoff et al., 1994), and still others showing hypothesized relationships only for some of the measured affective states (Annesi, 2005; Carels et al., 2006) or only among participants with low self-motivation, with opposite relationships among those with high self-motivation (Annesi, 2002a).
While these studies were among the first to explore the link between affective response to exercise and exercise adherence, this was not the sole purpose of any of the studies reviewed. Thus, the mixed findings may be attributed to methodology that was not ideal for examining this particular hypothesis, including the measurement of distinct affective states rather than basic affect (Annesi, 2002a, 2002b, 2005; Berger & Owen, 1992; Carels et al., 2006; Klonoff et al., 1994), measurement of affect before and after, rather than during exercise (Annesi, 2002a, 2002b, 2005; Berger & Owen, 1992; Carels et al., 2006; Klonoff et al., 1994), temporal overlap between measurement of affective response to exercise and exercise participation (Annesi, 2002a, 2002b, 2005; Berger & Owen, 1992), and lack of control for baseline physical activity levels (Berger & Owen, 1992) and pre-exercise affective states (Carels et al., 2006).
D. M. Williams and colleagues (2008) attempted to overcome the weaknesses of these previous studies by assessing basic affective response during a moderate intensity exercise stimulus and examining its relationship with adherence to a physical activity program 6 and 12 months later. Results showed that affective response to the moderate intensity exercise stimulus at baseline predicted physical activity participation both 6 and 12 months later when controlling for baseline and 6-month physical activity levels, respectively, as well as affect prior to the baseline exercise task and rated perceived exertion during the exercise task. While this study overcame a number of weaknesses of prior studies, it had its own weaknesses, including a small sample size (n = 31) and reliance on a single rating of affective valence as the independent variable.
A recent cross-sectional study (Kiviniemi et al., 2007) showed that affective associations with physical activity (i.e., “how participants feel when considering physical activity,” p. 154) were predictive of current physical activity behavior, above and beyond demographics, perceived behavioral control, perceived social norms, perceived severity and susceptibility to health problems, and perceived benefits and barriers of physical activity. Moreover, affective associations mediated the effects of these other cognitive variables on physical activity behavior. This study was novel in its examination of the mediational role of affect in the link between cognition and behavior. However, additional research, using prospective designs, is needed to understand how affective associations—which are based on past experiences with exercise—relate to acute affective responses to ongoing exercise, and ultimately, future exercise behavior.
Potential Pathways between Affective Response to Exercise and Exercise Adherence
Hedonic theory can also be used as a framework for understanding how affective response to exercise might influence adherence to exercise programs. In their models of hedonic processes, Kahneman (1999), Kirsch (1990), and Mellers (2000) posit that the expected affective response to a behavior or experience will determine whether the behavior/experience will be repeated (see also Gilbert & Wilson, 2007). Some researchers have called for the incorporation of anticipated affective response into existing social-cognitive models (Perugini & Bagozzi, 2001; van der Pligt & de Vries, 1998; D. M. Williams, Anderson, & Winett, 2005), and several studies have examined anticipated affective response—mostly anticipated regret—on risky sexual behavior (Richard, deVries, & van der Pligt, 1998; Richard, van der Pligt, & deVries, 1995, 1996a), driving and pedestrian safety (Evans & Norman, 2003; Parker, Manstead, & Stradling, 1995), alcohol and drug abuse (Murgraff, McDermott, White, & Phillips, 1999; Richard, van der Pligt, & de Vries, 1996b), and smoking (Moan, Rise, & Andersen, 2005). However, few studies have systematically studied the impact of expected affective response on future exercise behavior. In a pair of studies, Abraham and Sheeran (2003, 2004) showed that higher anticipated regret for not exercising in the next two weeks was correlated with intentions to exercise and subsequent exercise behavior and strengthened the relationship between behavioral intentions and exercise behavior. Additionally, in an experimental design, focusing on anticipated regret resulted in higher ratings of intentions to exercise (Abraham & Sheeran, 2004, study 2). While these studies provide an early indication that anticipated affective response to exercise may influence exercise behavior, more research is needed, particularly research that examines anticipated, immediate basic affective response to exercise as a determinant of exercise adherence.
Potential Public Health Implications
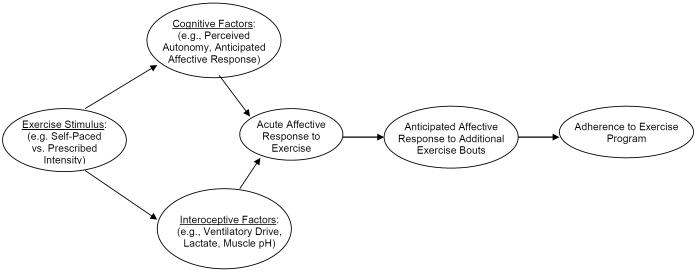
In the preceding sections, existing evidence was reviewed that provides preliminary support for an integrative model linking exercise intensity, affective response, and exercise adherence (Figure 1). In the context of this model, self-paced exercise was proposed as a potential means for enhancing affective response relative to imposed intensity exercise. As outlined above, more research is needed to support the proposed relationships. However, even if it is assumed that self-paced exercise will lead to better adherence than prescribed intensity exercise, is it also likely to lead to more positive health outcomes? This is ultimately an empirical question; but, is it one that is worth pursuing? That is, can a reasonable argument be made in favor of the health benefits of self-paced exercise? There are two general reasons to suspect that self-paced exercise may result in health benefits that are as good or better than prescribed intensity exercise.
Figure 1.
A model of self-paced exercise, affective response, and exercise adherence
First, as discussed earlier, self-paced exercise tends to approach, but not exceed the VT (Ekkekakis et al., 2006; Lind et al., 2005; Parfitt et al., 2006; Rose & Parfitt, 2007), albeit with evidence for some variability (Ekkekakis et al., 2006). In addition to avoidance of the decline in affective valence associated with exercise that exceeds the VT, exercise at or just below the VT has been shown to have significant health benefits (e.g., Astorino, 1997), potentially as good or better than those obtained when exercising above the VT (Aellen, Hollmann, & Boutellier, 1993; Boucher et al., 1985; Londeree, 1997; Tanaka et al., 1986; Weltman et al., 1992) or at moderate-intensity as defined by percent of maximal heart rate (Ahmaidi et al., 1998; for a review see Ekkekakis et al., 2004; Fabre, Masse-Biron, Ahmaidi, Adam, & Prefaut, 1997; Vallet et al., 1997). Given the heath benefits associated with exercise just below the VT, and the avoidance of a deterioration in affective response, simply prescribing exercise at an intensity just below the VT seems like a viable alternative to self-paced exercise, as well as traditional prescription schemes. However, prescribing exercise just below the VT is difficult because of the expensive and cumbersome procedures needed to ascertain the VT (i.e., exercise testing with collection of expired gases or blood samples). Moreover, as discussed earlier, because of potential cognitive mediators, self-paced exercise may lead to a more positive affective response, and thus better adherence, than exercise prescribed at similar intensities.
A second reason for the potential health benefits of self-paced exercise over traditional prescribed intensity exercise programs involves the distinction between exercise behavior and exercise prescriptions or recommendations. Numerous studies have examined the effects of exercise behavior on health outcomes (for reviews see Shaw, Gennat, O'Rourke, & Del Mar, 2006; Shepard et al., 2001). This research provides valuable information on the health benefits of exercise of varying intensities, assuming the exercise program is adhered to (Figure 2, Path B). However, few studies have examined the effects of various exercise prescriptions on naturally occurring exercise behavior (Figure 2, Path A). Preliminary evidence supports better adherence as a result of higher frequency (i.e., 5–7 days per week; Perri et al., 2002) and moderate intensity (versus vigorous intensity) exercise prescriptions (Cox, Burke, Gorely, Beilin, & Puddey, 2003; J. Y. Lee et al., 1996; Perri et al., 2002), which is consistent with existing public health guidelines (e.g., Haskell et al., 2007; Pate et al., 1995). However, no studies have compared adherence to self-paced exercise and prescribed intensity exercise. A study such as this would provide information on how self-paced versus, for example, moderate intensity exercise prescriptions impact health outcomes through their influence on adherence to the exercise program. Thus, even if moderate intensity exercise, as traditionally prescribed, resulted in greater health benefits per minute of exercise, the potential for better adherence to and maintenance of a self-paced exercise program could result in greater health benefits over the long term.
Figure 2.

Exercise prescription, compliance, and health outcomes
This may be especially true for overweight or obese adults, for whom energy expenditure is one of the primary benefits of exercise (Hu et al., 1999; I. M. Lee, Rexrode, Cook, Manson, & Buring, 2001; I. M. Lee & Skerrett, 2001; Manson et al., 1999). Indeed, physical activity among overweight and obese adults has particular public health significance as approximately 60% of the U.S. population is overweight or obese (Ogden et al., 2006), only 20% of overweight or obese adults meet the minimum national recommendations (30 minutes per day and five days per week of at least moderate intensity exercise; MMWR, 2000), and overweight or obese participants are more likely than normal weight participants to discontinue exercise programs (King et al., 1997; Kriska et al., 1986). Moreover, self-paced exercise may sometimes result in a lower exercise intensity than exercise prescribed at moderate intensity, as currently recommended in national guidelines (e.g., Focht & Hausenblas, 2003; D. M. Williams, 2007). Compared to moderate intensity exercise, the lower intensity of self-paced exercise will have negative effects on energy expenditure in the short term. However, because overweight adults are likely to experience exercise that exceeds the VT (e.g., brisk walking) as aversive (Ekkekakis & Lind, 2006; Mattsson, Larsson, & Rossner, 1997), self-paced exercise may eventually lead to greater energy expenditure, and associated health benefits (Hu et al., 1999; I. M. Lee et al., 2001; I. M. Lee & Skerrett, 2001; Manson et al., 1999), due to increased adherence to the exercise program. Finally, as people become more experienced exercisers, they may be more likely to increase their pace to a level that results in greater physiological adaptations.
The feasibility of a home-based, self-paced walking program was examined in a recent pilot study among 13 predominantly female (92%), Non-Hispanic Caucasian (85%) adults (mean age = 45.8, SD = 11.0; mean BMI = 29.9 kg/m2, SD = 6.2; D. M. Williams, 2007). At baseline, participants underwent a behavioral counseling program used previously in other exercise promotion trials (e.g., Marcus et al., 2007) focusing on social cognitive principles, such as goal setting and overcoming barriers. Participants, who were inactive or low active at baseline (i.e., exercised < 90 min/week, mean = 8.6, SD = 22.1), were instructed to walk 5–7 days/week for 30 min/day at a self-selected intensity, but to avoid exercise that elicited unpleasant feelings (Ekkekakis et al., 2004). Additionally, in order to help regulate their affect, participants were instructed to imagine their Feeling Scale ratings while walking to avoid a decrease in pleasure. Over the course of the one-month program participants increased to a mean of 142.2 min/week (SD = 72.8) of self-paced walking for exercise (completed in purposeful bouts of at least 10 min), with a mean intensity of 37% HRR (SD = 12.6%), as measured by heart rate monitors with recording capability (Timex Corporation). Program satisfaction ratings averaged 4.62 on a scale of 1 to 5, and all 13 participants said they would recommend the program to a friend. These findings show preliminary support for the feasibility of home-based, self-paced walking programs among predominantly overweight or obese women. However, additional research is needed to compare adherence to self-paced walking programs with adherence to programs with traditional exercise intensity prescriptions.
Conclusions and Future Directions
There is emerging evidence that self-paced exercise elicits a more positive affective response than prescribed intensity exercise, and that affective response to exercise leads to increased adherence to exercise programs. These findings are consistent with a model of exercise, affect, and adherence that represents an integration of the dual-mode model and hedonic theory (Figure 1). Given its focus on affect, it is not intended that the proposed model replace existing health behavior theories, which tend to focus more on cognitive and social factors (Ajzen, 1991; Bandura, 1986; King et al., 2002; Prochaska & DiClemente, 1984; Rogers, 1983). Self-regulatory strategies emphasized in these models, such as goal-setting, self-monitoring, and enlisting social support should be viewed as complimentary to the proposed model, which focuses exclusively on the mechanisms of the exercise stimulus (e.g., self-paced versus prescribed intensity). The potential for self-paced exercise to improve adherence to exercise programs among previously sedentary individuals is likely dependent on its effective integration with established programs of behavioral counseling (e.g., Williams, 2007). Additionally, while this paper has focused on exercise intensity, there may be other aspects of the exercise stimulus that influence affective processes and exercise adherence as indicated in the model, such as the exercise setting and mode.
The proposed model is intended as a vehicle to promote future research, not as a recommendation for practice, due to the premature nature of supporting evidence. Indeed, more research is needed to further support the relationships posited in the model. For example, what is the source of variability in affective response to self-paced exercise? Does overweight status moderate the effect of self-paced versus prescribed exercise on affective response? Do perceived autonomy and anticipated affective responses mediate the influence of self-paced exercise on affective response? Does affective response to exercise predict future exercise behavior? If so, does anticipated affective response mediate the affect-adherence link?
Many additional hypotheses can be generated from the multiple manifestations of hedonic theory. For example, do recent hedonic states impact the perceived pleasure/displeasure of exercise (Zellner, Allen, Henley, & Parker, 2006)? Do hedonic principles have less impact on exercise behavior in social situations (Erber & Erber, 2000)? To what extent do non-valence aspects of affective response to exercise influence future exercise behavior (Zemack-Rugar, Bettman, & Fitzsimons, 2007)? Can desire to exercise be increased by pairing exercise goals with positively valenced affective stimuli (Custers & Aarts, 2005)? How does partitioning of exercise into multiple daily bouts impact affective response (Ariely & Zauberman, 2003; Morewedge, Gilbert, Keysar, Berkovits, & Wilson, 2007)? What is the relationship between affective response to exercise and anticipated affective response; and, is anticipated affective response to exercise—and in turn, future exercise behavior—influenced by global, retrospective evaluations of affective responses to previous exercise or by aggregation of instantaneous affective states that occur during exercise (Kahneman, 1999)?
Finally, will existing laboratory research relevant to the proposed model generalize to more naturalistic settings? The rapidly developing field of ecological momentary assessment (EMA; Stone, Shiffman, Atienza, & Nebeling, 2007) may be a key to answering this critical question. EMA is characterized by repeated assessments of participants' experiences (e.g., behavior, cognition, emotion, surroundings) in real-time and in their natural environment (Stone, Shiffman, Atienza, & Nebeling, 2007), and, for a number of reasons, is an ideal methodology for examining the relationship between exercise and affective response. First, because EMA occurs in “real time,” it minimizes the considerable bias introduced when participants are asked to recall experiences retrospectively (M. D. Robinson & Clore, 2002; Shiffman, Hufford, & Hickcox, 1997). Second, EMA occurs in participants' natural environments, thus minimizing demand or expectancy bias that results from assessments conducted at the laboratory site (Pickering & Devereux, 1987). Third, EMA allows for repeated assessment over time, which provides a rich data set that allows for the fine-grained examination of complex patterns of experience. In addition to the advantages of EMA noted above, electronic diaries have the added advantage of time-stamping responses. This allows the researcher to know exactly when the response occurred and prevents the participant from “hoarding” numerous questionnaires and responding to them at a later time.
While there are a growing number of studies that have used EMA to assess exercise behavior (Atienza, Oliveira, Fogg, & King, 2006; Dunton, Whalen, Jamner, & Floro, 2007; Dunton, Whalen, Jamner, Henker, & Floro, 2005; Focht, Ewing, Gauvin, & Rejeski, 2002; Gauvin, Rejeski, & Reboussin, 2000; King et al., 2008; Liszka-Hackzell & Martin, 2004), few studies have examined affective responses to exercise via EMA (Focht, Gauvin, & Rejeski, 2004; Gauvin, Rejeski, & Norris, 1996; Vansteelandt, Rijmen, Pieters, Probst, & Vanderlinden, 2007; Yoshiuchi et al., 2007), and none have examined the link between acute affective response to exercise and exercise adherence. In addition to exploring some of the questions raised above, future research using EMA should address some of its limitations, such as need for technological savvy, difficulty promoting compliance with rigorous assessment schedules, technical problems, and potential for social desirability. The assessment of real-time affective responses to exercise in natural environments will be critical to fully understanding the relationships posited in the proposed model.
In closing, it is important to note that all exercise that is freely undertaken may be considered “self-paced,” including exercise that has been prescribed at a specific intensity. That is, exercisers are likely to be influenced by how the exercise stimulus makes them feel, regardless of whether or not they have been instructed to do so. What may vary is the extent to which they allow their affect to determine their selected intensity instead of personal or imposed expectations about what intensity they should be performing. A shift in physical activity guidelines, emphasizing performance of exercise at an intensity that feels good, rather than at a specific prescribed intensity, could result in a more sustainable exercise experience and enhanced health outcomes.
ACKNOWLEDGEMENTS
This project was supported in part through a postdoctoral fellowship award from the National Heart, Lung, and Blood Institute (F32 HL78709) and a career development award from the National Institute of Child Health and Human Development (K12 HD043447). I would like to thank Hollie A. Raynor for reviewing a prior version of this manuscript, two anonymous reviewers who provided excellent suggestions for revising the manuscript, and Barbara Doll for her assistance with manuscript preparation.
Footnotes
A brief summary of the critical points is provided here, and the reader is referred to the above references for more detail.
References
- Abraham C, Sheeran P. Acting on intentions: the role of anticipated regret. British Journal of Social Psychology. 2003;42(4):495–511. doi: 10.1348/014466603322595248. [DOI] [PubMed] [Google Scholar]
- Abraham C, Sheeran P. Deciding to exercise: the role of anticipated regret. British Journal of Health Psychology. 2004;9(2):269–278. doi: 10.1348/135910704773891096. [DOI] [PubMed] [Google Scholar]
- Aellen R, Hollmann W, Boutellier U. Effects of aerobic and anaerobic training on plasma lipoproteins. International Journal of Sports Medicine. 1993;14(7):396–400. doi: 10.1055/s-2007-1021198. [DOI] [PubMed] [Google Scholar]
- Ahmaidi S, Masse-Biron J, Adam B, Choquet D, Freville M, Libert JP, et al. Effects of interval training at the ventilatory threshold on clinical and cardiorespiratory responses in elderly humans. European Journal of Applied Physiology and Occupational Physiology. 1998;78(2):170–176. doi: 10.1007/s004210050403. [DOI] [PubMed] [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. 7th ed. Lippincott, Williams, & Wilkins; Philadelphia, PA: 2005. [Google Scholar]
- Annesi JJ. Relationship between changes in acute exercise-induced feeling states, self-motivation, and adults' adherence to moderate aerobic exercise. Perceptual and Motor Skills. 2002a;94(2):425–439. doi: 10.2466/pms.2002.94.2.425. [DOI] [PubMed] [Google Scholar]
- Annesi JJ. Self-motivation moderates effect of exercise-induced feelings on adherence. Perceptual and Motor Skills. 2002b;94(2):467–475. doi: 10.2466/pms.2002.94.2.467. [DOI] [PubMed] [Google Scholar]
- Annesi JJ. Relations of self-motivation, perceived physical condition, and exercise-induced changes in revitalization and exhaustion with attendance in women initiating a moderate cardiovascular exercise regimen. Women and Health. 2005;42(3):77–93. doi: 10.1300/j013v42n03_05. [DOI] [PubMed] [Google Scholar]
- Ariely D, Zauberman G. Differential partitioning of extended experiences. Organizational Behavior and Human Decision Processes. 2003;91:128–139. [Google Scholar]
- Astorino TA. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women? Journal of Sports Medicine and Physical Fitness. 1997;40:209–216. [PubMed] [Google Scholar]
- Atienza A, Oliveira B, Fogg B, King A. Using electronic diaries to examine physical activity and other health behaviors of adults age 50+ Journal of Aging and Physical Activity. 2006;14:192–202. doi: 10.1123/japa.14.2.192. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Prentiss-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Baranowski T, Anderson C, Carmack C. Mediating variable framework in physical activity interventions. How are we doing? How might we do better? American Journal of Preventive Medicine. 1998;15(4):266–297. doi: 10.1016/s0749-3797(98)00080-4. [DOI] [PubMed] [Google Scholar]
- Bell DE. Regret in decision making under uncertainty. Operations Research. 1982;30:961–981. [Google Scholar]
- Bell DE. Disappointment in decision making under uncertainty. Operations Research. 1985;33:1–27. [Google Scholar]
- Bentham J. An Introduction to the Principles of Morals and Legislation. Dover Publications; New York: 2007. Original work published 1789. [Google Scholar]
- Berger BG, Owen DR. Mood alteration with yoga and swimming: Aerobic exercise may not be necessary. Perceptual and Motor Skills. 1992;75(3 Pt 2):1331–1343. doi: 10.2466/pms.1992.75.3f.1331. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychological Review. 1974;81(3):199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology. 2005;180(2):306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Boucher CA, Anderson MD, Schneider MS, Murphy JH, Okada RD, Kanarek DJ. Left ventricular function before and after reaching the anaerobic threshold. Chest. 1985;87(2):145–150. doi: 10.1378/chest.87.2.145. [DOI] [PubMed] [Google Scholar]
- Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. American Journal of Preventive Medicine. 2002;23(2 Suppl):80–86. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Exertion and pleasure from an evolutionary perspective. In: Acevedo EO, Ekkekakis P, editors. Psychobiology of physical activity. Human Kinetics; Champaign, IL: 2006. [Google Scholar]
- Cabanac M, Leblanc J. Physiological conflict in humans: Fatigue vs. cold discomfort. American Journal of Physiology. 1983;244(5):R621–628. doi: 10.1152/ajpregu.1983.244.5.R621. [DOI] [PubMed] [Google Scholar]
- Carels RA, Berger B, Darby L. The association between mood states and physical activity in postmenopausal, obese, sedentary women. Journal of Aging and Physical Activity. 2006;14(1):12–28. doi: 10.1123/japa.14.1.12. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine and Tobacco Research. 2004;6(1):39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Research Reviews. 2006;51(1):85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40–65 years: The S.W.E.A.T. Study (Sedentary Women Exercise Adherence Trial) Preventive Medicine. 2003;36(1):17–29. doi: 10.1006/pmed.2002.1134. [DOI] [PubMed] [Google Scholar]
- Custers R, Aarts H. Positive affect as implicit motivator: on the nonconscious operation of behavioral goals. Journal of Personality and Social Psychology. 2005;89(2):129–142. doi: 10.1037/0022-3514.89.2.129. [DOI] [PubMed] [Google Scholar]
- Daley A, Maynard I. Preferred exercise mode and affective response in physically active adults. Psychology of Sport and Exercise. 2003;4:347–356. [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addictive Behaviors. 2007;32(2):425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. Plenum; New York: 1985. [Google Scholar]
- Desharnais R, Jobin J, Cote C, Levesque L, Godin G. Aerobic exercise and the placebo effect: a controlled study. Psychosomatic Medicine. 1993;55(2):149–154. doi: 10.1097/00006842-199303000-00003. [DOI] [PubMed] [Google Scholar]
- Dunton G, Whalen C, Jamner L, Floro J. Mapping the social and physical contexts of physical activity across adolescence using ecological momentary assessment. Annals of Behavioral Medicine. 2007;43(2):144–153. doi: 10.1007/BF02872669. [DOI] [PubMed] [Google Scholar]
- Dunton G, Whalen C, Jamner L, Henker B, Floro J. Using ecologic momentary assessment to measure physical activity during adolescence. American Journal of Preventive Medicine. 2005;29(4):281–287. doi: 10.1016/j.amepre.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P. Pleasure and displeasure from the body: Perspectives from exercise. Cognition and Emotion. 2003;17:213–239. doi: 10.1080/02699930302292. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P. The study of affective responses to acute exercise: The dual-mode model. In: Stelter R, Roessler KK, editors. New approaches to exercise and sport psychology. Meyer & Meyer Sport; Oxford, United Kingdom: 2005. pp. 119–46. [Google Scholar]
- Ekkekakis P, Acevedo EO. Affective responses to acute exercise: Toward a psychobiological dose-response model. In: Acevedo EO, Ekkekakis P, editors. Psychobiology of physical activity. Human Kinetics; Champaign, IL: 2006. pp. 91–109. [Google Scholar]
- Ekkekakis P, Backhouse SH, Gray C, Lind E. Walking is popular among adults but is it pleasant? A framework for clarifying the link between walking and affect as illustrated in two studies. Psychology of Sport and Exercise. 2008;9:246–264. [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. Practical markers of the transition from aerobic to anaerobic metabolism during exercise: Rationale and a case for affect-based exercise prescription. Preventive Medicine. 2004;38(2):149–159. doi: 10.1016/j.ypmed.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. Variation and homogeneity in affective responses to physical activity of varying intensities: An alternative perspective on dose-response based on evolutionary considerations. Journal of Sports Sciences. 2005;23(5):477–500. doi: 10.1080/02640410400021492. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: The impact of self-selected and imposed intensity on affect and exertion. International Journal of Obesity (Lond) 2006;30(4):652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Lind E, Joens-Matre RR. Can self-reported preference for exercise intensity predict physiologically defined self-selected exercise intensity? Research Quarterly for Exercise and Sport. 2006;77(1):81–90. doi: 10.1080/02701367.2006.10599334. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Petruzzello SJ. Acute aerobic exercise and affect: Current status, problems and prospects regarding dose-response. Sports Medicine. 1999;28(5):337–374. doi: 10.2165/00007256-199928050-00005. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Petruzzello SJ. Analysis of the affect measurement conundrum in exercise psychology. I. Fundamental issues. Psychology of Sport and Exercise. 2000;1(2):71–88. [Google Scholar]
- Ekkekakis P, Petruzzello SJ. Analysis of the affect measurement conundrum in exercise psychology: IV. A conceptual case for the affect circumplex. Psychology of Sport and Exercise. 2002;3:35–63. [Google Scholar]
- Erber R, Erber MW. The self-regulation of moods: second thoughts on the importance of happiness in everyday life. Psychological Inquiry. 2000;11(3):142–148. [Google Scholar]
- Evans D, Norman P. Predicting adolescent pedestrians' road-crossing intentions: an application and extension of the theory of planned behaviour. Health Education Research. 2003;18(3):267–277. doi: 10.1093/her/cyf023. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fabre C, Masse-Biron J, Ahmaidi S, Adam B, Prefaut C. Effectiveness of individualized aerobic training at the ventilatory threshold in the elderly. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52(5):B260–266. doi: 10.1093/gerona/52a.5.b260. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neuroscience and Biobehavioral Reviews. 2007;31(7):987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Focht B, Ewing M, Gauvin L, Rejeski W. The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Annals of Behavioral Medicine. 2002;24(3):201–210. doi: 10.1207/S15324796ABM2403_05. [DOI] [PubMed] [Google Scholar]
- Focht B, Gauvin L, Rejeski W. The contribution of daily experiences and acute exercise to fluctuations in daily feeling states among older, obese adults with knee osteoarthritis. Journal of Behavioral Medicine. 2004;27(2):101–121. doi: 10.1023/b:jobm.0000019847.80315.4d. [DOI] [PubMed] [Google Scholar]
- Focht BC, Hausenblas HA. State anxiety responses to acute exercise in women with high social physique anxiety. Journal of Sport and Exercise Psychology. 2003;25:123–144. [Google Scholar]
- Focht BC, Knapp DJ, Gavin TP, Raedeke TD, Hickner RC. Affective and self-efficacy responses to acute aerobic exercise in sedentary older and youger adults. Journal of Aging and Physical Activity. 2007;15:123–138. doi: 10.1123/japa.15.2.123. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Kahneman D. Duration neglect in retrospective evaluations of affective episodes. Journal of Personality and Social Psychology. 1993;65(1):45–55. doi: 10.1037//0022-3514.65.1.45. [DOI] [PubMed] [Google Scholar]
- Freud S. A General Introduction to Psychoanalysis. Washington Square Press; New York: 1952. Original work published 1920. [Google Scholar]
- Garg N, Wansink B, Inman JJ. The influence of incidental affect on consumers' food intake. Journal of Marketing. 2007;71:194–206. [Google Scholar]
- Gauvin L, Rejeski WJ. Disentangling substance from rhetoric: A rebuttal to Ekkekakis and Petruzzello. Psychology of Sport and Exercise. 2001;2:73–88. [Google Scholar]
- Gauvin L, Rejeski W, Norris J. A naturalistic study of the impact of acute physical activity on feeling states and affect in women. Health Psychology. 1996;15(5):391–397. doi: 10.1037//0278-6133.15.5.391. [DOI] [PubMed] [Google Scholar]
- Gauvin L, Rejeski W, Reboussin B. Contributions of acute bouts of vigorous physical activity to explaining diurnal variations in feeling states in active, middle-aged women. Health Psychology. 2000;19(4):365–375. doi: 10.1037//0278-6133.19.4.365. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317(5843):1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Darkes J, Del Boca FK. Expectancy mediation of biopsychosocial risk for alcohol use and alcoholism. In: Kirsch I, editor. How Expectancies Shape Experience. APA; Washington, DC: 1999. pp. 233–262. [Google Scholar]
- Hall EE, Ekkekakis P, Petruzzello SJ. The affective beneficence of vigorous exercise revisited. British Journal of Health Psychology. 2002;7(Pt 1):47–66. doi: 10.1348/135910702169358. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52(12):1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA. 1999;282(15):1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- Hull CL. A Behavior System. Yale University Press; New Haven, CT: 1952. [Google Scholar]
- Kahneman D. Objective happiness. In: Kahneman D, Diener E, Schwarz N, editors. Well-Being: Foundations of Hedonic Psychology. Russell-Sage; New York: 1999. [Google Scholar]
- Kahneman D, Fredrickson BL, Schreiber CA, Redelmeier DA. When more pain is preferred to less: adding a better end. Psychological Science. 1993;4(6):401–405. [Google Scholar]
- Kahneman D, Krueger AB, Schkade D, Schwarz N, Stone AA. Would you be happier if you were richer? A focusing illusion. Science. 2006;312(5782):1908–1910. doi: 10.1126/science.1129688. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decisions under risk. Econometrica. 1979;47:313–327. [Google Scholar]
- Kahneman D, Wakker PP, Sarin R. Back to Bentham? Explorations of experienced utility. Quarterly Journal of Economics. 1997;112(2):375–405. [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychological Review. 2005;112(2):446–467. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. Journal of Neuroscience. 2005;25(26):6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M, Kraemer R, Bartholomew J, Acevedo E, Jarreau D. Affective responses to exercise are dependent on intensity rather than total work. Medicine and Science in Sports and Exercise. 2007;39(8):1417–1422. doi: 10.1249/mss.0b013e31806ad73c. [DOI] [PubMed] [Google Scholar]
- King A, Ahn D, Oliveira B, Atienza A, Castro C, Gardner C. Promoting physical activity through hand-held computer technology. American Journal of Preventive Medicine. 2008;34(2):138–142. doi: 10.1016/j.amepre.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Kiernan M, Oman RF, Kraemer HC, Hull M, Ahn D. Can we identify who will adhere to long-term physical activity? Signal detection methodology as a potential aid to clinical decision making. Health Psychology. 1997;16(4):380–389. doi: 10.1037//0278-6133.16.4.380. [DOI] [PubMed] [Google Scholar]
- King AC, Stokols D, Talen E, Brassington GS, Killingsworth R. Theoretical approaches to the promotion of physical activity: Forging a transdisciplinary paradigm. American Journal of Preventive Medicine. 2002;23(2 Suppl):15–25. doi: 10.1016/s0749-3797(02)00470-1. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Changing Expectations: A Key to Effective Therapy. Wadsworth; Belmont, CA: 1990. [Google Scholar]
- Kirsch I. Response expectancy theory and application: a deccenial review. Applied and Preventive Psychology. 1997;6:69–80. [Google Scholar]
- Kiviniemi MT, Voss-Humke AM, Seifert AL. How do I feel about the behavior? The interplay of affective associations with behaviors and cognitive beliefs as influences on physical activity behavior. Health Psychology. 2007;26(2):152–158. doi: 10.1037/0278-6133.26.2.152. [DOI] [PubMed] [Google Scholar]
- Klonoff EA, Annechild A, Landrine H. Predicting exercise adherence in women: The role of psychological and physiological factors. Preventive Medicine. 1994;23(2):257–262. doi: 10.1006/pmed.1994.1036. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska AM, Bayles C, Cauley JA, LaPorte RE, Sandler RB, Pambianco G. A randomized exercise trial in older women: Increased activity over two years and the factors associated with compliance. Medicine and Science in Sports and Exercise. 1986;18(5):557–562. [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49(4):525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: Is “no pain, no gain” passe? JAMA. 2001;285(11):1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- Lee IM, Skerrett PJ. Physical activity and all-cause mortality: What is the dose-response relation? Medicine and Science in Sports and Exercise. 2001;33(6 Suppl):S459–471. doi: 10.1097/00005768-200106001-00016. discussion S493–454. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jensen BE, Oberman A, Fletcher GF, Fletcher BJ, Raczynski JM. Adherence in the training levels comparison trial. Medicine and Science in Sports and Exercise. 1996;28(1):47–52. doi: 10.1097/00005768-199601000-00013. [DOI] [PubMed] [Google Scholar]
- Lind E, Joens-Matre RR, Ekkekakis P. What intensity of physical activity do previously sedentary middle-aged women select? Evidence of a coherent pattern from physiological, perceptual, and affective markers. Preventive Medicine. 2005;40(4):407–419. doi: 10.1016/j.ypmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Liszka-Hackzell J, Martin D. An analysis of the relationship between activity and pain in chronic and acute low back pain. Anesthesia and Analgesia. 2004;99:477–481. doi: 10.1213/01.ANE.0000132696.15310.DD. [DOI] [PubMed] [Google Scholar]
- Londeree BR. Effect of training on lactate/ventilatory thresholds: A meta-analysis. Medicine and Science in Sports and Exercise. 1997;29(6):837–843. doi: 10.1097/00005768-199706000-00016. [DOI] [PubMed] [Google Scholar]
- Loomes G, Sugden R. Regret theory: an alternative of rationale choice under uncertainty. Economic Journal. 1982;92:805–824. [Google Scholar]
- Loomes G, Sugden R. Disappointment and dynamic consistency in choice under uncertainty. Review of Economic Studies. 1986;53:271–282. [Google Scholar]
- Lowenstein GF, Prelec D. Preferences for sequences of outcomes. Psychological Review. 1993;100(1):91–108. [Google Scholar]
- Macera CA, Ham SA, Yore MM, Jones DA, Ainsworth BE, Kimsey CD, et al. Prevalence of physical activity in the United States: Behavioral Risk Factor Surveillance System, 2001. Preventing Chronic Disease. 2005;2(2):A17. [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. New England Journal of Medicine. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Williams DM, Whiteley JA, Albrecht AE, Jakicic JM, Parisi AF, Hogan JW, Napolitano MA, Bock BC. Step into Motion: A randomized trial examining the relative efficacy of Internet vs. print-based physical activity interventions. Contemporary Clinical Trials. 2007;28:737–747. doi: 10.1016/j.cct.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? International Journal of Obesity and Related Metabolic Disorders. 1997;21(5):380–386. doi: 10.1038/sj.ijo.0800417. [DOI] [PubMed] [Google Scholar]
- McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exercise and Sport Sciences Reviews. 2000;28(2):85–88. [PubMed] [Google Scholar]
- McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: The Women's Health Initiative Cohort Study. Journal of the American Medical Association. 2003;290(10):1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- Mela DJ. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite. 2006;47(1):10–17. doi: 10.1016/j.appet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Mellers BA. Choice and the relative pleasure of consequences. Psychological Bulletin. 2000;126(6):910–924. doi: 10.1037/0033-2909.126.6.910. [DOI] [PubMed] [Google Scholar]
- Mellers BA, Schwartz A, Ho Katty, Ritov I. Decision affect theory: emotional reactions to the outcomes of risky options. Psychological Science. 1997;8(6):423–429. [Google Scholar]
- Mellers B, Schwartz A, Ritov I. Emotion-based choice. Journal of Experimental Psychology: General. 1999;128(3):332–345. [Google Scholar]
- MMWR Prevalence of leisure-time physical activity among overweight adults--United States, 1998. MMWR. Morbidity and Mortality Weekly Report. 2000;49(15):326–330. [PubMed] [Google Scholar]
- Moan IS, Rise J, Andersen M. Predicting parents' intentions not to smoke indoors in the presence of their children using an extended version of the theory of planned behaviour. Psychology and Health. 2005;20(3):353–371. [Google Scholar]
- Moller AC, Deci EL, Ryan RM. Choice and ego-depletion: The moderating role of autonomy. Personality and Social Psychology Bulletin. 2006;32(8):1024–1036. doi: 10.1177/0146167206288008. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Bovbjerg DH. Specific response expectancies predict anticipatory nausea during chemotherapy for breast cancer. Journal of Consulting and Clinical Psychology. 2001;69:831–835. doi: 10.1037//0022-006x.69.5.831. [DOI] [PubMed] [Google Scholar]
- Morewedge CK, Gilbert DT, Keysar B, Berkovits MJ, Wilson TD. Mispredicting the hedonic benefits of segregated gains. Journal of Experimental Psychology: General. 2007;136(4):700–709. doi: 10.1037/0096-3445.136.4.700. [DOI] [PubMed] [Google Scholar]
- Murgraff V, McDermott MR, White D, Phillips K. Regret is what you get: the effects of manipulating anticipated affect and time perspective on risky single-occasion drinking. Alcohol and Alcoholism. 1999;34(4):590–600. doi: 10.1093/alcalc/34.4.590. [DOI] [PubMed] [Google Scholar]
- Neef NA, Shade D, Miller MS. Assessing influential dimensions of reinforcers on choice in students with serious emotional disturbance. Journal of Applied Behavior Analysis. 1994;27(4):575–583. doi: 10.1901/jaba.1994.27-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Palange P, Ward SA, Whipp BJ. Basic Principles of clinical exercise testing. Breathe. 2006;3:159–163. [Google Scholar]
- Parfitt G, Eston R, Connolly D. Psychological affect at different ratings of perceived exertion in high- and low-active women: A study using a production protocol. Perceptual and Motor Skills. 1996;82(3 Pt 1):1035–1042. doi: 10.2466/pms.1996.82.3.1035. [DOI] [PubMed] [Google Scholar]
- Parfitt G, Gledhill C. The effect of choice of exercise mode on psychological responses. Psychology of Sport and Exercise. 2004;5:111–117. [Google Scholar]
- Parfitt G, Markland D, Holmes C. Responses to physical exertion in active and inactive males and females. Journal of Sport & Exercise Psychology. 1994;16:178–186. [Google Scholar]
- Parfitt G, Rose EA, Burgess WM. The psychological and physiological responses of sedentary individuals to prescribed and preferred intensity exercise. British Journal of Health Psychology. 2006;11(Pt 1):39–53. doi: 10.1348/135910705X43606. [DOI] [PubMed] [Google Scholar]
- Parfitt G, Rose EA, Markland D. The effect of prescribed and preferred intensity exercise on psychological affect and the influence of baseline measures of affect. Journal of Health Psychology. 2000;5(2):231–240. doi: 10.1177/135910530000500213. [DOI] [PubMed] [Google Scholar]
- Parker D, Manstead ASR, Stradling SG. Extending the theory of planned behaviour: the role of personal norm. British Journal of Social Psychology. 1995;34:127–137. [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE, et al. Adherence to exercise prescriptions: Effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychology. 2002;21(5):452–458. [PubMed] [Google Scholar]
- Perugini M, Bagozzi RP. The role of desires and anticipated emotions in goal-directed behaviours: broadening and deepening the theory of planned behaviour. British Journal of Social Psychology. 2001;40(1):79–98. doi: 10.1348/014466601164704. [DOI] [PubMed] [Google Scholar]
- Pickering T, Devereux R. Ambulatory monitoring of blood pressure as a predictor of cardiovascular risk. The American Heart Journal. 1987;114(4):925–928. doi: 10.1016/0002-8703(87)90589-8. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Self change processes, self efficacy and decisional balance across five stages of smoking cessation. Progress in Clinical and Biological Research. 1984;156:131–140. [PubMed] [Google Scholar]
- Raedeke TD, Focht BC, Scales D. Social environmental factors and psychological responses to acute exercise for socially physique anxious females. Psychology of Sport and Exercise. 2007;8:463–476. [Google Scholar]
- Redelmeier D, Kahneman D. Patients' memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;116:3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- Richard R, de Vries NK, van der Pligt J. Anticipated regret and precautionary sexual behavior. Journal of Applied Social Psychology. 1998;28(15):1411–1428. [Google Scholar]
- Richard R, van der Pligt J, de Vries N. The impact of anticipated affect on (risky) sexual behaviour. British Journal of Social Psychology. 1995;34:9–21. doi: 10.1111/j.2044-8309.1995.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Richard R, van der Pligt J, de Vries N. Anticipated regret and time perspective: changing sexual risk-taking behavior. Journal of Behavioral Decision Making. 1996a;9:185–199. [Google Scholar]
- Richard R, van der Pligt J, de Vries N. Anticipated affect and behavioral choice. Basic and Applied Social Psychology. 1996b;18(2):111–129. [Google Scholar]
- Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addictive Behaviors. 2007;32(3):543–561. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychological Bulletin. 2002;128(6):934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: A revised theory of protection motivation. In: Cacioppo JT, Petty RE, editors. Social psychophysiology: A sourcebook. Guilford; New York: 1983. pp. 153–176. [Google Scholar]
- Rose EA, Parfitt G. A quantitative analysis and qualitative explanation of the individual differences in affective responses to prescribed and self-selected exercise intensities. Journal of Sport and Exercise Psychology. 2007;29:281–309. doi: 10.1123/jsep.29.3.281. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Schkade DA, Kahneman D. Does living in California make people happy? A focusing illusion is judgments of life satisfaction. Psychological Science. 1998;9:340–346. [Google Scholar]
- Shaw K, Gennat H, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Systems Review. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TY, Weil KM, Sharp TA, Grunwald GK, Bell ML, Hill JO, et al. Occasional physical inactivity combined with a high-fat diet may be important in the development and maintenance of obesity in human subjects. American Journal of Clinical Nutrition. 2001;73(4):703–708. doi: 10.1093/ajcn/73.4.703. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology. 2006;184(3–4):608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997;65(2):292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Are theories of learning necessary? Psychological Review. 1950;57(4):193–216. doi: 10.1037/h0054367. [DOI] [PubMed] [Google Scholar]
- Slattery ML. Physical activity and colorectal cancer. Sports Medicine. 2004;34(4):239–252. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- Stevens A, Peschk I, Schwarz J. Implicit learning, executive function and hedonic activity in chronic polydrug abusers, currently abstinent polydrug abusers and controls. Addiction. 2007;102(6):937–946. doi: 10.1111/j.1360-0443.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S, Atienza A, Nebeling L. Historic roots and rationale of ecological momentary assessment (EMA) In: Stone AA, Shiffman S, Atienza AA, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. Oxford University Press; New York: 2007. pp. 3–10. [Google Scholar]
- Svedahl K, MacIntosh BR. Anaerobic threshold: The concept and methods of measurement. Canadian Journal of Applied Physiology. 2003;28(2):299–323. doi: 10.1139/h03-023. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yoshimura T, Sumida S, Mitsuzono R, Tanaka S, Konishi Y, et al. Transient responses in cardiac function below, at, and above anaerobic threshold. European Journal of Applied Physiology and Occupational Physiology. 1986;55(4):356–361. doi: 10.1007/BF00422733. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Watson D, Clark LA. On the dimensional and hierarchical structure of affect. Psychological Science. 1999;10(4):297–303. [Google Scholar]
- Thogersen-Ntoumani C, Ntoumanis N. The role of self-determined motivation in the understanding of exercise-related behaviours, cognitions and physical self-evaluations. Journal of Sports Sciences. 2006;24(4):393–404. doi: 10.1080/02640410500131670. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. Animal Intelligence. Macmillan; New York: 1911. [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: Review and update. Medicine and Science in Sports and Exercise. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Healthy people 2010: Understanding and improving health. 2nd ed. U.S. Government Printing Office; Washington, DC: 2000. [Google Scholar]
- Urala N, Lahteenmaki L. Hedonic ratings and perceived healthiness in experimental functional food choices. Appetite. 2006;47(3):302–314. doi: 10.1016/j.appet.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Vallet G, Ahmaidi S, Serres I, Fabre C, Bourgouin D, Desplan J, et al. Comparison of two training programmes in chronic airway limitation patients: Standardized versus individualized protocols. European Respiratory Journal. 1997;10(1):114–122. doi: 10.1183/09031936.97.10010114. [DOI] [PubMed] [Google Scholar]
- Van der Pligt J, de Vries N. Expectancy-value models of health behaviour: the role of salience and anticipated affect. Psychology and Health. 1998;13:289–305. [Google Scholar]
- Vansteelandt K, Rijmen F, Pieters G, Probst M, Vanderlinden J. Drive for thinness, affect regulation and physical activity in eating disorders: A daily life study. Behavior Research and Therapy. 2007;45:1717–1734. doi: 10.1016/j.brat.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Varey C, Kahneman D. Experiences extended across time: evaluation of moments and episodes. Journal of Behavioral Decision Making. 1992;5:169–185. [Google Scholar]
- Vogel-Sprott M, Fillmore MT. Expectancy and behavioral effects of socially used drugs. In: Kirsch I, editor. How Expectancies Shape Experience. APA; Washington, DC: 1999. pp. 215–232. [Google Scholar]
- Vuori IM. Health benefits of physical activity with special reference to interaction with diet. Public Health Nutrition. 2001;4(2B):517–528. doi: 10.1079/phn2001137. [DOI] [PubMed] [Google Scholar]
- Wasserman K. Determinants and detection of anaerobic threshold and consequences of exercise above it. Circulation. 1987;76(6 Pt 2):VI29–39. [PubMed] [Google Scholar]
- Wegener DT, Petty RE. Mood management across affective states: the hedonic contingency hypothesis. Journal of Personality and Social Psychology. 1994;66(6):1034–1048. doi: 10.1037//0022-3514.66.6.1034. [DOI] [PubMed] [Google Scholar]
- Welch AS, Hulley A, Ferguson C, Beauchamp MR. Affective responses of inactive women to a maximal incremental exercise test: A test of the dual-mode model. Psychology of Sport and Exercise. 2007;8:401–423. [Google Scholar]
- Weltman A, Seip RL, Snead D, Weltman JY, Haskvitz EM, Evans WS, et al. Exercise training at and above the lactate threshold in previously untrained women. International Journal of Sports Medicine. 1992;13(3):257–263. doi: 10.1055/s-2007-1021263. [DOI] [PubMed] [Google Scholar]
- Williams DM. Piloting a self-paced exercise program among previously sedentary women. Journal of Women's Health. 2007;16:1101. [Google Scholar]
- Williams DM, Anderson ES, Winett RA. A review of the outcome expectancy construct in physical activity research. Annals of Behavioral Medicine. 29:70–79. doi: 10.1207/s15324796abm2901_10. [DOI] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychology of Sport and Exercise. 2008;9:231–245. doi: 10.1016/j.psychsport.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of Personality and Social Psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychology. 1998;17(3):269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- Wininger SR. Improvement of affect following exercise: Methodological artifact or real finding? Anxiety, Stress, and Coping. 2007;20(1):93–102. doi: 10.1080/10615800601170540. [DOI] [PubMed] [Google Scholar]
- Yeung RR. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 1996;40(2):123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]
- Yoshiuchi K, Cook D, Ohashi K, Kumano H, Kubaki T, Yamamoto Y, et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiology & Behavior. 2007;92:936–968. doi: 10.1016/j.physbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PT. The role of hedonic processes in the organization of behavior. Psychological Review. 1952;59(4):249–262. doi: 10.1037/h0057176. [DOI] [PubMed] [Google Scholar]
- Young PT. The role of affective processes in learning and motivation. Psychological Review. 1959;66(2):104–125. doi: 10.1037/h0045997. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Allen D, Henley M, Parker S. Hedonic contrast and condensation: good stimuli make mediocre stimuli less good and less different. Psychonomic Bulletin and Review. 2006;13(2):235–239. doi: 10.3758/bf03193836. [DOI] [PubMed] [Google Scholar]
- Zemack-Rugar Y, Bettman JR, Fitzsimons GJ. The effects of nonconsciously priming emotion concepts on behavior. Journal of Personality and Social Psychology. 2007;93(6):927–939. doi: 10.1037/0022-3514.93.6.927. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Porac J, Lathin D, Smith R, Deci L. On the importance of self-determination for intrinsically-motivated behavior. Personality and Social Psychology Bulletin. 1978;4(3):443–446. [Google Scholar]