Abstract
A number of viral infections can cause hearing loss. Hearing loss induced by these viruses can be congenital or acquired, unilateral or bilateral. Certain viral infections can directly damage inner ear structures, others can induce inflammatory responses which then cause this damage, and still others can increase susceptibility or bacterial or fungal infection, leading to hearing loss. Typically, virus-induced hearing loss is sensorineural, although conductive and mixed hearing losses can be seen following infection with certain viruses. Occasionally, recovery of hearing after these infections can occur spontaneously. Most importantly, some of these viral infections can be prevented or treated. For many of these viruses, guidelines for their treatment or prevention have recently been revised. In this review, we outline many of the viruses that cause hearing loss, their epidemiology, course, prevention, and treatment.
Keywords: hearing loss, sensorineural hearing loss, sudden sensorineural hearing loss, cytomegalovirus, lymphocytic choriomeningitis virus, varicella zoster virus, herpes simplex type 1, herpes simplex type 2, rubella, measles, rubeola, HIV, West Nile virus, mumps
Introduction
Among the many causes of hearing loss, viruses often are ignored. Viral infections, in particular cytomegalovirus (CMV), cause up to 40% of all congenitally acquired hearing loss. Many viruses can be the cause of congenital or acquired hearing loss (Table 1). Typically, viruses cause sensorineural hearing loss (SNHL); however, a viral etiology has been proposed for otosclerosis. Infection with HIV can lead to conductive hearing loss (CHL) through bacterial and fungal infections, which become more frequent following the immunosuppression caused by that virus. Hearing loss caused by viruses can be mild or severe to profound, unilateral or bilateral. Mechanisms involved in the induction of hearing loss by different viruses vary greatly, ranging from direct damage to inner ear structures, including inner ear hair cells and organ of Corti (as seen in some of the classically described causes of viral hearing loss such as measles), to induction of host immune-mediated damage (Table 2). Following infections with certain viruses, hearing loss can be reversed or limited by appropriate antiviral therapy. Effective vaccines are available for many of the viruses that cause hearing loss, leading to substantial changes in the incidence of these infections and to their prevalence as causes of hearing loss. Although it may seem a daunting task, a working knowledge of the potential viral causes of hearing loss and their treatment is essential to the recognition of these entities and their appropriate management.
Table 1.
Viral Causes of Hearing Loss.
| Virus | Type of HL | Degree of HL | Incidence of HL | Prevention | Treatment | Hearing recovery |
|---|---|---|---|---|---|---|
| Congenital | ||||||
| CMV | Bilateral progressive SNHL | Severe | 6–23% if asymptomatic; 22–65% if symptomatic | None | Ganciclovir, valganciclovir, cidofovir, foscarnet | Only with antiviral therapy |
| Rubella | Bilateral SNHL | Mild to severe | 12–19% | MMR | None | None |
| LCMV | Bilateral SNHL | Severe to profound | 7.4% | Avoidance of exposure | Ribavirin, favipiravir | None |
| Congenital and acquired | ||||||
| HIV | SNHL, CHL, mixed | SNHL: mild to moderate; CHL: mild to maximal | 27.5–33.5% | Postexposure antiretroviral | HAART | Dependent on type of HL |
| HSV | Unilateral or bilateral SNHL | Moderate to profound | Up to 33% (congenital) | None | Acyclovir | None |
| Acquired | ||||||
| Measles | Bilateral SNHL | Profound | 0.1–3.4% | MMR, IVIg | None | None |
| VZV | Unilateral SNHL | Mild to moderate | 7–85% | Zostavax | Acyclovir, prednisone | Improves with Rx |
| Mumps | Unilateral SNHL | Variable | 0.005–4% | MMR | None | Spontaneous |
| WNV | Bilateral SNHL | Mild to profound | Rare | Vaccine in trials | None | Spontaneous |
Note. CHL = conductive hearing loss; CMV = cytomegalovirus; HAART = highly active antiviral therapy (for HIV); HL = hearing loss; HSV = herpes simplex virus (listed values for type I); IVIg = intravenous immunoglobulin; MMR = measles, mumps, rubella vaccine; Rx = treatment; SNHL = sensorineural hearing loss; VZV = varicella zoster virus; WNV = West Nile virus.
Table 2.
Potential Etiologies of Hearing Loss Due to Different Viral Infections.
| Virus | Direct |
Indirect |
Unknown | |||
|---|---|---|---|---|---|---|
| Stria vascularis | Organ of Corti | Neuronal (cochlear, central) | Decreased immunity and secondary infection | Host immune response to viral antigen | ||
| CMV | x | |||||
| LCMV | x | |||||
| HIV | x | x | x | |||
| HSV | x | |||||
| Rubeolaa | x | x | ||||
| Mumps | x | x | x | |||
| Rubella | x | x | ||||
| WNV | x | |||||
| VZV | x | |||||
Note. CMV = cytomegalovirus; HSV = herpes simplex virus; LCMV = lymphocytic choriomeningitis virus; VZV = varicella zoster virus; WNV = West Nile virus.
Rubeola is also hypothesized to cause otosclerosis via stimulation of abnormal osteoblastic activity in endochondral bone of the inner ear.
In this review, we discuss many of the common viral causes of hearing loss and the interventions available for their prevention and treatment. Viruses causing congenital hearing losses are discussed first, followed by those that cause both congenital and acquired hearing loss, and finally those that exclusively cause acquired hearing loss. Within each category, the most common viral causes of hearing loss are discussed first, and then infrequent or emerging viruses that have been shown to cause hearing loss are discussed.
Viruses Causing Congenital Hearing Loss
Cytomegalovirus
CMV is an extremely common viral infection with nearly 100% prevalence. Despite its high prevalence, CMV rarely causes symptomatic disease in immunocompetent older infants or adults. CMV is a member of the herpesvirus family, along with herpes simplex virus (HSV), varicella zoster virus (VZV), and Epstein–Barr virus. Like all herpesviruses, CMV is a double-stranded enveloped DNA virus that can remain latent in the body long after primary infection. CMV can reactivate and cause disease in immunocompromised hosts. During reactivation, the virus again begins to make copies of its DNA and transmission to other people can occur.
CMV is typically acquired early in life and may be acquired in utero. In the United States, up to 1% of newborns are infected (Fowler et al., 1997; Madden et al., 2005; Smith, Bale, & White, 2005). CMV transmission to fetuses can occur during primary maternal infection (accounting for 40–50% of cases of congenital CMV) or reactivation during pregnancy (1% of cases of congenital CMV) (Adler, 2005; Bale, 2012). Congenitally acquired CMV is included as one of the TORCHS, an acronym for frequently occurring infectious teratogens (toxoplasmosis, rubella, CMV, herpes simplex, and syphilis, respectively). All of the TORCHS infections can cause similar signs before and after birth as well as similar birth defects. These include reduced intrauterine growth, microcephaly, seizures, mental retardation, visual defects, and cerebral palsy (Madden et al., 2005; Smith et al., 2005). Overall, the TORCHS are one of the most common causes of hearing loss that lead to pediatric cochlear implantation (Smith et al., 2005).
Maternal infection with CMV earlier in pregnancy increases the risk of symptomatic infection (Bale, 2012; Pass, Fowler, Boppana, Britt, & Stagno, 2006). Only 5% to 10% of infected neonates will show signs of CMV infection at birth (Fowler et al., 1997). Among these symptomatic infants, mortality is high (5%), and infants who survive often have permanent neurological defects such as SNHL, visual deficits, and seizures (Bale, 2012).
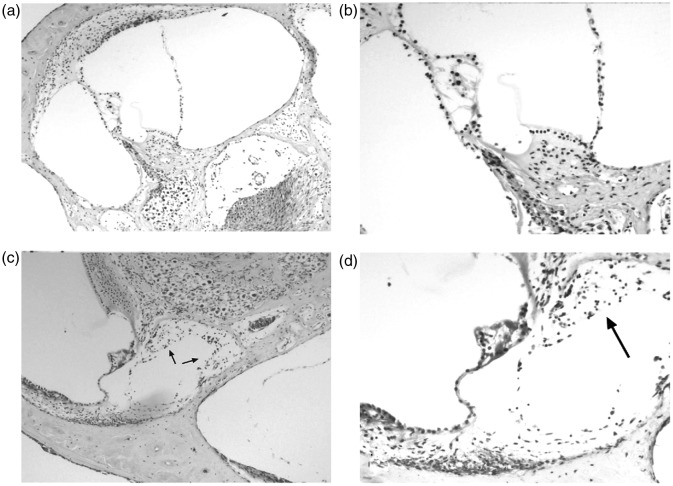
CMV is the leading nongenetic cause of childhood SNHL. Hearing loss can occur in both symptomatic and asymptomatically infected children. Delayed manifestations of congenital CMV infection, particularly SNHL, can manifest months or years after birth (Fowler et al., 1997). Initial hearing screens will miss the majority of cases of SNHL in CMV-infected children (Fowler, Dahle, Boppana, & Pass, 1999). The average age of diagnosis of hearing impairment in congenitally infected children is 27 to 33 months. Hearing loss may be diagnosed many years later, and thus CMV may be the cause underlying many cases of idiopathic SNHL in children (Fowler et al., 1997; Grosse, Ross, & Dollard, 2008). Of children born with symptomatic CMV infection, 14% will develop hearing loss and 5% will have severe, bilateral SNHL (Grosse et al., 2008). Risk factors for the development of SNHL in initially asymptomatic CMV-infected infants have not been identified, and so periodic screening of infected children is necessary (Barbi, Binda, Caroppo, & Primache, 2006; Fowler et al., 1999; Smith et al., 2005). The etiology of SNHL resulting from CMV infection is not fully understood. Temporal bone studies demonstrate inflammation and edema of the cochlea and spiral ganglion, and viral antigens in the spiral ganglion, organ of Corti, scala media, and Reissner’s membrane (Fowler et al., 1997; Schraff et al., 2007; Figure 1). Whether damage to inner ear structures is due to the direct effects of the virus or the innocent bystander effects of the patient’s immune system reacting to the presence of viral antigens in these areas is still an area of active research. Recent research in a guinea pig model system demonstrates that CMV expresses proteins that trigger an immune response that leads to hearing loss and inflammation within the cochlea (Schraff et al., 2007). This theory of pathogenesis may not fully explain progressive or later onset hearing losses seen in patients following CMV infection in utero.
Figure 1.
Temporal bone histology of guinea pigs either mock infected (a,b) or infected with CMV (c,d).
Note. Inflammatory cells and early fibrosis are found within the scala tympani in animals infected with CMV (arrows).
Source: Temporal bone pathology used with permission from Schraff et al. (2007).
The presentation, severity, progression, and audiographic pattern of SNHL resulting from congenital CMV infection are highly variable (Fowler et al., 1997; Grosse et al., 2008). Following identification of SNHL in children with CMV, continued audiographic monitoring is necessary due to frequent progression (Barbi et al., 2006; Foulon, Naessens, Foulon, Casteels, & Gordts, 2008). Generally, SNHL in children with initially asymptomatic CMV infections involves high frequencies and fluctuates. Hearing loss in children who were initially symptomatic tends to be bilateral, more severe, and more likely to progress (Fowler & Boppana, 2006; Madden et al., 2005).
Ganciclovir is the treatment for both early and delayed SNHL resulting from congenital CMV infection. Ganciclovir prevents SNHL progression and sometimes can improve hearing status. This medication must be administered intravenously and can be associated with neutropenia (Kimberlin et al., 2003). Other options include valganciclovir (a prodrug of ganciclovir that can be given orally), cidofovir, and foscarnet. Studies are currently underway testing the efficacy and duration of treatment of infected neonates with valganciclovir (Shin, Keamy, & Steinberg, 2011). Ganciclovir is teratogenic in animal studies and so cannot be used to treat pregnant women with active CMV infection. Other treatment modalities also exist. In vitro and animal studies support the use of CMV hyperimmune globulin during pregnancy (Carlson, Norwitz, & Stiller, 2010). SNHL that does not respond to antiviral medications can be treated with hearing aids or cochlear implantation depending on hearing severity. Cochlear implantation can significantly improve hearing loss due to CMV infection; however, the extent of improvement in speech and language skills following cochlear implantation may not be as great as in non-CMV-infected children with severe to profound hearing loss (Shin et al., 2011).
Despite multiple attempts at vaccine development, there is not currently an effective CMV vaccine. Prevention of primary infection in previously uninfected pregnant women is therefore the mainstay of limiting congenital CMV infection. Pregnant women are encouraged to frequently wash their hands and to avoid contact with saliva or urine of children younger than 6 years, particularly if they are enrolled in daycare. The U.S. Centers for Disease Control and Prevention (CDC) does not advocate CMV screening due to concerns that CMV IgM testing is not sufficiently specific and because treatment of CMV during pregnancy is controversial (Carlson et al., 2010).
Rubella
Rubella, also known as the German measles, is a member of the Togaviridae family of viruses. The genome of this virus is a single-stranded RNA and is enclosed in an icosahedral nonenveloped capsid. Rubella is most commonly transmitted via contaminated upper respiratory secretions during coughing, sneezing, and talking. In immunocompetent adults, the virus has a self-limiting course marked by low-grade fever, eye pain with movement, conjunctivitis, sore throat, malaise, headache, nausea, decreased appetite, transient arthritis, and tender lymphadenopathy (Lee & Bowden, 2000; McLean, Fiebelkorn, Temte, & Wallace, 2013). In contrast, if the virus is acquired during pregnancy it is a potent teratogen and one of the TORCHS infections (McLean et al., 2013). Congenital rubella syndrome manifests as hearing loss, congenital cataracts, microcephaly, mental retardation, thrombocytopenia, cardiac anomalies, and a characteristic rash (the so-called blueberry muffin spots; Pandey, Dudeja, Datta, Singla, & Saili, 2013).
SNHL is the most common sequela of congenital rubella infection (58%) and is most often seen when maternal rubella infection occurs within the first 16 weeks of pregnancy. Vestibular function is spared (Webster, 1998). Hearing loss typically manifests in the first 6 to 12 months of life, although it can present at birth (Dammeyer, 2010; Donley, 1993). Audiograms often show a flat, uniform mild to severe SNHL, but isolated high-frequency hearing loss has been reported (Dammeyer, 2010; Sheridan, 1964).
While the mechanism of rubella-induced hearing loss has not been fully explained, the virus causes direct cochlear damage and cell death in the organ of Corti and stria vascularis (Lee & Bowden, 2000). Alterations in the composition of endolymph due to strial damage have also been described (Webster, 1998). Depending on the severity of hearing loss, treatment options include the use of hearing aids and cochlear implantation (Smith et al., 2005).
Vaccination of women prior to or during reproductive age is extremely effective at prevention of congenital rubella in their offspring (De Leenheer et al., 2011). In areas without routine rubella vaccination, congenital rubella remains a common cause of severe to profound bilateral SNHL. In a recent Brazilian study, congenital rubella was thought to be the cause of hearing loss in 32% of patients with deafness (da Silva, Queiros, & Lima, 2006). Following the institution of a “school girl” vaccination program in Western Australia, the rate of congenital rubella syndrome dropped to 0% in vaccinated mothers (Stanley, Sim, Wilson, & Worthington, 1986). Despite vaccination, however, rare cases of congenital rubella syndrome have been documented, delivered to previously vaccinated mothers who had received only one dose of the vaccine (Miller, Cradock-Wilson, & Pollack, 1982). There is also one published case of bilateral profound hearing loss occurring in an adult following measles–rubella vaccination that has been attributed to rubella infection from the vaccine strain, although this was not confirmed by isolation of the virus (Hulbert, Larsen, Davis, & Holtom, 1991).
The U.S. CDC recommends rubella vaccination at age 12 to 15 months of age with a booster at 4 to 6 years, given as a part of the combined measles, mumps, and rubella (MMR) vaccine. Since the vaccine contains an attenuated live form of rubella, it should not be used to vaccinate during or 1 month prior to planned pregnancy (McLean et al., 2013). Published cases of accidental vaccination during pregnancy yielded no cases of congenital rubella syndrome, although 4.5% of children had serologic evidence of rubella infection (Ergenoglu et al., 2012; Nasiri, Yoseffi, Khajedaloe, Sarafraz Yazdi, & Delgoshaei, 2009; Sato et al., 2011). If a woman not known to be pregnant is vaccinated, no intervention is currently recommended. Congenital rubella syndrome has not been reported following birth from mothers who were asymptomatically infected during pregnancy. Due to successful vaccination programs, rubella is currently considered eliminated in the United States; however, cases can still occur due to importation of the infection from other countries (McLean et al., 2013).
Lymphocytic Choriomeningitis Virus
Lymphocytic choriomeningitis virus (LCMV) is a single-stranded enveloped RNA virus. LCMV is a member of the Arenaviridae family and has been identified as an emerging teratogen (Barton, Mets, & Beauchamp, 2002). Rodents, including the common house mouse, are the natural hosts and serve as reservoirs of LCMV (Jamieson, Kourtis, Bell, & Rasmussen, 2006). Infection is transmitted to humans through contact with rodent urine, feces, or saliva, and occurs more commonly in winter months when mice seek shelter indoors (Bonthius, 2012). The virus is not typically spread between humans; however, there have been cases of transmission via organ transplantation (Jamieson et al., 2006).
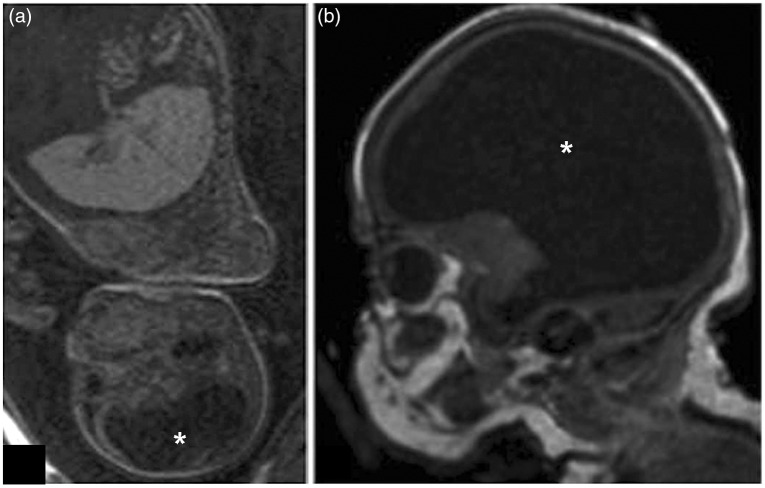
In immunocompetent adults, LCMV infection is typically either asymptomatic or associated with upper respiratory tract infection symptoms (fever, headache, nausea, and vomiting). Rarely, complications such as aseptic meningitis and meningoencephalitis occur. LCMV infection in pregnancy greatly increases the risk of spontaneous abortion. LCMV infection can also be teratogenic, especially if the virus is contracted during the first or second trimester, and is associated with microcephaly, hydrocephalus, ventriculomegaly, pachygyra, cerebellar hypoplasia, chorioretinitis, periventricular calcification, and hearing loss (Anderson et al., 2013; Bonthius, 2012; Jamieson et al., 2006; Figure 2). In contrast to congenital CMV or rubella, visual impairment and microcephaly are much more common than hearing loss in congenital LCMV infection. LCMV can also be distinguished from these other congenital viral causes of hearing loss by the lack of hepatosplenomegaly (Barton et al., 2002).
Figure 2.
Fetal (28 weeks gestation) (a) and postnatal (week of life 1) (b) MRI of infant with congenital LCMV infection, demonstrating severe ventriculomegaly (asterisks).
Note. The patient had severe growth and developmental delays, mild myopia with chorioretinopathy, profound hearing loss on the left side, and severe loss of hearing on the right.
Source: MRI images used with permission from Anderson et al. (2013).
Enzyme-linked immunosorbent assay (ELISA) for LCMV IgG and IgM antibodies can establish the diagnosis of congenital LCMV infection. Hearing loss in these patients is relatively rare, can vary in severity between ears, and ranges from severe to profound SNHL (Anderson et al., 2013; Barton et al., 2002).
Ribavirin, a nucleoside inhibitor used to stop viral RNA synthesis and capping, has been used to treat LCMV infection in adults. However, ribavirin efficacy against LCMV has not been proven in clinical trials and is associated with significant side effects such as hemolytic anemia. Ribavirin is a teratogen in many animal models and should not be used to treat pregnant women (Jamieson et al., 2006). Favipiravir, an antiviral drug that may target RNA-dependent RNA polymerase and is therefore effective against a wide range of RNA viruses, may be a future treatment option but at present has only been tested against LCMV in vitro (Bonthius, 2012).
Treatment of hearing loss in affected children with hearing aids and other assistive listening devices is indicated when appropriate. Treatment of severe to profound SNHL in children with congenital LCMV may be limited in patients in whom involvement of the vestibulocochlear nerve is the cause of hearing loss; however, because severe visual impairment is seen in all children with congenital LCMV infection, it should be attempted.
Viruses Causing Congenital and Acquired Hearing Loss
Human Immunodeficiency Virus
HIV is the retrovirus that causes AIDS. HIV is composed of a single-stranded RNA genome, which is converted to double-stranded DNA after infection of the host cell. HIV can infect a wide range of cell types but preferentially infects neurons and immune cells, particularly CD4+ T-cells. Over time, death of helper T-cells results in immunosuppression, with resultant development of opportunistic infections and cancers (Prasad, Bhojwani, Shenoy, & Prasad, 2006).
Symptoms of the initial infection are nonspecific and include fever, headache, sore throat, and myalgias. As the disease progresses and immune deficiency develops, patients develop opportunistic infections and other manifestations of HIV in multiple organ systems. Common symptoms within the temporal bone include hearing loss, tinnitus, chronic otitis media, facial nerve palsy, and malignancies (Palacios et al., 2008; Prasad et al., 2006; Rarey, 1990).
The prevalence of auditory symptoms in patients with HIV is 14% to 49% (Chandrasekhar et al., 2000; Marra et al., 1997; Rarey, 1990; van der Westhuizen, Swanepoel de, Heinze, & Hofmeyr, 2013). In a study of 200 HIV-positive South African adults, many patients complained of hearing loss (27.5%) and vertigo (25%; van der Westhuizen et al., 2013). Audiograms performed in a 1997 U.S. study of 99 HIV-positive patients detected significant hearing loss in 29%. The risk of developing hearing loss increases with the patient’s age after the third decade of life (Marra et al., 1997). Infants can develop hearing loss following either infection or exposure in utero without infection (Torre et al., 2012). In a study of 370 HIV-positive children in Uganda (mean age 38 months, range 6 months to 5 years), 33% developed hearing loss. Two-thirds of the children with hearing loss in this study had SNHL (Christopher, Edward, Sabrina, & Agnes, 2013).
Hearing loss associated with HIV infection can be unilateral or bilateral, progressive or sudden, and conductive, sensorineural, or mixed. Hearing loss in HIV-infected patients can be caused by a number of factors, including the direct effects of HIV, increased susceptibility to opportunistic infections in the middle ear and brain, and treatment with potentially ototoxic medications (Khoza-Shangase et al., 2011). CHL often results from recurrent otitis media, otitis externa, acquired aural atresia, cholesteatoma, formation of aural polyps, or malignancy (Rarey, 1990). SNHL is more common in adults and can have a variety of causes, including direct damage to the auditory system by HIV, opportunistic infections, and ototoxicity. SNHL can occur early in the course of HIV infection, even in the absence of prior treatment with antivirals or depression of CD4 counts (Mathews, Albert, & Job, 2012). Rarely, SNHL may be the only presenting symptom of HIV infection (Timon & Walsh, 1989). Typically, SNHLs are mild to moderate and predominantly include high frequencies (Chandrasekhar et al., 2000; van der Westhuizen et al., 2013). Severity of hearing loss has been correlated with disease progression and decline in CD4 counts (van der Westhuizen et al., 2013). This might lead some to suspect that hearing loss in patients with HIV might be prevented by antiretroviral therapy. However, hearing loss is still seen at higher levels in patients treated with highly active antiretroviral therapy (HAART) than in controls (Quidicomo & Matas, 2013).
A variety of studies have demonstrated that HIV may affect the auditory system both centrally and peripherally. HIV has been detected in auditory and vestibular hair cells, strial cells, and along the tectorial membrane (Pappas, Chandra, Lim, & Hillman, 1994). However, patients with HIV have abnormal brainstem responses (ABRs), suggesting involvement of the auditory nerve (Grimaldi et al., 1993; Rarey, 1990). SNHL may occur in patients with HIV as the result of increased vulnerability to other pathogens, such as syphilis, CMV, HSV, toxoplasmosis, and herpes zoster oticus (HZO), or as a result of the ototoxic medications used to treat these conditions. Because SNHL in patients with HIV may be caused by other infections as well as primary effects of HIV itself, computed tomography (CT) or magnetic resonance imaging (MRI) should be performed to rule out other central nervous system infections (Kohan, Hammerschlag, & Holliday, 1990; Torre et al., 2012). Because HIV-positive patients often present with multiple risk factors for hearing loss, it can be impossible to ethically determine the exact etiology of the observed hearing losses. Thus, development of an animal model for these studies would significantly improve understanding of the direct role of HIV in hearing loss.
HIV prevention is predominantly guided by avoidance of contact with infected blood and bodily secretions. Use of antiretroviral cocktails reduces HIV transmission from infected mothers to fetuses and breastfeeding children from 25% to 48% to 1% to 2% (Sturt, Dokubo, & Sint, 2010). Two- or three-drug antiviral regimens initiated as soon as possible (ideally within 24–36 hr) after accidental exposure to HIV-infected body fluids also greatly decreases the risk of HIV infection (Tolle & Schwarzwald, 2010). The use of HAART has dramatically improved morbidity and mortality associated with HIV infection. By increasing CD4+ T-cell counts, HAART can protect patients from opportunistic infections that can cause hearing loss. HAART use does not significantly reverse hearing loss, and one study suggested a positive correlation between hearing loss and use of antiretroviral medications in patients older than 35 years (Marra et al., 1997). However, a prospective observational study of hearing loss in patients treated with either zidovudine or didanosine concluded that antiretroviral therapy is not detrimental to hearing (Schouten, Lockhart, Rees, Collier, & Marra, 2006).
Hearing aids can be used in the treatment of HIV-infected patients with mild to moderate SNHL. For patients with severe to profound SNHL, cochlear implantation can be successful (Vincenti et al., 2005). In HIV-positive patients with stable CD4 counts, otologic surgery can be performed without additional operative risks and have favorable results (Kohan & Giacchi, 1999; Truitt & Tami, 1999). Following cochlear implantation, these patients should be closely followed to avoid development of chronic otitis media and its related complications (Vincenti et al., 2005). Chronic otitis media and cholesteatoma in HIV-infected patients can be successfully surgically treated as well (Kohan & Giacchi, 1999). However, in our experience, treatment of acquired canal atresias in HIV-infected patients has at least up to a 50% rate of late restenosis, even when CD4+ T-cell counts are stable on HAART; skin grafts, canalplasty, and meatoplasty are performed; and close follow-up with rapid treatment of otitis externa is maintained.
HSV Types 1 and 2
HSV types 1 and 2 have been implicated as causes of hearing loss. Both are encapsulated, double-stranded DNA viruses of the herpesvirus family. Infection follows contact of mucous membranes or broken skin surfaces with infected fluids from herpes sores or with other body fluids of patients with herpes. The initial infection may be asymptomatic or can be associated with symptoms. For HSV1, these symptoms can include painful blisters on the lips and tongue that eventually rupture, dysphagia, fever, myalgias, and sore throat. In the case of HSV2 primary infection, symptoms include tingling in the affected areas, followed by an erythematous papular rash that evolves into blisters and then ruptured open lesions, as well as a viral prodrome and headache. The viruses can latently infect nerve cells innervating the initially infected tissue. Months to years later, the viruses can reactivate, leading to recurrent disease. HSV type 1 is typically associated with labial herpes and type 2 with genital herpes, although either virus can infect and manifest in the other’s typical territory (Whitley & Roizman, 2001).
Congenital herpes infection typically arises due to exposure to HSV1 or HSV2 during delivery. Neonatal infection is more frequent from women who develop infection late during pregnancy or who have active herpetic lesions in the birth canal. However, 30% of pregnant women without prior history of HSV2 may be serologically positive and have asymptomatic viral shedding, which can lead to neonatal infection. Many (62%) of HSV2-infected mothers are HSV1 positive as well. Neonatal HSV1 infection occurs in 1/2,000 to 1/8,000 and HSV2 in 5.9/100,000 live births (Muller, Jones, & Koelle, 2010; Westerberg, Atashband, & Kozak, 2008). Sequelae of neonatal infection range from eye and mucous membrane involvement to disseminated disease, encephalitis, hearing loss, mental retardation, microcephaly, and death. Many infected infants will not have a vesicular rash and so may not be tested for HSV infection (reviewed in Westerberg et al., 2008).
HSV1 infection is much more frequently associated with encephalitis and hearing loss following infection in neonates compared with HSV2 (al Muhaimeed & Zakzouk, 1997). However, hearing loss following HSV1 infection is relatively rare and typically associated with concomitant severe neurological complications. Hearing loss following neonatal infection can be bilateral or unilateral severe to profound SNHL (Westerberg et al., 2008). One case series described four children with mild to moderate SNHL on ABRs but severe auditory agnosia and bilateral auditory cortex lesions on MRI after herpes encephalitis (Kaga, Kaga, Tamai, & Shindo, 2003).
HSV1 infection has also been associated with hearing loss following infection or reactivation of latent infection after infancy. Some of these cases are associated with herpes simplex meningitis or encephalitis. Hearing loss in this setting is bilateral and severe (Lavi & Sklar, 2001; Mimura et al., 2002; Montano, Melley, & Karam, 1983; Rabinstein, Jerry, Saraf-Lavi, Sklar, & Bradley, 2001). In one case, a 3-year-old boy suddenly developed a profound bilateral hearing loss following a mild febrile illness. IgG and IgM were positive for HSV1 and indicative of primary infection. He was treated with prednisone and acyclovir with partial recovery of hearing (Chand, Jan, & Vyas, 1993). In another report, a 61-year-old woman who developed oral herpes lesions and subsequent bilateral profound hearing loss had no recovery of hearing following treatment with prednisone and acyclovir. Her serological testing was consistent with HSV1 reactivation (Rabinstein et al., 2001).
Animal studies have confirmed that herpes simplex infections can cause hearing loss and vestibular symptoms. Following infection with HSV1 or HSV2, fibrosis of the scala tympani and vestibule, loss of outer hair cells, and atrophy of the stria vascularis and tectorial membrane were found in these animals. Viral antigens were located throughout the cochlea, and viral capsids were found within cochlear nerve fibers, including both afferent and efferent nerve endings. These findings were similar to those found in human temporal bone studies of patients with deafness following known viral infection with measles or rubella (Nomura, Kurata, & Saito, 1985). When treated with acyclovir and steroids, animals infected with HSV had less severe hearing loss and decreased damage to intracochlear structures than untreated, infected animals (Stokroos, Albers, & Schirm, 1999). In all of these animal studies, temporal bone changes induced by HSV infection were similar to those seen in humans with sudden SNHL (SSNHL; Esaki et al., 2011; Nomura et al., 1985; Stokroos et al., 1999).
HSV1 and HSV2 have been associated with SSNHL in some, but not all, human studies in which testing for these viruses following onset of hearing loss has been performed (Garcia Berrocal, Ramirez-Camacho, Portero, & Vargas, 2000; Koide, Yanagita, Hondo, & Kurata, 1988; Sugiura et al., 2004; Veltri, Wilson, Sprinkle, Rodman, & Kavesh, 1981; Wilson, 1986; Yoshida, Yamauchi, Shinkawa, Horiuchi, & Sakai, 1996). Additionally, HSV1 has been suggested as the etiologic agent in Meniere’s disease, although not all studies support this association (Arnold & Niedermeyer, 1997; Calenoff et al., 1995; Gartner, Bossart, & Linder, 2008; Selmani, Marttila, & Pyykko, 2005; Takahash et al., 2001; Vrabec, 2003; Welling, Miles, Western, & Prior, 1997; Yazawa, Suzuki, Hanamitsu, Kimura, & Tooyama, 2003). A randomized placebo controlled trial of the use of famciclovir, an antiherpetic agent, for patients with Meniere’s disease found a trend toward reduction in hearing fluctuation but no difference in number of episodes of dizziness that these patients experienced (Derebery, Fisher, & Iqbal, 2004).
There currently are no approved vaccines for HSV1 or HSV2. Prevention of neonatal HSV infection can be achieved by preventing transmission of herpes simplex from infected mothers to their infants during delivery. Women with primary HSV1 or HSV2 contracted during pregnancy should be treated with 400 mg of oral acyclovir three times a day (TID) for 7 to 10 days, regardless of the timing of the occurrence. In addition, the American College of Obstetricians and Gynecologists recommends herpes suppressive therapy for these women from 36 weeks gestation to delivery. Similarly, women with recurrent herpes should receive herpes suppressive therapy from 36 weeks until delivery. Cesarean delivery is recommended for women with active herpes lesions at the time of delivery (Jaiyeoba, Amaya, Soper, & Kilby, 2012).
Treatment of hearing loss associated with HSV1 or HSV2 infections includes treatment with antiherpetic agents and steroids. In the setting of encephalitis and hearing loss, standard therapy for HSV1 encephalitis (10–15 mg/kg acyclovir TID) should be performed; in the setting of meningitis and hearing loss, therapy for HSV1 meningitis may be adequate (5–10 mg/kg acyclovir TID or valacyclovir 1,000 mg TID; Studahl et al., 2013). Hearing loss that does not recover following treatment with steroids and antiherpetic agents can be remediated with hearing aids or cochlear implantation, depending on the severity of loss.
Viruses Causing Acquired Hearing Loss
Measles (Rubeola)
The measles virus (rubeola) is an enveloped single-stranded RNA virus in the paramyxovirus family. It is very easily transmitted through contact with respiratory secretions from patients with measles. Symptoms include fever, cough, nasal congestion, erythematous maculopapular rash, conjunctivitis, and pathognomonic Koplik spots on the buccal mucosa. Hearing loss is a common complication of measles infection; prior to widespread vaccination, measles accounted for 5 to 10% of cases of profound hearing loss in the United States (McKenna, 1997). In 2000, measles was declared eliminated from the United States, although cases resulting from immigration or importation of the virus continue to occur (McLean et al., 2013). Measles continues to be a common cause of profound hearing loss in areas in which measles vaccination is rare (Dunmade, Segun-Busari, Olajide, & Ologe, 2007).
Fortunately, most children recover from measles without long-term side effects. However, due to measles’ virulence, it was a nearly ubiquitous viral infection of childhood prior to the development of vaccines, and so complications that appear to be infrequent on a percentage based on the total number of individuals infected actually affected a large number of people. Prior to the effective vaccination, measles was thought to be the cause of severe to profound hearing loss in 4% to 9% of deaf patients (Suboti, 1976). Hearing loss resulting from measles infection is typically bilateral, moderate to profound SNHL and may follow measles encephalitis (McKenna, 1997). Temporal bone studies on infected humans and in animal models have shown degeneration of cochlear neurons most prominently in the basal turn, degeneration of the organ of Corti and stria vascularis, and cellular infiltration of the cochlea (Fukuda, Ishikawa, & Inuyama, 1994; McKenna, 1997; Suboti, 1976). Measles is associated with a high incidence of otitis media (in up to 8.5–25% of infected people, higher in developing countries), possibly due to a transient decrease in the immune response to infection (Stephenson, 2002). Sequelae of bacterial superinfection may account for some cases of hearing loss associated with measles infection (Suboti, 1976).
The mainstay of measles prevention is through MMR vaccination, with the first dose at age 12 to 15 months and the second at 4 to 6 years of age (the third dose of MMR in the teens/early twenties is indicated for prevention of mumps). Prior to the development of widespread vaccination, measles would infect >90% of susceptible children in epidemics. Compilation of infection data from multiple countries shows 70.9 cases of rubeola/100,000 unvaccinated people compared with 0.9 cases/100,000 people in countries in which 90% to 100% of the target population of children had received just one dose of measles vaccine (Hall & Jolley, 2011). As noted previously, MMR includes live-attenuated measles virus, and so cannot be used in immunocompromised patients, although recent guidelines have been revised to recommend MMR vaccination in patients aged ≥12 months with HIV who do not have severe immunosuppression (McLean et al., 2013). MMR and other anti-measles vaccinations are rarely associated with SNHL, with a time course of onset corresponding to incubation period of measles infection and an incidence of 1 case per 6 to 8 million vaccine doses. Because MMR vaccine also includes live attenuated mumps virus, it is possible that the measles vaccine is not the sole cause of these cases. SNHL associated with vaccination ranges in severity, can be bilateral or unilateral, and has been reported in both pediatric and adult populations. In some of these patients, symptoms such as fever, rash, headache, and ataxia have been reported after vaccination and prior to onset of hearing loss, consistent with the possibility that hearing loss is the result of vaccination-induced encephalitis (Asatryan et al., 2008; Brodsky & Stanievich, 1985). A review by the Institute of Medicine concluded that while hearing loss due to the measles vaccine strain was possible, there was insufficient evidence to make conclusions regarding a causal relationship (Stratton, Howe, & Johnston, 1994).
Postexposure prophylaxis is through the use of intramuscularly administered measles immune globulin for infants aged 0 to 6 months and in older immunocompetent people. In pregnant woman without evidence of measles immunity and in severely immunocompromised patients, the measles immune globulin should be administered intravenously (McLean et al., 2013). Treatment for measles infection is supportive, including intravenous fluids for dehydration and diarrhea. Secondary infections, including pneumonia, can occur and should be treated promptly (Sabella, 2010).
Treatment of hearing loss resulting from measles depends on the degree and type of hearing loss noted. Hearing aids can be used by patients with mild to moderate SNHL, while cochlear implantation is effective for patients with severe to profound SNHL (McKenna, 1997).
Measles infection has been hypothesized to cause otosclerosis, which causes stapes fixation and subsequent CHL as well as SNHL due to the formation of abnormal foci of bone remodeling in the middle and inner ear. Measles antigens have been identified within otosclerotic lesions, and histological and polymerase chain reaction studies of otosclerotic stapes footplates are suggestive of measles infection (Karosi, Konya, Petko, & Sziklai, 2005). Rates of otosclerosis are higher in patients who have not been vaccinated against measles (Niedermeyer & Arnold, 2008). Patients with otosclerosis can be treated with either hearing amplification or stapedotomy for their conductive loss and with hearing aids for SNHL. For patients with severe SNHL from otosclerosis, cochlear implantation or stapedotomy for far advanced otosclerosis plus a postoperative hearing aid can rehabilitate hearing (Semaan et al., 2012).
Varicella Zoster Virus
VZV is a double-stranded enveloped DNA virus of the Herpesviridae family. VZV is a highly contagious virus that is transmitted either by droplets from coughing or sneezing of actively infected individuals or by direct contact with fluid from herpetic vesicles. VZV-infected patients are infectious from 2 days prior to appearance of the viral rash until after all the vesicles have crusted. VZV first causes a primary infection that, when symptomatic, manifests with fever, an erythematous macular rash, and pustules (chickenpox). In some individuals, the primary infection is asymptomatic. The virus can subsequently remain latent in neurons in various parts of the body for an extended period, reactivating years later. Symptoms of VZV reactivation (e.g., zoster or shingles) include both systematic symptoms (fever and malaise) as well as local symptoms (severe pain and a vesicular rash) that are limited to the area innervated by the neurons in which the virus reactivated. Risk factors for viral reactivation include age >50 years, pregnancy/postpartum states, and immunocompromise (Kansu & Yilmaz, 2012; Sweeney & Gilden, 2001).
Reactivation of latent VZV within the geniculate ganglion causes Ramsay Hunt syndrome or HZO through the development of geniculate ganglionitis and inflammation of the facial nerve. Eighth nerve involvement results from transfer of the virus from the nearby geniculate ganglion or directly from the facial nerve within the internal auditory canal. Symptoms include facial nerve paralysis, herpetic vesicles, severe otalgia, SNHL (24% of affected patients), tinnitus (48%), and vertigo (30%; Sweeney & Gilden, 2001; Figure 3). Additional symptoms such as ipsilateral loss of lacrimation and impaired sense of taste in the ipsilateral two thirds of the tongue result from damage to the facial nerve. Erythematous vesicles that ultimately erupt and crust can typically be seen on the pinna, external auditory canal, tympanic membrane, hard palate, and tongue (Kansu & Yilmaz, 2012). Rarely, patients can present with all of the signs and symptoms of HZO without a herpetiform rash (zoster sine herpete; Sweeney & Gilden, 2001). Although the facial, trigeminal, and vestibular nerves are most commonly affected, cases have been reported involving the glossopharyngeal and vagus nerves with resulting dysphagia and hoarseness (Lin, Kao, & Wang, 2011).
Figure 3.
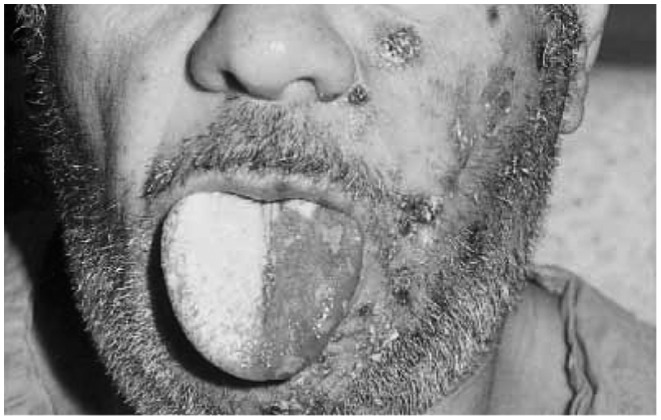
Herpes zoster oticus, with crusted vesicles on the face in the left V2 distribution and vesicles and swelling of the left tongue.
Source: Clinical photograph used with permission from Braverman Uri, and Greenberg (2000).
SNHL is unilateral and can range from a mild, high frequency loss to profound; however, it is usually mild to moderate. Occasionally, SNHL can occur suddenly as the first manifestation of HZO, and so must be included in the differential diagnosis of SSNHL (Wayman, Pham, Byl, & Adour, 1990). Patients with HZO have been found to have ABR changes consistent with both cochlear and retrocochlear dysfunction (Abramovich & Prasher, 1986).
The diagnosis of HZO is usually made based on history and physical examination. Enhancement of the seventh and eighth cranial nerves on gadolinium-enhanced, T1-weighted MRI can be observed (Gantz, Redleaf, Perry, & Gubbels, 2010). Hemorrhage of the cochlear nerve and destruction of the apex of the organ of Corti have been reported. Temporal bone studies of affected individuals have shown loss of geniculate ganglion cells with neuronal swelling and chromatolysis, perivascular lymphocytic infiltrates, neural demyelination, and axonal loss (Aleksic, Budzilovich, & Lieberman, 1973).
Hearing loss from HZO may improve following treatment, while facial nerve recovery is reported less frequently (Ohtani, Furuta, Aizawa, & Fukuda, 2006; Wayman et al., 1990). Current therapy for HZO involves high doses of both antiherpetic agents and corticosteroids. In one retrospective study of treatment outcomes, facial nerve recovery from HZO occurred in 75% of patients in whom therapy with prednisone and acyclovir was initiated within 72 hr of onset of symptoms. If treatment was initiated before 10 days of onset of HZO, 52% recovered facial function to House–Brackmann grade I. In this study, 22 of 80 patients complained of hearing loss, and of those, 12 patients were monitored with repeated audiograms. Complete hearing recovery was noted in six patients, near complete in three, and no improvement in six (Murakami et al., 1997). Topical lidocaine or gabapentin can be used to alleviate otalgia. Diazepam has been used to treat vertigo associated the viral labyrinthitis in HZO (Nalamachu & Morley-Forster, 2012).
Negative prognostic factors for hearing recovery in HZO include older age, male gender, the presence of vertigo, and hearing impairment within speech frequencies (Wayman et al., 1990). High-frequency SNHL indicates a better prognosis for complete recovery. The severity of facial nerve paralysis does not necessarily correlate with the severity or prognosis of auditory and vestibular symptoms (Gantz et al., 2010).
Control of primary varicella infection may decrease the incidence of HZO. Prior to the advent of vaccination for varicella, chickenpox was an extremely common childhood disease with an annual incidence of 15 to 16 per 1,000 population. The U.S. CDC recommends vaccination of children using one of two licensed vaccines. VARIVAX, a single-antigen live-attenuated varicella vaccine, has been available in the United States since 1995 for use in healthy patients aged 12 months and older. A second vaccine, ProQuad (MMRV), includes the live-attenuated antivaricella vaccine as well as the MMR vaccine. These vaccines are thought to reduce the incidence of primary VZV infection by up to 96%. Recommendations include two doses of the vaccine, the first at 12 to 15 months of age and the second at age 4 to 6 years. For teens without evidence of immunity, the recommendations include two doses of VARIVAX separated by 4 to 8 weeks between doses. Because the vaccines include live-attenuated virus, they should not be administered to patients with decreased humoral immunity or to pregnant women. However, these vaccines can be administered to patients with HIV and breastfeeding mothers (Marin, Guris, Chaves, Schmid, & Seward, 2007). Accidental VZV exposure of immunocompetent adults and children who do not have serologic evidence of immunity to varicella is countered by administration of VARIVAX; patients with impaired B-cell immunity should be treated with intravenous antivaricella immunoglobulin following VZV exposure (CDC, 2012).
Prevention of VZV reactivation is also possible via immunization with Zostavax, a live-attenuated antivaricella vaccine that currently is recommended in the United States for patients aged 50 years and older (CDC, 2011). This vaccine utilizes the same attenuated strain as the VARIVAX and MMRV vaccines but at a higher potency. Fifteen percent to 30% of individuals who were infected with VZV experience reactivation later in life (up to 4.2 per 1,000 U.S. population); Zostavax decreases this incidence by 50% (Harpaz, Ortega-Sanchez, & Seward, 2008). While the direct impact of varicella immunization on HZO has not been measured, presumably the incidence of these cases will also decrease.
Permanent hearing impairment occurs in about 5% of cases of HZO (Murakami et al., 1997; Wayman et al., 1990). In these cases, SNHL results from damage to both the cochlea and more proximal auditory pathways (Abramovich & Prasher, 1986; Kaberos, Balatsouras, Korres, Kandiloros, & Economou, 2002). Nonpharmacological treatment is focused on the use of hearing aids to remediate mild to moderately severe SNHL.
Mumps
The mumps virus is an enveloped single-stranded RNA virus that belongs to the paramyxovirus family, which also includes measles. Mumps is transmitted through infected respiratory secretions and is highly contagious (Gupta, Best, & MacMahon, 2005). While mumps is one of the most common causes of acquired SNHL, its incidence varies greatly between studies. Estimates of the incidence of hearing loss following mumps infection range from 1 per 1,000 to 1 per 30,000 to as high as 3 per 100 in the 1984 Israeli epidemic (Hashimoto, Fujioka, & Kinumaki, 2009; Kanra et al., 2002). Differences in vaccination practices between nations may underlie this variance in SNHL. For instance, Hashimoto et al. (2009) have reported an incidence of 0.1% in Japan, where measles–rubella vaccine was more commonly administered than the MMR.
Initially, mumps presents with symptoms of a flu-like illness, followed by bilateral swelling of the parotid glands (Elliman, Sengupta, El Bashir, & Bedford, 2007). Mumps can additionally cause SNHL, pancreatitis, orchitis, oophoritis, and infertility (Senanayake, 2008). Aseptic meningitis and encephalitis can also occur, and their presence increases the risk of SNHL (Kanra et al., 2002). Mumps increases the risk of spontaneous abortion if acquired during the first trimester of gestation. However, mumps is not believed to cause congenital abnormalities (Gupta et al., 2005).
The diagnosis of mumps can usually be made by the history and physical examination. Confirmation of the diagnosis is obtained through salivary anti-IgM testing (Gupta et al., 2005).
SNHL tends to occur suddenly 4 to 5 days after the onset of flu-like symptoms and parotitis (Hall & Richards, 1987). Typically, hearing loss is unilateral and reversible but can be severe and permanent (Elliman et al., 2007; Hashimoto et al., 2009). Everberg (1957) calculated that permanent profound hearing loss occurred in 0.05 of 1,000 cases in patients who had not been vaccinated. Temporal bone studies of patients with mumps SNHL demonstrate nonspecific pathology and are difficult to differentiate from studies of patients with rubella or measles (Westmore, Pickard, & Stern, 1979). The virus has been detected in both endolymph and perilymph (Kanra et al., 2002). Proposed mechanisms of SNHL include atrophy of hair cells in the organ of Corti and stria vascularis, and damage to the myelin sheath around the vestibulocochlear nerve (Kanra et al., 2002; McKenna, 1997; Westmore et al., 1979). Brainstem regions may also be involved (Tsubota, Shojaku, Ishimaru, Fujisaka, & Watanabe, 2008). Reversible vestibular dysfunction has also been reported in patients with mumps, suggesting that the eighth nerve is involved in the pathology because the utricle, saccule, and semicircular canals are not affected (McKenna, 1997; Tsubota et al., 2008).
The risk of SNHL following mumps infection is not correlated with severity of the infection or presence of parotitis (Hall & Richards, 1987). Asymptomatic cases of mumps can result in sudden SNHL, as demonstrated by positive antimumps IgM antibodies (Hashimoto et al., 2009; Nomura, Harada, Sakata, & Sugiura, 1988).
The MMR vaccine, introduced in 1949, has drastically reduced the incidence of mumps. Despite the availability of an effective vaccine, several recent outbreaks have occurred, including a 2005 epidemic of 56,000 cases in the United Kingdom among young adults who did not receive adequate immunization (Gupta et al., 2005; Senanayake, 2008). The epidemiology of recent cases differs from that seen in the pre-MMR era, as most cases are observed in patients between 15 and 24 years, rather than in younger children (Senanayake, 2008). Today, three doses of MMR vaccine are recommended for mumps prevention—the first at one dose at age 12 to 15 months, the second at 4 to 6 years of age, and the third dose in the late teens/early twenties. Because mumps vaccine contains live, attenuated virus, it is not recommended for severely immunocompromised patients, although current guidelines recommend MMR vaccination for patients with HIV as long as they do not fall into the severely immunocompromised category (McLean et al., 2013). Recent outbreaks of mumps have occurred despite vaccination, mainly associated with patients who had received only two doses of the vaccine and who were in crowded conditions, such as densely populated schools. In the 2006 outbreak in the United States, 63% of those affected had received two doses of the mumps vaccine. Eleven out of the 4,039 patients who contacted mumps developed unilateral or bilateral severe to profound SNHL. Of the 10 patients with hearing loss with complete data, none developed permanent hearing loss, suggesting that vaccination may limit the number of patients with mumps who develop permanent severe hearing impairment (Dayan et al., 2008).
There is no specific treatment against mumps virus, so interventions are mostly supportive (Gupta et al., 2005). For treatment of SNHL related to mumps, hearing aids can be utilized for mild to severe cases. In severe bilateral cases of SNHL, cochlear implantation has been shown to be efficacious (Suzuki et al., 2009; Wang, Cao, Yang, Wei, & Zheng, 2003).
West Nile Virus
West Nile virus (WNV) is a member of the Flaviviridae family which also includes dengue and yellow fever virus. WNV, like other members of this viral family, has a single-stranded RNA genome and is transmitted by an insect vector (Hayes, Sejvar, et al., 2005). Viral transmission of WNV typically occurs through mosquito bites, but may also be acquired congenitally, through breastfeeding, or through blood transfusions from infected individuals. WNV was initially identified in Uganda in 1940 and is most prevalent in Africa and the Middle East. WNV infection was not seen in the United States until 1999, when an outbreak occurred in New York City. Since that initial outbreak, instances of WNV have been reported throughout the East Coast and the midwestern United States (Hayes, Komar, et al., 2005).
Only 20% of WNV infections are symptomatic, and most commonly present with a flu-like illness (Hayes, Sejvar, et al., 2005). However, neurological complications, such as meningitis, encephalitis, and acute flaccid paralysis, occur in less than 1% of cases. These manifestations are more common in elderly or immunocompromised patients. In most cases, the severe neurologic complications eventually resolve with only supportive treatment, although some patients may suffer from persistent flaccid paralysis (Sejvar et al., 2003).
Hearing loss resulting from WNV infection is extremely rare. Similar to other neurologic symptoms, hearing loss is also reported more frequently in immunocompromised patients and often recovers spontaneously (Jamison, Michaels, Ratard, Sweet, & Deboisblanc, 2007; McBride, Gill, & Wiviott, 2006). However, at least one case of SNHL in a WNV-infected, immunocompetent man did not resolve, leaving this patient with a permanent downsloping moderate to severe SNHL (Casetta et al., 2011).
There is no currently Food and Drug Administration-approved vaccine against WNV, although efforts to approve an inactivated WNV vaccine are ongoing. Prevention of WNV is focused on mosquito control programs because bites from infected mosquitoes are the most common route of infection (Hayes, Komar, et al., 2005). There is also no specific treatment approved for WNV-infected patients. Ribavarin, interferon alpha 2 b, and intravenous immunoglobulin have been used for patients with severe neurological manifestations of WNV on a compassionate basis, but none of these treatments have been proven in clinical trials (Hayes, Sejvar, et al., 2005).
Conclusion
A number of viral infections can cause hearing loss. A baseline knowledge of these viruses is critical in the recognition of their involvement in hearing loss in affected patients. Because some of these infections and the hearing loss that they cause can be treated with specific therapy, knowledge of these entities becomes even more important in the evaluation and management of patients with hearing loss. Hearing health care providers may encounter frequent questions from parents of children with hearing loss, questioning whether specific viral infections or vaccinations for these viruses have caused their child’s hearing loss.
In this review, we have discussed some of the more commonly known viral causes of hearing loss as well as a few emerging viral infections that ultimately may be shown to be frequent causes of deafness. Some cause congenital hearing loss due to infection of the fetus in utero. Others cause hearing loss as a result of infection in childhood or adulthood. Hearing loss following viral infection is often sensorineural, although it may be mixed (CMV, measles) or conductive (measles). Auditory system damage is typically intracochlear; however, some viruses can affect the auditory brainstem as well. Mechanisms of injury to the peripheral auditory system can include direct viral damage to the organ of Corti, stria vascularis, or spiral ganglion; damage mediated by the patient’s immune system against virally expressed proteins (CMV); and immunocompromise leading to secondary bacterial infection of the ear (HIV, measles). Hearing loss due to HZO or CMV infection can be treated medically with stabilization or improvement in hearing thresholds. Common childhood vaccines can prevent several of the viral infections discussed within this review and should be recommended to patients and parents. The incidence of hearing loss following vaccination with live-attenuated virus vaccines, such as the MMR and MMRV, is extremely rare. Rehabilitation of hearing loss due to other viruses typically involves hearing aids, with cochlear implantation for patients with severe to profound hearing loss.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health/National Institute of Deafness and Other Common Disorders Grant K08 DC009288.
References
- Abramovich S., Prasher D. K. (1986) Electrocochleography and brain-stem potentials in Ramsay Hunt syndrome. Archives of Otolaryngology Head and Neck Surgery 112(9): 925–928. [DOI] [PubMed] [Google Scholar]
- Adler S. P. (2005) Congenital cytomegalovirus screening. Pediatric Infectious Disease Journal 24(12): 1105–1106. [DOI] [PubMed] [Google Scholar]
- Aleksic S. N., Budzilovich G. N., Lieberman A. N. (1973) Herpes zoster oticus and facial paralysis (Ramsay Hunt syndrome). Clinicopathologic study and review of literature. Journal of the Neurological Sciences 20(2): 149–159. [DOI] [PubMed] [Google Scholar]
- al Muhaimeed H., Zakzouk S. M. (1997) Hearing loss and herpes simplex. Journal of Tropical Pediatrics 43(1): 20–24. [DOI] [PubMed] [Google Scholar]
- Anderson, J. L., Levy, P. T., Leonard, K. B., Smyser, C. D., Tychsen, L., & Cole, F. S. (2013). Congenital lymphocytic choriomeningitis virus: When to consider the diagnosis. Journal of Child Neurology, 29(6), 837–842. [DOI] [PMC free article] [PubMed]
- Arnold W., Niedermeyer H. P. (1997) Herpes simplex virus antibodies in the perilymph of patients with Meniere disease. Archives of Otolaryngology Head and Neck Surgery 123(1): 53–56. [DOI] [PubMed] [Google Scholar]
- Asatryan A., Pool V., Chen R. T., Kohl K. S., Davis R. L., Iskander J. K. The VAERS team (2008) Live attenuated measles and mumps viral strain-containing vaccines and hearing loss: Vaccine Adverse Event Reporting System (VAERS), United States, 1990–2003. Vaccine 26(9): 1166–1172 7. [DOI] [PubMed] [Google Scholar]
- Bale J. F. Jr. (2012) Cytomegalovirus infections. Seminars in Pediatric Neurology 19(3): 101–106. [DOI] [PubMed] [Google Scholar]
- Barbi M., Binda S., Caroppo S., Primache V. (2006) Neonatal screening for congenital cytomegalovirus infection and hearing loss. Journal of Clinical Virology 35(2): 206–209. [DOI] [PubMed] [Google Scholar]
- Barton L. L., Mets M. B., Beauchamp C. L. (2002) Lymphocytic choriomeningitis virus: Emerging fetal teratogen. American Journal of Obstetrics and Gynecology 187(6): 1715–1716. [DOI] [PubMed] [Google Scholar]
- Bonthius D. J. (2012) Lymphocytic choriomeningitis virus: An underrecognized cause of neurologic disease in the fetus, child, and adult. Seminars in Pediatric Neurology 19(3): 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman I., Uri N., Greenberg E. (2000) Trigeminal herpes zoster/chocolate-vanilla tongue. Otolaryngology Head and Neck Surgery 122(3): 463. [DOI] [PubMed] [Google Scholar]
- Brodsky L., Stanievich J. (1985) Sensorineural hearing loss following live measles virus vaccination. International Journal of Pediatric Otorhinolaryngology 10: 159–163. [DOI] [PubMed] [Google Scholar]
- Calenoff E., Zhao J. C., Derlacki E. L., Harrison W. H., Selmeczi K., Dutra J. C., Hanson D. G. (1995) Patients with Meniere’s disease possess IgE reacting with herpes family viruses. Archives of Otolaryngology Head and Neck Surgery 121(8): 861–864. [DOI] [PubMed] [Google Scholar]
- Carlson A., Norwitz E. R., Stiller R. J. (2010) Cytomegalovirus infection in pregnancy: Should all women be screened? Review of Obstetrics & Gynecology 3(4): 172–179. [PMC free article] [PubMed] [Google Scholar]
- Casetta I., Ciorba A., Cesnik E., Trevisi P., Tugnoli V., Bovo R. (2011) West Nile virus neuroinvasive disease presenting with acute flaccid paralysis and bilateral sensorineural hearing loss. Journal of Neurology 258(10): 1880–1881. [DOI] [PubMed] [Google Scholar]
- Chand R. P., Jan A., Vyas H. (1993) Acute sensorineural deafness following herpes simplex infection. European Journal of Pediatrics 152(4): 379. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S. S., Connelly P. E., Brahmbhatt S. S., Shah C. S., Kloser P. C., Baredes S. (2000) Otologic and audiologic evaluation of human immunodeficiency virus-infected patients. American Journal of Otolaryngology 21(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Christopher N., Edward T., Sabrina B. K., Agnes N. (2013) The prevalence of hearing impairment in the 6 months-5 years HIV/AIDS-positive patients attending paediatric infectious disease clinic at Mulago Hospital. International Journal of Pediatric Otorhinolaryngology 77(2): 262–265. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) Update on herpes zoster vaccine: Licensure for persons aged 50 through 59 years. MMWR Morbidity and Mortality Weekly Report 60(44): 1528. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2012) FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella. MMWR Morbidity and Mortality Weekly Report 61(12): 212. [PubMed] [Google Scholar]
- Dammeyer J. (2010) Congenital rubella syndrome and delayed manifestations. International Journal of Pediatric Otorhinolaryngology 74(9): 1067–1070. [DOI] [PubMed] [Google Scholar]
- da Silva L. P. A., Queiros F., Lima I. (2006) Etiology of hearing impairment in children and adolescents of a reference center APADA in the city of Salvador, state of Bahia. Revista Brasileira de Otorrinolaringologia 72(1): 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan G. H., Quinlisk P., Parker A. A., Barskey A. E., Harris M. L., Schwartz J. M. H., Seward J. F. (2008) Recent resurgence of mumps in the United States. New England Journal of Medicine 335(15): 1580–1589. [DOI] [PubMed] [Google Scholar]
- De Leenheer E. M., Janssens S., Padalko E., Loose D., Leroy B. P., Dhooge I. J. (2011) Etiological diagnosis in the hearing impaired newborn: Proposal of a flow chart. International Journal of Pediatric Otorhinolaryngology 75(1): 27–32. [DOI] [PubMed] [Google Scholar]
- Derebery M. J., Fisher L. M., Iqbal Z. (2004) Randomized double-blinded, placebo-controlled clinical trial of famciclovir for reduction of Meniere’s disease symptoms. Otolaryngology Head and Neck Surgery 131(6): 877–884. [DOI] [PubMed] [Google Scholar]
- Donley D. K. (1993) TORCH infections in the newborn. Seminars in Neurology 13(1): 106–115. [DOI] [PubMed] [Google Scholar]
- Dunmade A. D., Segun-Busari S., Olajide T. G., Ologe F. E. (2007) Profound bilateral sensorineural hearing loss in Nigerian children: Any shift in etiology? Journal of Deaf Studies and Deaf Education 12(1): 112–118. [DOI] [PubMed] [Google Scholar]
- Elliman D., Sengupta N., El Bashir H., Bedford H. (2007) Measles, mumps, and rubella: Prevention. Clinical Evidence (Online): 316. [PubMed] [Google Scholar]
- Ergenoglu A. M., Yeniel A. O., Yildirim N., Kazandi M., Akercan F., Sagol S. (2012) Rubella vaccination during the preconception period or in pregnancy and perinatal and fetal outcomes. Turkish Journal of Pediatrics 54(3): 230–233. [PubMed] [Google Scholar]
- Esaki S., Goshima F., Kimura H., Ikeda S., Katsumi S., Kabaya K., Murakami S. (2011) Auditory and vestibular defects induced by experimental labyrinthitis following herpes simplex virus in mice. Acta Otolaryngologica 131(7): 684–691. [DOI] [PubMed] [Google Scholar]
- Everberg G. (1957) Deafness following mumps. Acta Otolaryngologica 58(5–6): 397–403. [DOI] [PubMed] [Google Scholar]
- Foulon I., Naessens A., Foulon W., Casteels A., Gordts F. (2008) A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. Journal of Pediatrics 153(1): 84–88. [DOI] [PubMed] [Google Scholar]
- Fowler K. B., Boppana S. B. (2006) Congenital cytomegalovirus (CMV) infection and hearing deficit. Journal of Clinical Virology 35(2): 226–231. [DOI] [PubMed] [Google Scholar]
- Fowler K. B., Dahle A. J., Boppana S. B., Pass R. F. (1999) Newborn hearing screening: Will children with hearing loss caused by congenital cytomegalovirus infection be missed? Journal of Pediatrics 135(1): 60–64. [DOI] [PubMed] [Google Scholar]
- Fowler K. B., McCollister F. P., Dahle A. J., Boppana S., Britt W. J., Pass R. F. (1997) Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. Journal of Pediatrics 130(4): 624–630. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Ishikawa K., Inuyama Y. (1994) Acute measles infection in the hamster cochlea. Acta Otolaryngologica Supplement 514: 111–116. [DOI] [PubMed] [Google Scholar]
- Gantz B. J., Redleaf M., Perry B. P., Gubbels S. P. (2010) Chapter 28: Management of Bell’s Palsy and Ramsay Hunt Syndrome. In: Brackmann D. E., Shelton C., Arriaga A. A. (eds) Otologic surgery, 3rd ed Philadelphia, PA: Saunders Elsevier, pp. 335–346. [Google Scholar]
- Garcia Berrocal J. R. G., Ramirez-Camacho R., Portero F., Vargas J. A. (2000) Role of viral and Mycoplasma pneumoniae infection in idiopathic sudden sensorineural hearing loss. Acta Otolaryngologica 120(7): 835–839. [DOI] [PubMed] [Google Scholar]
- Gartner M., Bossart W., Linder T. (2008) Herpes virus and Meniere’s disease. ORL Journal of Oto-Rhino-laryngology and Its Related Specialties 70(1): 28–31 ; discussion 31. [DOI] [PubMed] [Google Scholar]
- Grimaldi L. M., Luzi L., Martino G. V., Furlan R., Nemni R., Antonelli A., Pozza G. (1993) Bilateral eighth cranial nerve neuropathy in human immunodeficiency virus infection. Journal of Neurology 240(6): 363–366. [DOI] [PubMed] [Google Scholar]
- Grosse S. D., Ross D. S., Dollard S. C. (2008) Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: A quantitative assessment. Journal of Clinical Virology 41(2): 57–62. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Best J., MacMahon E. (2005) Mumps and the UK epidemic 2005. British Medical Journal 330(7500): 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R., Jolley D. (2011) International measles incidence and immunization coverage. The Journal of Infectious Diseases 204: S158–S163. [DOI] [PubMed] [Google Scholar]
- Hall R., Richards H. (1987) Hearing loss due to mumps. Archives of Disease in Childhood 62(2): 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz R., Ortega-Sanchez I. R., Seward J. F. (2008) Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 57(RR-5): 1–30 quiz CE32–34. [PubMed] [Google Scholar]
- Hashimoto H., Fujioka M., Kinumaki H. (2009) An office-based prospective study of deafness in mumps. The Pediatric Infectious Disease Journal 28(3): 173–175. [DOI] [PubMed] [Google Scholar]
- Hayes E. B., Komar N., Nasci R. S., Montgomery S. P., O’Leary D. R., Campbell G. L. (2005) Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infectious Diseases 11(8): 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. B., Sejvar J. J., Zaki S. R., Lanciotti R. S., Bode A. V., Campbell G. L. (2005) Virology, pathology, and clinical manifestations of West Nile virus disease. Emerging Infectious Diseases 11(8): 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert T. V., Larsen R. A., Davis C. L., Holtrom P. D. (1991) Bilateral hearing loss after measles and rubella vaccination in an adult. New England Journal of Medicine 325(2): 134. [DOI] [PubMed] [Google Scholar]
- Jaiyeoba O., Amaya M. I., Soper D. E., Kilby J. M. (2012) Preventing neonatal transmission of herpes simplex virus. Clinical Obstetrics and Gynecology 55(2): 510–520. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Kourtis A. P., Bell M., Rasmussen S. A. (2006) Lymphocytic choriomeningitis virus: An emerging obstetric pathogen? American Journal of Obstetrics & Gynecology 194(6): 1532–1536. [DOI] [PubMed] [Google Scholar]
- Jamison S. C., Michaels S. R., Ratard R., Sweet J. M., Deboisblanc B. P. (2007) A 41-year-old HIV-positive man with acute onset of quadriplegia after West Nile virus infection. South African Medical Journal 100(10): 1051–1053. [DOI] [PubMed] [Google Scholar]
- Kaberos A., Balatsouras D. G., Korres S. G., Kandiloros D., Economou C. (2002) Audiological assessment in Ramsay Hunt syndrome. Annals of Otology, Rhinology & Laryngology 111(1): 68–76. [DOI] [PubMed] [Google Scholar]
- Kaga K., Kaga M., Tamai F., Shindo M. (2003) Auditory agnosia in children after herpes encephalitis. Acta Otolaryngologica 123(2): 232–235. [DOI] [PubMed] [Google Scholar]
- Kanra G., Kara A., Cengiz A. B., Isik P., Ceyhan M., Atas A. (2002) Mumps meningoencephalitis effect on hearing. Pediatric Infectious Disease Journal 21(12): 1167–1169. [DOI] [PubMed] [Google Scholar]
- Kansu L., Yilmaz I. (2012) Herpes zoster oticus (Ramsay Hunt syndrome) in children: Case report and literature review. International Journal of Pediatric Otorhinolaryngology 76(6): 772–776. [DOI] [PubMed] [Google Scholar]
- Karosi T., Konya J., Petko M., Sziklai I. (2005) Histologic otosclerosis is associated with the presence of measles virus in the stapes footplate. Otology & Neurotology 26(6): 1128–1133. [DOI] [PubMed] [Google Scholar]
- Kimberlin D. W., Lin C. Y., Sanchez P. J., Demmler G. J., Dankner W., Shelton M. the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (2003) Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: A randomized, controlled trial. Journal of Pediatrics 143(1): 16–25. [DOI] [PubMed] [Google Scholar]
- Kohan D., Giacchi R. J. (1999) Otologic surgery in patients with HIV-1 and AIDS. Otolaryngology Head and Neck Surgery 121(4): 355–360. [DOI] [PubMed] [Google Scholar]
- Kohan D., Hammerschlag P. E., Holliday R. A. (1990) Otologic disease in AIDS patients: CT correlation. Laryngoscope 100(12): 1326–1330. [DOI] [PubMed] [Google Scholar]
- Koide J., Yanagita N., Hondo R., Kurata T. (1988) Serological and clinical study of herpes simplex virus infection in patients with sudden deafness. Acta Otolaryngologica Supplement 456: 21–26. [DOI] [PubMed] [Google Scholar]
- Lavi E. S., Sklar E. M. (2001) Enhancement of the eighth cranial nerve and labyrinth on MR imaging in sudden sensorineural hearing loss associated with human herpesvirus 1 infection: Case report. AJNR American Journal of Neuroradiology 22(7): 1380–1382. [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Bowden D. S. (2000) Rubella virus replication and links to teratogenicity. Clinical Microbiology Reviews 13(4): 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Y., Kao C. H., Wang C. H. (2011) Varicella zoster virus infection of the pharynx and larynx with multiple cranial neuropathies. Laryngoscope 121(8): 1627–1630. [DOI] [PubMed] [Google Scholar]
- Madden C., Wiley S., Schleiss M., Benton C., Meinzen-Derr J., Greinwald J., Choo D. (2005) Audiometric, clinical and educational outcomes in a pediatric symptomatic congenital cytomegalovirus (CMV) population with sensorineural hearing loss. International Journal of Pediatric Otorhinolaryngology 69(9): 1191–1198. [DOI] [PubMed] [Google Scholar]
- Marin M., Guris D., Chaves S. S., Schmid S., Seward J. F. (2007) Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 56(RR-4): 1–40. [PubMed] [Google Scholar]
- Marra C. M., Wechkin H. A., Longstreth W. T., Jr., Rees T. S., Syapin C. L., Gates G. A. (1997) Hearing loss and antiretroviral therapy in patients infected with HIV-1. Archives of Neurology 54(4): 407–410. [DOI] [PubMed] [Google Scholar]
- Mathews S. S., Albert R. R., Job A. (2012) Audio-vestibular function in human immunodeficiency virus infected patients in India. Indian Journal of Sexually Transmitted Diseases 33(2): 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W., Gill K. R., Wiviott L. (2006) West Nile Virus infection with hearing loss. Journal of Infection 53(5): e203–e205. [DOI] [PubMed] [Google Scholar]
- McKenna M. J. (1997) Measles, mumps, and sensorineural hearing loss. Annals of the New York Academy of Sciences 830: 291–298. [DOI] [PubMed] [Google Scholar]
- McLean H. Q., Fiebelkorn A. P., Temte J. L., Wallace G. S. (2013) Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 62(RR-04): 1–34. [PubMed] [Google Scholar]
- Miller E., Cradock-Wilson J. E., Pollock T. M. (1982) Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 2(8302): 781–784. [DOI] [PubMed] [Google Scholar]
- Mimura T., Amano S., Nagahara M., Oshika T., Tsushima K., Nakanishi N., Tanino T. (2002) Corneal endotheliitis and idiopathic sudden sensorineural hearing loss. American Journal of Ophthalmology 133(5): 699–700. [DOI] [PubMed] [Google Scholar]
- Montano J. J., Melley C. C., Karam D. B. (1983) Encephalitis herpes simplex: Aural rehabilitation following bilateral deafness. Archives of Physical Medicine and Rehabilitation 64(10): 479–480. [PubMed] [Google Scholar]
- Muller W. J., Jones C. A., Koelle D. M. (2010) Immunobiology of herpes simplex virus and cytomegalovirus infections of the fetus and newborn. Current Immunology Reviews 6(1): 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Hato N., Horiuchi J., Honda N., Gyo K., Yanagihara N. (1997) Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: Significance of early diagnosis and treatment. Annals of Neurology 41(3): 353–357. [DOI] [PubMed] [Google Scholar]
- Nalamachu S., Morley-Forster P. (2012) Diagnosing and managing postherpetic neuralgia. Drugs Aging 29(11): 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiri R., Yoseffi J., Khajedaloe M., Sarafraz Yazdi M., Delgoshaei F. (2009) Congenital rubella syndrome after rubella vaccination in 1–4 weeks periconceptional period. The Indian Journal of Pediatrics 76(3): 279–282. [DOI] [PubMed] [Google Scholar]
- Niedermeyer H. P., Arnold W. (2008) Otosclerosis and measles virus – association or causation? ORL Journal of Oto-Rhino-Laryngology and Its Related Specialties 70(1): 63, 69–69 ; discussion. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Harada T., Sakata H., Sugiura A. (1988) Sudden deafness and asymptomatic mumps. Acta Otolaryngologica Supplement 456: 9–11. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kurata T., Saito K. (1985) Cochlear changes after herpes simplex virus infection. Acta Otolaryngologica 99(3–4): 419–427. [DOI] [PubMed] [Google Scholar]
- Ohtani F., Furuta Y., Aizawa H., Fukuda S. (2006) Varicella-zoster virus load and cochleovestibular symptoms in Ramsay Hunt syndrome. The Annals of Otology, Rhinology, and Laryngology 115(3): 233–238. [DOI] [PubMed] [Google Scholar]
- Palacios G. C., Montalvo M. S., Fraire M. I., Leon E., Alvarez M. T., Solorzano F. (2008) Audiologic and vestibular findings in a sample of human immunodeficiency virus type-1-infected Mexican children under highly active antiretroviral therapy. International Journal of Pediatric Otorhinolaryngology 72(11): 1671–1681. [DOI] [PubMed] [Google Scholar]
- Pandey M., Dudeja A., Datta V., Singla B., Saili A. (2013) Congenital rubella syndrome. The Indian Journal of Pediatrics 80(7): 613–614. [DOI] [PubMed] [Google Scholar]
- Pappas D. G., Jr., Chandra H. K., Lim J., Hillman D. E. (1994) Ultrastructural findings in the cochlea of AIDS cases. American Journal of Otolaryngology 15(4): 456–465. [PubMed] [Google Scholar]
- Pass R. F., Fowler K. B., Boppana S. B., Britt W. J., Stagno S. (2006) Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. Journal of Clinical Virology 35(2): 216–220. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Bhojwani K. M., Shenoy V., Prasad S. C. (2006) HIV manifestations in otolaryngology. American Journal of Otolaryngology 27(3): 179–185. [DOI] [PubMed] [Google Scholar]
- Quidicomo S., Matas C. G. (2013) Study of hearing functions in individuals with HIV/AIDS submitted and not submitted to antiretroviral therapies. Audiology Communication Research 18(1): 10–16. [Google Scholar]
- Rabinstein A., Jerry J., Saraf-Lavi E., Sklar E., Bradley W. G. (2001) Sudden sensorineural hearing loss associated with herpes simplex virus type 1 infection. Neurology 56(4): 571–572. [DOI] [PubMed] [Google Scholar]
- Rarey K. E. (1990) Otologic pathophysiology in patients with human immunodeficiency virus. American Journal of Otolaryngology 11(6): 366–369. [DOI] [PubMed] [Google Scholar]
- Sabella C. (2010) Measles: Not just a childhood rash. Cleveland Clinic Journal of Medicine 77(3): 207–213. [DOI] [PubMed] [Google Scholar]
- Sato H. K., Sanajotta A. T., Moraes J. C., Andrade J. Q., Duarte G., Cervi M. C. Sao Paulo Study Group for the Effects of Rubella Vaccination During Pregnancy (2011) Rubella vaccination of unknowingly pregnant women: The Sao Paulo experience, 2001. The Journal of Infectious Diseases 204(Suppl. 2): S737–S744. [DOI] [PubMed] [Google Scholar]
- Schouten J. T., Lockhart D. W., Rees T. S., Collier A. C., Marra C. M. (2006) A prospective study of hearing changes after beginning zidovudine or didanosine in HIV-1 treatment-naive people. BMC Infectious Diseases 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraff S. A., Schleiss M. R., Brown D. K., Meinzen-Derr J., Choi K. Y., Greinwald J. H., Choo D. I. (2007) Macrophage inflammatory proteins in cytomegalovirus-related inner ear injury. Otolaryngology Head and Neck Surgery 137(4): 612–618. [DOI] [PubMed] [Google Scholar]
- Sejvar J. J., Haddad M. B., Tierney B. C., Campbell G. L., Marfin A. A., Van Gerpen J. A., Petersen L. R. (2003) Neurologic manifestations and outcome of West Nile virus infection. The Journal of the American Medical Association 290(4): 511–515. [DOI] [PubMed] [Google Scholar]
- Selmani Z., Marttila T., Pyykko I. (2005) Incidence of virus infection as a cause of Meniere’s disease or endolymphatic hydrops assessed by electrocochleography. European Archives of Oto-Rhino-Laryngology 262(4): 331–334. [DOI] [PubMed] [Google Scholar]
- Semaan M. T., Gehani N. C., Tummala N., Coughlan C., Fares S. A., Hsu D. P., Megerian C. A. (2012) Cochlear implantation outcomes in patients with far advanced otosclerosis. American Journal of Otolaryngology 33(5): 608–614. [DOI] [PubMed] [Google Scholar]
- Senanayake S. N. (2008) Mumps: A resurgent disease with protean manifestations. Medical Journal of Australia 189(8): 456–459. [DOI] [PubMed] [Google Scholar]
- Sheridan M. D. (1964) Final report of a prospective study of children whose mothers had rubella in early pregnancy. British Medical Journal 2(5408): 536–539. [PMC free article] [PubMed] [Google Scholar]
- Shin J. J., Keamy D. G., Jr., Steinberg E. A. (2011) Medical and surgical interventions for hearing loss associated with congenital cytomegalovirus: A systematic review. Otolaryngology Head and Neck Surgery 144(5): 662–675. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Bale J. F., Jr., White K. R. (2005) Sensorineural hearing loss in children. Lancet 365(9462): 879–890. [DOI] [PubMed] [Google Scholar]
- Stanley F. J., Sim M., Wilson G., Worthington S. (1986) The decline in congenital rubella syndrome in Western Australia: An impact of the school girl vaccination program? The American Journal of Public Health 76(1): 35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. (2002) Will the current measles vaccines ever eradicate measles? Expert Review of Vaccines 1(3): 355–362. [DOI] [PubMed] [Google Scholar]
- Stokroos R. J., Albers F. W., Schirm J. (1999) Therapy of idiopathic sudden sensorineural hearing loss: Antiviral treatment of experimental herpes simplex virus infection of the inner ear. Annals of Otology, Rhinology, and Laryngology 108(5): 423–428. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Howe C. J., Johnston R. B. (1994) Adverse events associated with childhood vaccines other than pertussis and rubella. The Journal of the American Medical Association 271: 1602–1605. [PubMed] [Google Scholar]
- Studahl M., Lindquist L., Eriksson B. M., Gunther G., Bengner M., Franzen-Rohl E., Aurelius E. (2013) Acute viral infections of the central nervous system in immunocompetent adults: Diagnosis and management. Drugs 73(2): 131–158. [DOI] [PubMed] [Google Scholar]
- Sturt A. S., Dokubo E. K., Sint T. T. (2010) Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database of Systematic Reviews 3: CD008440.. doi: 10.1002/14651858.CD008440. [DOI] [PubMed] [Google Scholar]
- Suboti R. (1976) Histopathological findings in the inner ear caused by measles. The Journal of Laryngology & Otology 90(2): 173–181. [DOI] [PubMed] [Google Scholar]
- Sugiura S., Yoshikawa T., Nishiyama Y., Morishita Y., Sato E., Beppu R., Nakashima T. (2004) Detection of herpesvirus DNAs in perilymph obtained from patients with sensorineural hearing loss by real-time polymerase chain reaction. Laryngoscope 114(12): 2235–2238. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ogawa H., Baba Y., Suzuki T., Yamada N., Omori K. (2009) Cochlear implantation in a case of bilateral sensorineural hearing loss due to mumps. Fukushima Journal of Medical Science 55(1): 32–38. [DOI] [PubMed] [Google Scholar]
- Sweeney C. J., Gilden D. H. (2001) Ramsay Hunt syndrome. The Journal of Neurology, Neurosurgery, and Psychiatry 71(2): 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahash K., Aono T., Shichinohe M., Tamura M., Iwata Y., Yamanishi K., Shigeta S. (2001) Herpesvirus DNA in peripheral blood mononuclear cells of some patients with Meniere’s disease. Microbiology and Immunology 45(9): 635–638. [DOI] [PubMed] [Google Scholar]
- Timon C. I., Walsh M. A. (1989) Sudden sensorineural hearing loss as a presentation of HIV infection. The Journal of Laryngology & Otology 103(11): 1071–1072. [DOI] [PubMed] [Google Scholar]
- Tolle M. A., Schwarzwald H. L. (2010) Postexposure prophylaxis against human immunodeficiency virus. American Family Physician 82(2): 161–166. [PubMed] [Google Scholar]
- Torre P., 3rd., Zeldow B., Hoffman H. J., Buchanan A., Siberry G. K., Rice M., et al. (2012) Hearing loss in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents. Pediatric Infectious Disease Journal 31(8): 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt T. O., Tami T. A. (1999) Otolaryngologic manifestations of human immunodeficiency virus infection. Medical Clinics of North America 83(1): 303–315, xii. [DOI] [PubMed] [Google Scholar]
- Tsubota M., Shojaku H., Ishimaru H., Fujisaka M., Watanabe Y. (2008) Mumps virus may damage the vestibular nerve as well as the inner ear. Acta Otolaryngologica 128(6): 644–647. [DOI] [PubMed] [Google Scholar]
- van der Westhuizen Y., Swanepoel de W., Heinze B., Hofmeyr L. M. (2013) Auditory and otological manifestations in adults with HIV/AIDS. International Journal of Audiology 52(1): 37–43. [DOI] [PubMed] [Google Scholar]
- Veltri R. W., Wilson W. R., Sprinkle P. M., Rodman S. M., Kavesh D. A. (1981) The implication of viruses in idiopathic sudden hearing loss: Primary infection or reactivation of latent viruses? Otolaryngology Head and Neck Surgery 89(1): 137–141. [DOI] [PubMed] [Google Scholar]
- Vincenti V., Pasanisi E., Bacciu A., Giordano D., Di Lella F., Guida M., Bacciu S. (2005) Cochlear implantation in a human immunodeficiency virus-infected patient. Laryngoscope 115(6): 1079–1081. [DOI] [PubMed] [Google Scholar]
- Vrabec J. T. (2003) Herpes simplex virus and Meniere’s disease. Laryngoscope 113(9): 1431–1438. [DOI] [PubMed] [Google Scholar]
- Wang Y., Cao K., Yang W., Wei C., Zheng Z. (2003) [Bilateral total deafness due to mumps and the outcome of cochlear implantation]. Lin Chuang Er Bi Yan Hou Ke Za Zhi 17(10): 602–603. [PubMed] [Google Scholar]
- Wayman D. M., Pham H. N., Byl F. M., Adour K. K. (1990) Audiological manifestations of Ramsay Hunt syndrome. The Journal of Laryngology & Otology 104(2): 104–108. [DOI] [PubMed] [Google Scholar]
- Webster W. S. (1998) Teratogen update: Congenital rubella. Teratology 58(1): 13–23. [DOI] [PubMed] [Google Scholar]
- Welling D. B., Miles B. A., Western L., Prior T. W. (1997) Detection of viral DNA in vestibular ganglia tissue from patients with Meniere’s disease. American Journal of Otolaryngology 18(6): 734–737. [PubMed] [Google Scholar]
- Westerberg B. D., Atashband S., Kozak F. K. (2008) A systematic review of the incidence of sensorineural hearing loss in neonates exposed to Herpes simplex virus (HSV). International Journal of Pediatric Otorhinolaryngology 72(7): 931–937. [DOI] [PubMed] [Google Scholar]
- Westmore G. A., Pickard B. H., Stern H. (1979) Isolation of mumps virus from the inner ear after sudden deafness. British Medical Journal 1(6155): 14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R. J., Roizman B. (2001) Herpes simplex virus infections. Lancet 357(9267): 1513–1518. [DOI] [PubMed] [Google Scholar]
- Wilson W. R. (1986) The relationship of the herpesvirus family to sudden hearing loss: A prospective clinical study and literature review. Laryngoscope 96(8): 870–877. [DOI] [PubMed] [Google Scholar]
- Yazawa Y., Suzuki M., Hanamitsu M., Kimura H., Tooyama I. (2003) Detection of viral DNA in the endolymphatic sac in Meniere’s disease by in situ hybridization. ORL Journal of Oto-Rhino-Laryngology and Its Related Specialties 65(3): 162–168. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Yamauchi S., Shinkawa A., Horiuchi M., Sakai M. (1996) Immunological and virological study of sudden deafness. Auris Nasus Larynx 23: 63–68. [DOI] [PubMed] [Google Scholar]