Abstract
Impulsivity, a tendency toward immediate action without consideration of future consequences, is associated with a wide array of problematic behaviors. Response impulsivity, a type of behaviorally-assessed impulsivity characterized by behavioral disinhibition, is also associated with health risk behaviors. Response impulsivity is distinct from choice impulsivity, which is characterized by intolerance for delay. Lewis rats have higher levels of choice impulsivity than Fischer rats (Anderson & Woolverton, 2005; Madden et al., 2008; Stein et al., 2012). However, no studies have examined whether Lewis and Fischer rats have different levels of response impulsivity. The present research examined response impulsivity in the two rat strains. Subjects were 16 male Lewis and Fischer rats. Rats’ response impulsivity was measured using the Five Choice Serial Reaction Time Task (5-CSRTT). In addition, their locomotor activity was measured in locomotor activity chambers. Lewis rats had more premature responses than Fischer rats during the 5-CSRTT assessment [F(1, 14) = 5.34, p < 0.05], indicating higher levels of response impulsivity. Locomotor activity did not differ between rat strain groups [F(1, 14) = 3.05, p = .10], suggesting that overall movement did not account for group differences in response impulsivity on the 5-CSRTT. It can be concluded from this research that Lewis rats have higher levels of response impulsivity than Fischer rats, and therefore provide a valid rat model of individual differences in impulsivity.
Keywords: Response impulsivity, Lewis, Fischer, Rats, Five Choice Serial Reaction Time Task (5-CSRTT)
1. Introduction
Impulsivity involves a tendency to act rapidly with diminished regard for future consequences (Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001) and is associated with multiple risk behaviors including substance use, gambling, drunk-driving, violence, and disordered eating (Dawe & Loxton, 2004; de Wit, 2009; Kalichman, Greenberg, & Abel, 1997; Perry & Carroll, 2008; Potenza, 2008). Impulsivity can be deconstructed into two types of behaviorally-assessed impulsivity, response impulsivity and choice impulsivity (Winstanley, Eagle, & Robbins, 2006). Response impulsivity is characterized by behavioral disinhibition and is indexed by a diminished ability or willingness to withhold a prepotent response. Response impulsivity differs from choice impulsivity, a diminished ability or willingness to tolerate delay. Response impulsivity and choice impulsivity are two distinct dimensions of impulsivity that frequently correlate weakly or not at all (Lane, Cherek, Rhoades, Pietras, & Tcheremissine, 2003; Meda et al., 2009; Reynolds, Ortengren, Richards, & de Wit, 2006), and each deserves focused research attention given their relationships with clinically relevant measures in people. However, the present research was focused specifically on behaviorally-assessed response impulsivity because of its relationships with drug use and addiction (Belin, Mar, Dalley, Robbins, & Everitt, 2008; de Wit, 2009), conditions in which disinhibition is a main component.
Response impulsivity is measured by tasks that require inhibition of a behavioral response until the presentation of a stimulus, such as a light or tone, signals that the appropriate time for responding has begun. The Five Choice Serial Reaction Time Task (5-CSRTT) is a commonly-used task that measures response impulsivity in rat models; premature responding on the task provides an index of response impulsivity (Robbins, 2002). The 5-CSRTT has been used to investigate response impulsivity in rats of various strains and ages, including adolescent and adult Sprague–Dawley rats (Burton & Fletcher, 2012; Jentsch & Taylor, 2003), adult Lister-hooded rats (Belin et al., 2008), and adult Wistar rats (Amitai & Markou, 2011; Diergaarde, Pattij, Nawijn, Schoffelmeer, & De Vries, 2009). However, no studies have examined the differences in response impulsivity between two rat strains, a research question that has utility for identifying a rat model of response impulsivity.
The Lewis and Fischer rat strains differ on variables that are relevant to addiction and other risk behaviors. Lewis rats have a higher intake of and preference for drugs including cocaine, morphine, ethanol, and nicotine (Horan, Smith, Gardner, Lepore, & Ashby, 1997; Kosten & Ambrosio, 2002; Suzuki, George, & Meisch, 1988; Suzuki, Otani, Koike, & Misawa, 1988). They also demonstrate the differences from Fischer rats in stress measures (including corticosterone levels), drug responsiveness (including amphetamine-induced locomotion) and brain function (including ventral striatal differences), and these differences have been linked to specific genetic locations between the different strains (Potenza et al., 2004; Potenza et al., 2008). Lewis and Fischer rats differ with respect to dopamine (DA) neurotransmission, with Lewis rats having higher levels of DA release in response to stimulants (Camp, Browman, & Robinson, 1994; Strecker, Eberle, & Ashby, 1995), as well as lower levels of DA receptors and DA transporters (Flores, Wood, Barbeau, Quirion, & Srivastava, 1998) than Fischer rats. Because DA neurotransmission is implicated in choice impulsivity and response impulsivity (van Gaalen, Brueggeman, Bronius, Schoffelmeer, & Vanderschuren, 2006; van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006), each of these differences in DA neurotransmission may predispose Lewis rats to elevated levels of impulsivity. Lewis rats also demonstrate higher levels of choice impulsivity than Fischer rats (Anderson & Woolverton, 2005; Madden, Smith, Brewer, Pinkston, & Johnson, 2008). In addition, Lewis rats were found to have a superior performance to Fischer rats on cognitive measures, including measures of attention, learning, and memory (Fole et al., 2011; Richards et al., 2013; van der Staay, Schuurman, van Reenen, & Korte, 2009). However, no studies have directly compared response impulsivity in Lewis and Fischer rats.
Both choice impulsivity and response impulsivity have been associated with relevant aspects of addictive behaviors across species (Fineberg et al., 2014). The gravity of the consequences of risk behaviors associated with response impulsivity highlights the importance of examining response impulsivity in Lewis and Fischer rats, strains that might be used to examine for biological (including genetic) differences relating to this construct and substance-use behaviors. Toward this end, the performance of Lewis and Fischer rats on the 5-CSRTT was compared in the present research. It was hypothesized that response impulsivity would be greater in Lewis rats than in Fischer rats.
2. Methods
2.1. Subjects and housing
Subjects in the experiment were 8 adult male Lewis rats and 8 adult male Fischer rats (Charles River Laboratories). Within rat strain, animals were pair-housed in standard rat cages (42.5 × 20.5 × 20 cm) on hardwood chip bedding (Pine-Dri) with access to food (Harlan Teklad 4% Mouse/Rat Diet 7001) and water. Rats were pair-housed to avoid potentially stressful effects of crowding (Brown & Grunberg, 1995) or isolation (Parker & Radlow, 1974). Cagemates were housed together throughout the entire training and testing phases. Rats were approximately 26 days old upon arrival, and approximately 46 days old at the start of the 5-CSRTT training. Sixteen Lewis and 16 Fischer rats were trained on the 5-CSRTT, and 8 Lewis rats and 8 Fischer rats were included in the experiment based on whether they met the training criterion (described below). At the start of the experiment (after the 5-CSRTT training had concluded), rats were approximately 144 days old; the Fischer rats’ mean weight was 274.3 g while the Lewis rats’ mean weight was 388.8 g. The strain difference in body weights was expected because Lewis rats are generally larger than Fischer rats (Gomez-Serrano, Tonelli, Listwak, Sternberg, & Riley, 2001). Animals were maintained at 85% to 90% of free-feeding body weight to motivate performance in the 5-CSRTT, which is an operant task with a food reward. Free-feeding body weight was determined by feeding ad libitum two additional pairs of Lewis rat cagemates and Fischer rat cagemates (a total of four rats) that were the same age as the experimental rats, and weighing them daily. Restricting food intake is a standard procedure in the experiments using operant tasks with a food reward to ensure that animals are sufficiently motivated to work in order to obtain the food reward (Bari, Dalley, & Robbins, 2008; Blondel, Sanger, & Moser, 2000; Burton & Fletcher, 2012; Carli, Robbins, Evenden, & Everitt, 1983; Diergaarde et al., 2009; Humby, Wilkinson, & Dawson, 2005).
Housing room was maintained at 68–72 °F with 40% humidity and a 12 h reverse light cycle, with lights off at 7:00 a.m. Because rats are nocturnal animals, maintaining a reverse light cycle caused their active (dark) phase to occur during the daytime, allowing all daytime behavioral testing to take place during the rats’ active (dark) phase. This experimental protocol was approved by the USUHS Institutional Animal Care and Use Committee and was conducted in full compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH, 1996).
2.2. 5-CSRTT
2.2.1. Apparatus
The 5-CSRTT equipment consisted of four operant conditioning chambers, each housed in a sound-attenuating box (Med Associates, Inc.). The rear wall of each chamber was a curved metal surface containing a row of five nose-poke apertures. An infra-red photocell beam traversed each aperture to detect nose pokes, and a yellow LED light was fixed at the rear of each aperture. In each chamber, on the opposite wall from the apertures, a pellet dispenser delivered 45 mg pellets (Noyes precision pellets) into a food-hopper. Chamber illumination was provided by a house light located above the food tray. Data collection and presentation of stimuli and rewards were controlled by a computer (Med-PC version 4.0, Med Associates, Inc.). In the 5-CSRTT, rats were required to respond to brief flashes of light randomly presented in one of the five apertures by making a nose-poke in the illuminated aperture. In the 5-CSRTT, the total number of premature responses indexed response impulsivity, with more premature responses indicating more response impulsivity. Premature responses were responses that occurred before a cue-light was illuminated, or during a time-out period. The accuracy variable is a measure of the capacity of the rat to sustain spatial attention divided among multiple locations and multiple trials. The accuracy measure is the proportion of correct detections plus errors of commission (i.e., incorrect responses in apertures where the visual target had not been presented (Robbins, 2002)). Omissions could reflect sensory, motor, or motivational factors (Robbins, 2002). An omission was recorded when a rat failed to make a nose-poke response in an aperture either when the aperture was illuminated or in the 2-second period immediately following the illumination.
2.2.2. Training
Rats were trained on the 5-CSRTT following the procedures of van Gaalen, Brueggeman, Bronius, Schoffelmeer, and Vanderschuren (2006) and van Gaalen, van Koten, Schoffelmeer, and Vanderschuren (2006). Training lasted approximately 12 weeks and consisted of five phases: two acquisition (autoshaping) phases, two training phases, and a discrimination phase. During the first acquisition phase, pellets were placed in each of the nose-poke holes so that the rats would approach the holes. During the second acquisition phase, a pellet was released into the food hopper when a nose-poke response was made in any hole. Next, rats received a pellet only when they responded in a hole that was illuminated. Later in the acquisition phase, rats were only reinforced with a food pellet when they responded exclusively and quickly to cue lights of a progressively shorter duration (16, 8, 4, 2, 1.5, and 1 s). When rats’ performances were stable for at least 7 sessions when a 1 second stimulus duration was used, their stimulus detection accuracy was assessed using the following calculation: [number correct trials/(correct + incorrect trials)] × 100 (Bari et al., 2008). Inclusion in the experiment was dependent upon whether a rat met the training criterion of at least 80% accuracy (Bari et al., 2008). During the final phase of training, the session parameters were identical to those used during testing. The timeline of the training phase is depicted in Table 1.
Table 1.
Timeline of the training phase procedures.
| Training week | Training phase |
|---|---|
| 1 | 5-CSRTT and locomotor acclimation |
| 2 | 5-CSRTT acquisition phases 1 and 2 |
| 3 | 5-CSRTT training phases 1 and 2 |
| 4 | 5-CSRTT training phases 1 and 2 |
| 5 | 5-CSRTT training phases 1 and 2 |
| 6 | 5-CSRTT training phases 1 and 2 |
| 7 | 5-CSRTT discrimination phase |
| 8 | 5-CSRTT discrimination phase |
| 9 | 5-CSRTT discrimination phase |
| 10 | 5-CSRTT discrimination phase |
| 11 | 5-CSRTT discrimination phase |
| 12 | 5-CSRTT discrimination phase |
2.2.3. Testing
Within a session, each trial began with a 5-second inter-trial interval (ITI) during which time only the house light was illuminated, which was followed by the illumination of one cue light in pseudorandom order for 1 s. There were 100 trials in a session. A correct response was one that occurred during stimulus presentation or within a limited hold of 2 s after the stimulus light was extinguished. Correct responses were rewarded with food pellet delivery, and followed by extinguishment of the stimulus light (if necessary) and initiation of a 5-second ITI during which time only the house light was illuminated. Premature responses were recorded when responses occurred before a cue-light was illuminated, or during a time-out period. Incorrect responses were counted when responses were made in a non-illuminated hole. Omissions were recorded when a rat did not respond during the cue-light illumination or 2-second limited hold. Incorrect and premature responses were followed by the extinguishment of the stimulus light in the correct hole. In addition, incorrect responses, premature responses, and omissions were punished by a 5-second time-out period, during which time all stimulus lights and the house light were turned off. The 5-second time-out period was followed by a 5-second ITI during which time only the house light was illuminated. Nose-pokes during an ITI or time-out period resulted in the initiation of a new time-out period. The timeline of the experiment is depicted in Table 2.
Table 2.
Timeline of the experimental procedures.
| Experimental day | Procedure | Duration |
|---|---|---|
| 1 | Locomotor testing | 1 h |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | 5-CSRTT testing | 30 min maximum |
2.3. Locomotor activity
Locomotor activity measurements provide information about a rat’s pattern of movement in an open field arena (Boguszewski & Zagrodzka, 2002; Campbell, Lin, DeVries, & Lambert, 2003; Elliott & Grunberg, 2005; Hamilton, Berger, Perry, & Grunberg, 2009). Measurement of locomotor activity allowed for the determination of whether any differences in performance on the 5-CSRTT were accounted for by differences in general movement. Locomotor activity was assessed for 1 h to remain consistent with previous research from our laboratory (Hamilton, Perry, Berger, & Grunberg, 2010; Hamilton, Starosciak, Chwa, & Grunberg, 2012; Hamilton et al., 2009). However, activity over five-minute intervals during this hour was also assessed to provide more information about the patterns of movement. During the training phase, rats were acclimated to the open field arena. Data were not collected during the locomotor acclimation. During the experimental phase, locomotor activity was assessed three days prior to the 5-CSRTT testing.
2.3.1. Apparatus
Locomotor activity was measured using electronic physical activity monitoring chambers of the Accuscan/Omnitech Electronics Digiscan infrared photocell system (Test box model RXYZCM [16 TAO]). The sixteen activity chambers were located in a designated testing room separate from the housing room. Lights were turned off during data collection. Each rat was placed into an individual chamber for 1 h to measure open-field locomotor activity and record horizontal movement via a grid of infra-red light beams. Equally spaced beams traversed the plastic arenas (40 × 40 × 30 cm) from front to back and left to right. The body of the rat in the chamber broke the beams, revealing movement in the chamber. The main activity-related variable examined was total horizontal activity.
2.4. Data analysis
2.4.1. 5-CSRTT impulsivity data
The premature response parameter on the 5-CSRTT indexed response impulsivity. A one-way ANOVA was conducted with rat strain as the between-subjects factor to determine whether differences existed in premature responses between groups. Tests were two-tailed with α level of p = 0.05.
2.4.2. Locomotor activity data
A multivariate ANOVA (MANOVA) with rat strain as the between-subjects factor was conducted to compare horizontal activity and vertical activity and total distance traveled in Lewis and Fischer rats. Repeated-measures ANOVAs with strain as the between-subjects variable and 5-minute interval as the within-subject variable (e.g., 1–5 min, 6–10 min) were performed to examine whether there were strain differences in horizontal activity and vertical activity and total distance traveled throughout the duration of the testing session. A Greenhouse–Geissner correction was used in the event of a violation of the assumption of sphericity. In addition, separate one-way ANOVAs were conducted to determine whether there were strain differences in each 5-minute interval of horizontal activity and vertical activity and total distance traveled. All tests were two-tailed with α level of p =0.05.
3. Results
3.1. Impulsivity
3.1.1. Stability of baseline phase response impulsivity
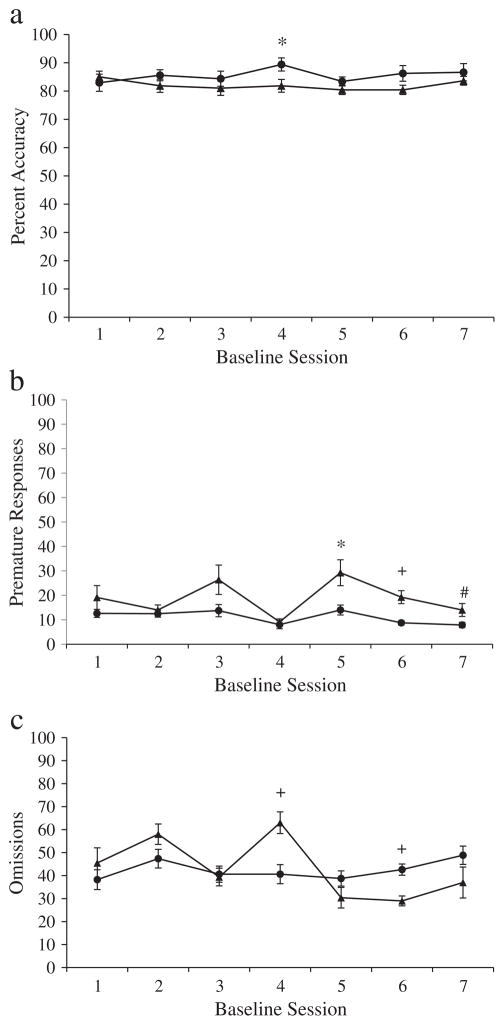
At the conclusion of the training period, the performances of all rats were systematically evaluated to determine which rats had met the training criterion (>80% accuracy; e.g. Bari et al., 2008). Twelve Lewis rats and 8 Fischer rats met the training criterion. To ensure equal group sizes, of the twelve Lewis rats exceeding 80% accuracy, the two Lewis rats with the highest accuracy level and the two Lewis rats with the lowest accuracy level were removed from the experiment. As a result of this procedure, 8 Lewis rats and 8 Fischer rats were included in the experiment, all of which met the >80% accuracy training criterion (see Fig. 1). While previous researchers have also included less than 20% omissions as a training criterion (e.g., Bari et al., 2008), the majority of subjects in the present experiment did not meet this criterion, which likely resulted from home-cage feedings that were scheduled immediately following the 5-CSRTT sessions. However, notably high levels of accurate responses despite greater than 20% omissions in the present experiment indicated that rats were sufficiently trained on the 5-CSRTT. Levels of accuracy [F(4.42, 61.81) = 1.42, p = .22] and premature responses [F(2.56, 35.82) = 1.65, p = 0.20] were stable throughout the baseline phase, and baseline levels of accuracy did not differ between the two rat strains [F(1, 14) = 1.81, p = .20]. Levels of response impulsivity were higher in Lewis [M ± SEM = 18.73 ± 1.2] than Fischer rats [M ± SEM = 11.07 ± 1.2] during the baseline phase [F(1, 14) = 19.17, p < .01] and did not change in either rat strain across time [F(2.56, 35.82) = 1.65, p = .20], suggesting that Lewis and Fischer rats are characterized by high and low levels of impulsivity, respectively, in a trait-like fashion. While omissions were somewhat unstable for the week prior to testing [F(4.03, 56.46) = 5.42, p < .001], the relatively high levels of omissions in the present experiment in both Lewis [43.18 ± 2.25] and Fischer [42.45 ± 2.25] rats did not differ by rat strain [F(1, 14) = .05, p = .82]. Similarly high levels of omissions in the two strains suggested they resulted from a factor common to all subjects, such as the home-cage feeding schedule, rather than a rat strain difference in motivation. Therefore, omissions greater than 20% did not exclude rats from the present experiment, as the omissions did not differ by rat strain and were concurrent with stable levels of accuracy in both strains (Table 3).
Fig. 1.
a. Percent accuracy. Average percent accuracy (±Standard Error of the Mean (SEM)) of Lewis and Fischer rats across 7 stable baseline sessions. Lewis rats are represented by triangles and Fischer rats are represented by circles. * indicates p < .05. b. Premature responses. Average premature responses (±SEM) of Lewis and Fischer rats across 7 stable baseline sessions. Lewis rats are represented by triangles and Fischer rats are represented by circles. + indicates p < .01; * indicates p ≤ .05; # indicates p = .05. c. Omissions. Average omissions (±SEM) of Lewis and Fischer rats across 7 stable baseline sessions. Lewis rats are represented by triangles and Fischer rats are represented by circles. + indicates p < 0.01.
Table 3.
Baseline performance. Average premature responses (PR), percent accuracy (% Acc), and omissions for Lewis (L) and Fischer (F) rats across 7 stable baseline sessions.
| Rat | PR | (SEM) | % Acc | (SEM) | Om | (SEM) | Rat | PR | (SEM) | % Acc | (SEM) | Om | (SEM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 17 | 3.8 | 85 | 2.7 | 36 | 7.4 | F1 | 15 | 3.0 | 80 | 3.1 | 35 | 4.1 |
| L2 | 19 | 5.0 | 80 | 2.1 | 42 | 3.7 | F2 | 9 | 1.5 | 92 | 1.1 | 38 | 3.4 |
| L3 | 21 | 3.6 | 81 | 2.5 | 39 | 6.7 | F3 | 14 | 1.5 | 83 | 3.1 | 42 | 2.3 |
| L4 | 25 | 3.7 | 82 | 1.2 | 33 | 5.2 | F4 | 12 | 1.1 | 81 | 2.2 | 36 | 3.8 |
| L5 | 14 | 2.2 | 85 | 1.5 | 49 | 7.3 | F5 | 10 | 1.3 | 83 | 2.7 | 51 | 1.3 |
| L6 | 19 | 6.5 | 84 | 2.2 | 46 | 7.5 | F6 | 9 | 1.8 | 88 | 1.7 | 41 | 6.8 |
| L7 | 22 | 7.8 | 82 | 3.1 | 49 | 7.7 | F7 | 11 | 2.6 | 87 | 2.3 | 51 | 1.9 |
| L8 | 12 | 2.5 | 87 | 2.3 | 51 | 7.9 | F8 | 8 | 0.7 | 90 | 1.8 | 45 | 2.8 |
3.1.2. 5-CSRTT test day assessment
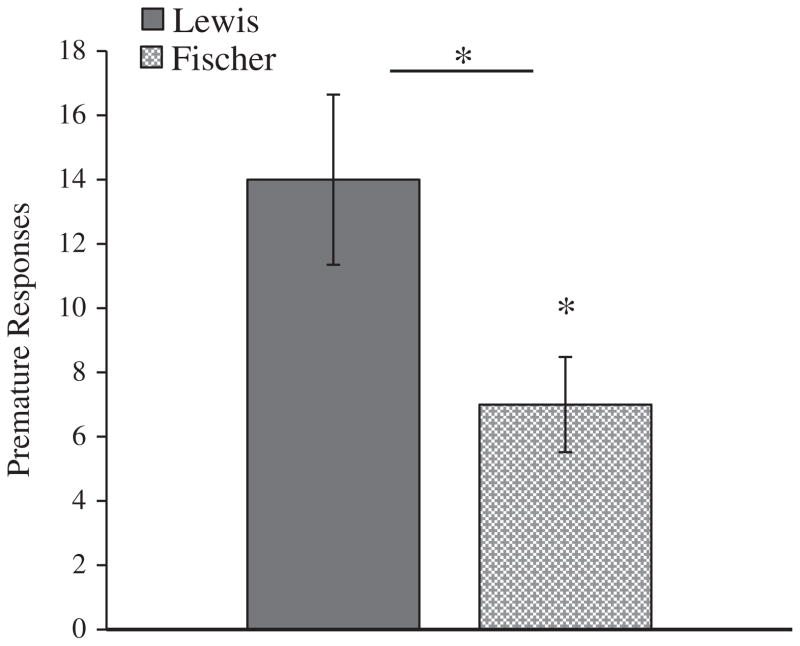
Lewis rats had more premature responses than Fischer rats during the 5-CSRTT assessment [F(1, 14) = 5.34, p < 0.05] (Fig. 2), indicating higher levels of response impulsivity. In addition, the results of the experiment remained statistically significant when the original 12 Lewis rats and 8 Fischer rats that met the training criterion were included in the analyses [F(1, 18) = 6.25, p < 0.05]. There was no significant difference in total latency to reward [F(1, 14) = 1.61, p = .23] between Lewis [Mean = 72.4 ± 23.2 s] and Fischer rats [Mean = 86.0 ± 25.1 s] during the 5-CSRTT assessment.
Fig. 2.
Response impulsivity. Average premature responses (±SEM) emitted by Lewis and Fischer rats during the testing session. * indicates p < 0.05.
3.2. Locomotor activity
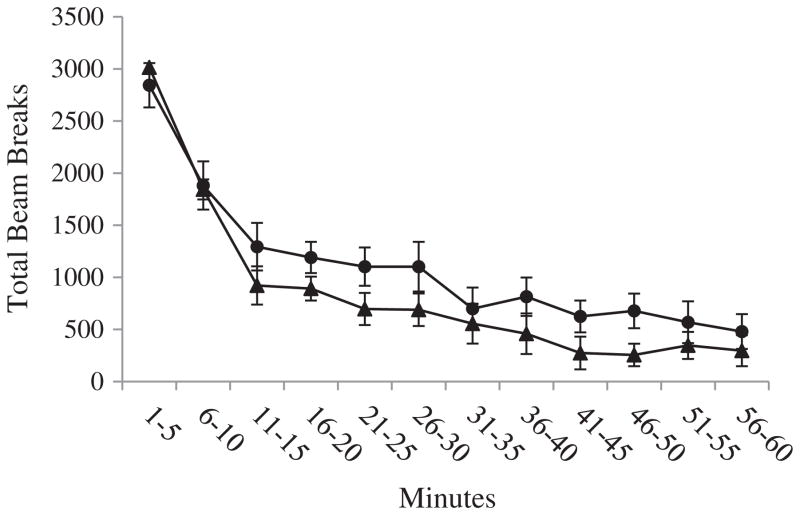
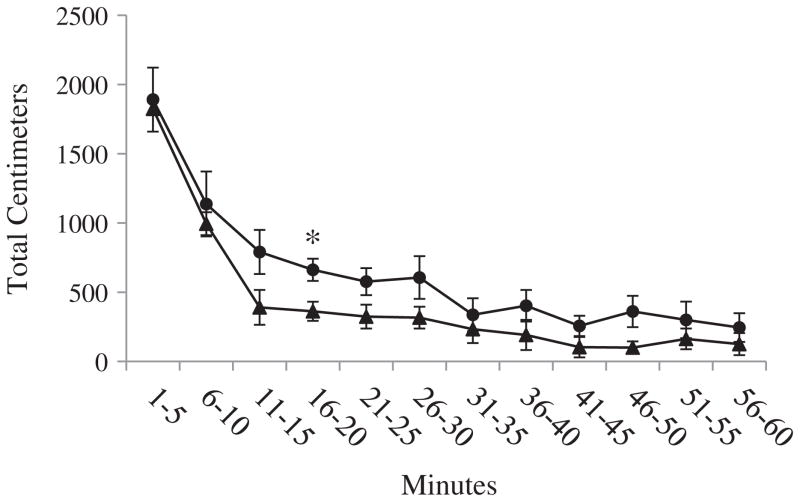
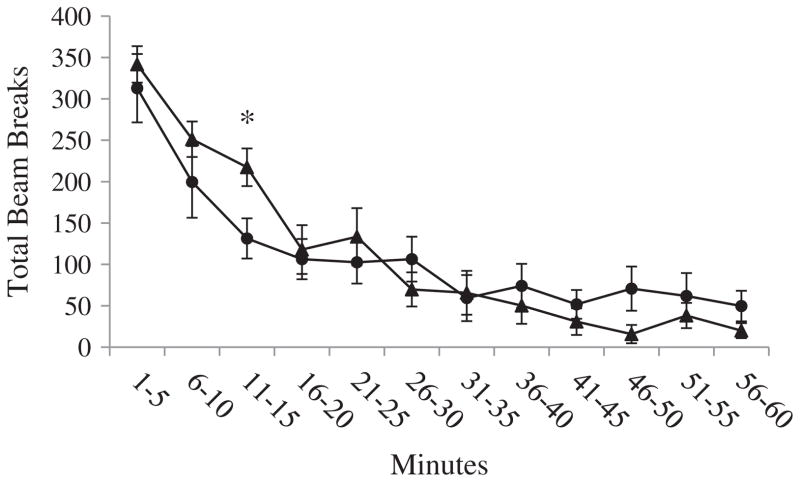
Overall, there were no rat strain differences in horizontal activity [F(1, 14) = 3.05, p = .10] and vertical activity [F(1, 14) = 0.01, p = .92], or total distance traveled [F(1, 14) = 3.55, p = .08]. There were also no strain differences in the patterns of activity over the duration of the testing session in horizontal activity [F(11, 154) = .85, p = .59] and vertical activity [F(4.88, 68.35) = 1.82, p = .12], or total distance traveled [F(11, 154) = .56, p = .86]. There were also no strain differences in horizontal activity when each 5-minute interval of data collection was considered separately. There was a strain difference during the 11-to-15-minute interval in vertical activity [F(1, 14) = 6.65, p < .05], and a strain difference during the 16-to-20-minute interval in total distance traveled [F(1, 14) = 8.00, p < .05]. The statistics for the individual 5-minute intervals are presented in Tables A, B, and C in Appendix A, and the locomotor activity across the hour of assessment is depicted in Figs. A, B, and C in Appendix A.
Fig. A.
Horizontal activity. Average horizontal activity (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session. Data were analyzed using one-way ANOVA.
Fig. B.
Total distance traveled. Average total distance traveled (cm) (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session. Data were analyzed using one-way ANOVA. * indicates p < .05.
Fig. C.
Vertical activity. Average vertical activity (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session. Data were analyzed using one-way ANOVA. *indicates p < .05.
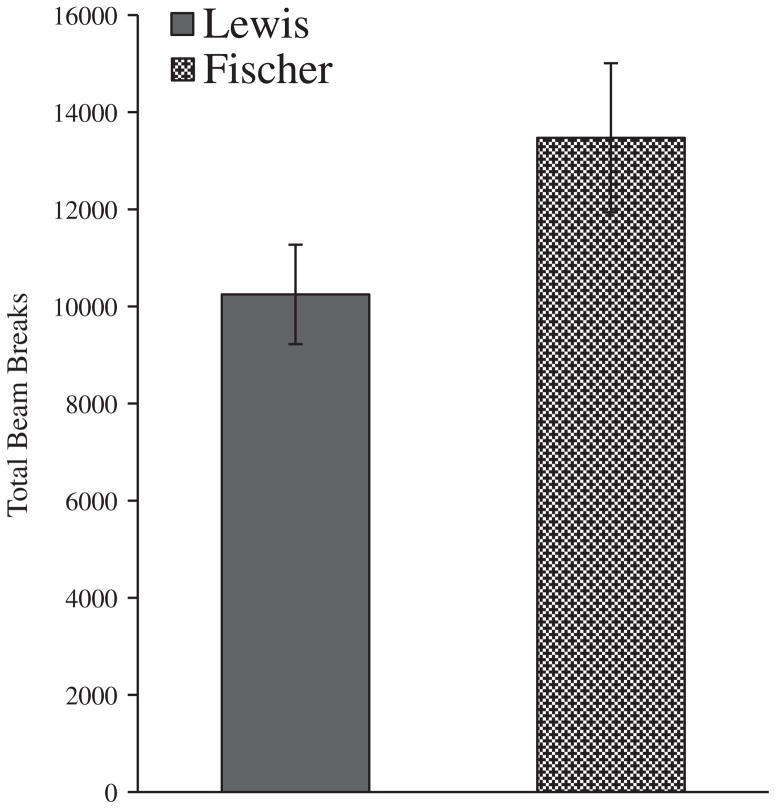
Locomotor activity did not differ between rat strain groups [F(1, 14) = 3.05, p = .10]. The lack of group differences in locomotor activity suggests that group differences in measures of response impulsivity on the 5-CSRTT were not accounted for by any group differences in overall general movement (Fig. 3).
Fig. 3.
Locomotor activity. Average locomotor activity (±SEM) of Lewis and Fischer rats during the open field session.
4. Discussion
As hypothesized, Lewis rats demonstrated greater response impulsivity than did Fischer rats. This is the first report of higher levels of response impulsivity in Lewis as compared with Fischer rats. Throughout the baseline phase and during the testing day assessment, Lewis rats consistently demonstrated higher levels of response impulsivity than Fischer rats, an effect that did not result from rat strain differences in general movement or patterns of movement over time. The consistency of this effect suggests that elevated response impulsivity in Lewis rats may reflect a stable, trait-like characteristic, although longer-term longitudinal studies are needed to directly test this hypothesis. Previous research reported that Lewis rats demonstrated more choice impulsivity than Fischer rats on a delay-discounting task (Anderson & Woolverton, 2005; Madden et al., 2008). Taken together, these separate lines of research suggest the utility of Lewis and Fischer rats as animal model for testing strain-related differences in multiple forms of impulsivity. Given that these strains are well characterized on multiple biological and behavioral measures relating to stress and substance use, they represent an important model for examining impulsivity in addiction, and particularly how stress might influence addictive behaviors.
Behavioral differences that manifest in Lewis and Fischer rats may result from underlying neurobiological and physiological differences between the two rat strains (Kosten & Ambrosio, 2002). Mesolimbic DA differences between Lewis and Fischer rats correspond to mesolimbic DA differences in humans with different levels of choice impulsivity and response impulsivity (Eisenberg et al., 2007). Lewis rats have more prolonged elevation of DA levels in the ventral striatum following methamphetamine and cocaine administration (Camp et al., 1994; Strecker et al., 1995) and have lower nucleus accumbens DA D2 and D3 receptor densities than do Fischer rats (Flores et al., 1998). Additionally, Lewis rats have lower levels of DA transporters (DATs) in the nucleus accumbens compared to Fischer rats (Flores et al., 1998). Given the well-established role of DA neurotransmission in choice impulsivity and response impulsivity (van Gaalen, Brueggeman, Bronius, Schoffelmeer, & Vanderschuren, 2006; van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006), each of these differences in DA neurotransmission may predispose Lewis rats to elevated levels of impulsivity, which is consistent with strain differences in response impulsivity in the present research as well as previously reported strain differences in choice impulsivity (Anderson & Woolverton, 2005; Madden et al., 2008; Stein, Pinkston, Brewer, Francisco, & Madden, 2012).
Interestingly, of the original sixteen Lewis and sixteen Fischer rats that were trained on the task, 12 Lewis rats met the training criterion while only 8 Fischer rats met the training criterion. This difference is concordant with previous research demonstrating better attention, task acquisition, learning, and memory in Lewis rats compared with Fischer rats (Fole et al., 2011; Richards et al., 2013; van der Staay et al., 2009). Rat strain differences in the brain reward system in Lewis and Fischer rats may contribute to other reported individual differences in variables that are relevant to impulsivity. Compared to Fischer rats, Lewis rats have demonstrated higher self-administration and conditioned place preference for cocaine, morphine, ethanol, and nicotine (Horan et al., 1997; Kosten & Ambrosio, 2002; Suzuki, George, & Meisch, 1988; Suzuki, Otani, Koike, & Misawa, 1988). These differences underscore the usefulness of Lewis and Fischer rats as an animal model of individual differences in various factors, including impulsivity, relating to risk for and progression and severity of addiction.
Given the established relationship of impulsivity to DA neurotransmission (Eisenberg et al., 2007; van Gaalen, Brueggeman, Bronius, Schoffelmeer, & Vanderschuren, 2006; van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006), future research with Lewis and Fischer rats should measure impulsivity and various aspects of the mesolimbic DA system (e.g., DATs, DA release, DA receptor densities) to determine the contributions of strain differences in DA to impulsivity. Additional research could examine these factors in relation to substance use and other measures (e.g., stress responsiveness) that have been shown to differ between the strains and interrelate to impulsivity in people (Ansell, Gu, Tuit, & Sinjha, 2012; Hamilton, Ansell, Reynolds, Potenza, & Sinha, 2013). Such research could provide a foundation for using Lewis and Fischer rats to test novel pharmacological interventions that might target addictive behaviors directly or through intermediary mechanisms (relating to impulsivity and stress, for example).
The present research has several limitations. First, home-cage feedings that immediately followed 5-CSRTT training and testing sessions may have contributed to the relatively high level of omissions observed in the present experiment. If rats learned that they would be fed immediately after each training or testing session, then this procedure may have decreased their motivation to perform for a food reward during the session. Latencies to reward that were slightly higher than those reported in previous research (Bari et al., 2008) and that did not differ between Lewis and Fischer rats may also suggest a decreased level of motivation to perform for a food reward. It also is possible that the high level of omissions in the present experiment could reflect sensory or motor factors (Robbins, 2002). Less than 20% omissions have been used as a training criterion in previous research (Bari et al., 2008), although this criterion was not used in the present experiment. Therefore, results should be interpreted with caution, given the high level of omissions in the present experiment. However, despite the high level of omissions, all rats in this research demonstrated stable levels of accurate performance, as well as stable levels of response impulsivity within each rat strain throughout the baseline period, which increases our confidence in the validity of the present results. Second, cagemates were separated during the 5-CSRTT testing, a difference from their normal housing conditions. Separation of the rats during testing was necessary, however, because the 5-CSRTT procedure can only accommodate one rat at a time. Steps were taken to minimize the possible effects of separation stress by testing cagemates at the same time, so that the time spent apart was not prolonged by the absence of one cagemate, and no rat spent time in the homecage alone. Third, only male rats were studied. Although this is a common practice in studies of Fischer and Lewis rats (Potenza et al., 2004, 2008), future research examining impulsivity in female Fischer and Lewis rats and sex differences would be valuable, particularly given the sex differences in impulsivity, motivations and addictive behaviors that are observed in people (Chapple & Johnson, 2007; Cross, Copping, & Campbell, 2011; Stoltenberg, Batien, & Birgenheir, 2008).
The purpose of the present research was to compare response impulsivity in Fischer and Lewis rats. Response impulsivity as assessed by the 5-CSRTT was greater in Lewis rats than in Fischer rats. Fischer and Lewis rats differ on their measures of multiple types of impulsivity, substance-use behaviors and other clinically relevant measures, and they represent an important resource for examining addictive behaviors.
HIGHLIGHTS.
Lewis and Fischer rats were compared on a measure of response impulsivity.
Lewis rats had greater response impulsivity than Fischer rats.
Lewis and Fischer rats provide a valid rodent model of response impulsivity.
Acknowledgments
Role of Funding Sources
This work was supported by the USUHS DoD grants T072KN and R072JK, the National Center for Responsible Gaming (NCRG), and the NIH P20-DA027844. The funding sources had no role in the preparation of the manuscript, nor did they have a role in the decision to submit the manuscript. The content of the manuscript reflects the contributions and thoughts of the authors and does not necessarily reflect the views of the funding agencies.
The authors’ private opinions or assertions contained herein should not to be construed as official or reflecting the views of the funding agencies or of the Department of Defense (DoD), Uniformed Services University of the Health Sciences (USUHS), University of Maryland (UMD), or Yale University School of Medicine. This work was supported by the USUHS DoD grants T072KN and R072JK, the National Center for Responsible Gaming (NCRG), and the NIH P20-DA027844. The authors would like to thank Dr. Amy Starosciak, Dr. Cynthia Rose, Angela Chwa, Angela Yarnell, Kevin Cravedi, Dr. Jennifer Rinker, Dr. Mary Anne Hutchinson, Erin Lowrey, and Raksha Bangalore for their assistance with this research.
Appendix A
Table A.
Horizontal activity. Average horizontal activity (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session.
| Minutes | Lewis | Fischer | F statistic | p value |
|---|---|---|---|---|
| 1–5 | 3014 ± 41 | 2843 ± 213 | .62 | .44 |
| 6–10 | 1843 ± 96 | 1881 ± 232 | .02 | .88 |
| 11–15 | 922 ± 184 | 1294 ± 229 | 1.61 | .23 |
| 16–20 | 892 ± 114 | 1190 ± 150 | 2.50 | .14 |
| 21–25 | 696 ± 154 | 1102 ± 184 | 2.87 | .11 |
| 26–30 | 689 ± 156 | 1102 ± 238 | 2.11 | .17 |
| 31–35 | 556 ± 191 | 698 ± 203 | .26 | .62 |
| 36–40 | 459 ± 195 | 814 ± 184 | 1.75 | .21 |
| 41–45 | 274 ± 157 | 624 ± 153 | 2.57 | .13 |
| 46–50 | 255 ± 108 | 678 ± 166 | 4.57 | .05 |
| 51–55 | 347 ± 130 | 569 ± 200 | .87 | .37 |
| 56–60 | 296 ± 149 | 480 ± 167 | .68 | .42 |
Table B.
Total distance traveled. Average total distance traveled (cm) (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session.
| Minutes | Lewis | Fischer | F statistic | p value |
|---|---|---|---|---|
| 1–5 | 1825 ± 47 | 1891 ± 231 | .08 | .78 |
| 6–10 | 995 ± 83 | 1137 ± 235 | .33 | .58 |
| 11–15 | 391 ± 126 | 791 ± 159 | 3.89 | .07 |
| 16–20 | 363 ± 69 | 662 ± 80 | 8.00 | .01* |
| 21–25 | 324 ± 86 | 577 ± 98 | 3.76 | .07 |
| 26–30 | 317 ± 78 | 606 ± 154 | 2.79 | .12 |
| 31–35 | 234 ± 100 | 337 ± 119 | .44 | .52 |
| 36–40 | 191 ± 109 | 403 ± 114 | 1.79 | .20 |
| 41–45 | 104 ± 74 | 257 ± 74 | 2.16 | .16 |
| 46–50 | 100 ± 45 | 361 ± 113 | 4.61 | .05 |
| 51–55 | 163 ± 75 | 301 ± 132 | .82 | .38 |
| 56–60 | 126 ± 80 | 245 ± 103 | .83 | .38 |
indicates p < .05.
Table C.
Vertical activity. Average vertical activity (±SEM) of Lewis and Fischer rats for each 5-minute interval of the open field session.
| Minutes | Lewis | Fischer | F statistic | p value |
|---|---|---|---|---|
| 1–5 | 342 ± 22 | 313 ± 41 | .38 | .55 |
| 6–10 | 251 ± 21 | 200 ± 43 | 1.14 | .30 |
| 11–15 | 217 ± 23 | 131 ± 24 | 6.65 | .02* |
| 16–20 | 118 ± 29 | 106 ± 24 | .09 | .77 |
| 21–25 | 134 ± 35 | 103 ± 26 | .51 | .49 |
| 26–30 | 70 ± 21 | 106 ± 27 | 1.16 | .30 |
| 31–35 | 66 ± 27 | 59 ± 28 | .03 | .87 |
| 36–40 | 50 ± 22 | 74 ± 27 | .48 | .50 |
| 41–45 | 31 ± 16 | 52 ± 17 | .78 | .39 |
| 46–50 | 16 ± 11 | 71 ± 27 | 3.63 | .08 |
| 51–55 | 38 ± 15 | 62 ± 28 | .56 | .47 |
| 56–60 | 20 ± 9 | 50 ± 18 | 2.09 | .17 |
indicates p < .05.
Footnotes
Contributors
Dr. Hamilton designed the study, wrote the protocol, conducted literature searches and statistical analyses and wrote the first draft of the manuscript. Dr. Grunberg provided guidance on the study design and protocol. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest. Dr. Potenza has consulted for and advised Boehringer Ingelheim, Lundbeck and Ironwood; has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling and legal entities on issues related to addictions or impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Contributor Information
Kristen R. Hamilton, Email: khamilt4@umd.edu.
Marc N. Potenza, Email: marc.potenza@yale.edu.
Neil E. Grunberg, Email: neil.grunberg@usuhs.edu.
References
- Amitai N, Markou A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behavioral Neuroscience. 2011;125:764–774. doi: 10.1037/a0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacology, Biochemistry, and Behavior. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell EB, Gu P, Tuit K, Sinjha R. Effects of cumulative stress and impulsivity on smoking status. Human Psychopharmacology. 2012;27:200–208. doi: 10.1002/hup.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nature Protocols. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: Antagonist studies. Psychopharmacology. 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Boguszewski P, Zagrodzka J. Emotional changes related to age in rats—A behavioral analysis. Behavioural Brain Research. 2002;133:323–332. doi: 10.1016/s0166-4328(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Brown K, Grunberg N. Effects of housing on male and female rats: Crowding stresses males but calms females. Physiology and Behavior. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behavioural Brain Research. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Research. 1994;668:180–193. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiology and Behavior. 2003;78:495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins T, Evenden J, Everitt B. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction time task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behavioural Brain Research. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chapple CL, Johnson KA. Gender differences in impulsivity. Youth Violence and Juvenile Justice. 2007;5:221–234. [Google Scholar]
- Cross C, Copping L, Campbell A. Sex differences in impulsivity: A meta-analysis. Psychological Bulletin. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton N. The role of impulsivity in the development of substance use and eating disorders. Neuroscience and Biobehavioral Reviews. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. 2009;1:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Nawijn L, Schoffelmeer AN, De Vries TJ. Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behavioral Neuroscience. 2009;123:794–803. doi: 10.1037/a0016504. [DOI] [PubMed] [Google Scholar]
- Eisenberg DA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 TaqI A and DRD4 48-bp VNTR association study. Behavioral and Brain Functions. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague–Dawley rats. Behavioural Brain Research. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fineberg N, Chamberlain S, Goudriaan A, Stein D, Vandershuren L, Gillan C, et al. New developments in human neurocognition: Clinical, genetic and brain imaging correlates of impulsivity and compulsivity. CNS Spectrums. 2014;19(1):69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: A comparison of dopamine transporter and receptors levels. Brain Research. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Fole A, Gonzalez-Martin C, Huarte C, Alguacil LF, Ambrosio E, Del Olmo N. Effects of chronic cocaine administration on spatial learning and hippocampal spine density in two genetically different strains of rats. Neurobiology of Learning and Memory. 2011;95:491–497. doi: 10.1016/j.nlm.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Gomez-Serrano M, Tonelli L, Listwak S, Sternberg E, Riley AL. Effects of cross fostering on open-field behavior, acoustic startle, lipopolysaccharide-induced corticosterone release, and body weight in Lewis and Fischer rats. Behavior Genetics. 2001;31:427–436. doi: 10.1023/a:1012742405141. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources. National Research Council: National Academies Press; 1996. [PubMed] [Google Scholar]
- Hamilton KR, Ansell EB, Reynolds B, Potenza MN, Sinha R. Self-reported impulsivity, but not behavioral choice or response impulsivity, partially mediates the effect of stress on drinking behavior. Stress. 2013;16:3–15. doi: 10.3109/10253890.2012.671397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacology, Biochemistry, and Behavior. 2009;92:51–59. doi: 10.1016/j.pbb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Perry ME, Berger SS, Grunberg NE. Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine & Tobacco Research. 2010;12:1236–1245. doi: 10.1093/ntr/ntq179. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Starosciak AK, Chwa A, Grunberg NE. Nicotine behavioral sensitization in Lewis and Fischer male rats. Experimental and Clinical Psychopharmacology. 2012;20:345–351. doi: 10.1037/a0029088. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–94. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, Dawson G. Current Protocols in Neuroscience. Unit 8.5H. 2005. Assaying aspects of attention and impulse control in mice using the five-choice serial reaction time task. [DOI] [PubMed] [Google Scholar]
- Jentsch J, Taylor J. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behavioral Neuroscience. 2003;117:76–83. doi: 10.1037//0735-7044.117.1.76. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Greenberg J, Abel GG. Sexual compulsivity among HIV positive men who engage in high-risk sexual behavior with multiple partners: An exploratory study. AIDS Care. 1997;9 doi: 10.1080/09540129750124984. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: Insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addictive Disorders and Their Treatment. 2003;2:33–40. [Google Scholar]
- Madden G, Smith N, Brewer A, Pinkston J, Johnson P. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: Between-condition delay manipulations. Journal of the Experimental Analysis of Behavior. 2008;90:333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. The American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Parker L, Radlow B. Isolation stress and volitional ethanol consumption in the rat. Physiology and Behavior. 1974;12:1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Brodkin ES, Joe B, Luo X, Remmers EF, Wilder RL, et al. Genomic regions controlling corticosterone levels in rats. Biological Psychiatry. 2004;55:634–641. doi: 10.1016/j.biopsych.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Brodkin ES, Yang B, Birnbaum SG, Nestler EJ, Gelernter J. Quantitative trait locus analysis identifies rat genomic regions related to amphetamine-induced locomotion and Gαi3 levels in nucleus accumbens. Neuropsychopharmacology. 2008;33:2735–2746. doi: 10.1038/sj.npp.1301667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40:305–315. [Google Scholar]
- Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, et al. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes, Brain, and Behavior. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: Behavioral pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Stein JS, Pinkston JW, Brewer AT, Francisco MT, Madden GJ. Delay discounting in Lewis and Fischer 344 rats: Steady-state and rapid-determination adjusting-amount procedures. Journal of the Experimental Analysis of Behavior. 2012;97:305–321. doi: 10.1901/jeab.2012.97-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg S, Batien B, Birgenheir D. Does gender moderate associations among impulsivity and health-risk behaviors? Addictive Behaviors. 2008;33:252–265. doi: 10.1016/j.addbeh.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker RE, Eberle WF, Ashby CR. Extracellular dopamine and its metabolites in the nucleus accumbens of Fischer and Lewis rats: Basal levels and cocaine-induced changes. Life Sciences. 1995;56:135–141. doi: 10.1016/0024-3205(94)00913-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. Journal of Pharmacology and Experimental Therapeutics. 1988;245:164–170. [PubMed] [Google Scholar]
- Suzuki T, Otani K, Koike Y, Misawa M. Genetic differences in preferences for morphine and codeine in Lewis and Fischer 344 inbred rat strains. Japanese Journal of Pharmacology. 1988;47:425–431. doi: 10.1254/jjp.47.425. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Schuurman T, van Reenen CG, Korte SM. Emotional reactivity and cognitive performance in aversively motivated tasks: A comparison between four rat strains. Behavioral and Brain Functions. 2009;5:50. doi: 10.1186/1744-9081-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen M, Brueggeman R, Bronius P, Schoffelmeer A, Vanderschuren L. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- van Gaalen M, van Koten R, Schoffelmeer A, Vanderschuren L. Critical involvement of dopamine neurotransmission in impulsive decision making. Biological Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]