Abstract
The increased use of nano-sized materials is likely to result in the release of these particles into the environment. It is, however, unclear if these materials are harmful to aquatic animals. In this study, the sub-lethal effects of exposure of low and high concentrations of titanium dioxide nanoparticles (TiO2 NPs) on goldfish (Carassius auratus) were investigated. Tissues, including intestine, gills, muscle, and brain were analyzed for Ti content by ICP-MS. Accumulation of TiO2 NPs increased from 42.71 to 110.68 ppb in the intestine and from 4.10 to 9.86 ppb in the gills of the goldfish with increasing exposure dose from 10 to 100 mg/L TiO2 NPs. No significant accumulation in the muscle and brain of the fish was detected. Malondialdehyde (MDA) as a biomarker of lipid oxidation was detected in the liver of the goldfish. Moreover, TiO2 NPs exposure inhibited growth of the goldfish. Although there was an increase (8.1%) in the body weights of the goldfish for the control group, in the low and high exposure groups 1.8% increase and 19.7 % decrease were measured respectively.
Keywords: Nanoparticles, Titanium dioxide, Bioaccumulation, Oxidative strees, Goldfish
INTRODUCTION
Nanomaterials are used in a wide range of domestic appliances and household products, in the manufacture of textiles and electronics, as well as in medical products and bioremediation technology. There are also concerns about the environmental risks of nanotechnology which need to be balanced against their undoubted benefits to human society (Crane and Handy, 2007; Owen and Handy, 2007). Handy and Shaw (2003) reviewed the risks to human health and identified a number of exposure routes including the discharge of nanoparticles (NPs) to water and agricultural land. The chemistry and physical characteristics of the NPs themselves are key elements in determining their fate and behavior in aquatic systems. The large surface area, crystalline structure and reactivity of some NPs may facilitate transport of these toxic materials in the environment (Zhang, 2003). Certain conditions such as presence of humic and fulvic acids, pH and specific cation concentrations may favor the stabilization of NPs in the water column (Baalousha et al., 2008). The aquatic species are also at risk of exposure to the NPs and there is currently little known about their uptake, potential toxic effects, and behavior in aquatic systems.
Titanium dioxide (TiO2) NPs have been widely used in several industries. Nanoparticulate TiO2 has been utilized as an ultraviolet radiation absorber in transparent sunscreen formulations (Nohynek et al., 2007) and in specialist photocatalytic coatings for glass (Medina-Valtierra et al., 2009). The environmental chemistry of TiO2 NPs has been partly investigated. TiO2 NPs can be dispersed in freshwater by sonication (Federici et al., 2007), but the primary particles tend to form aggregates of a few 100 nm dimensions, and the aggregates gradually precipitate from the water column over a few hours. TiO2 has two major crystal structures (rutile and anastase), and the surface reactivity of the NP is closely defined by the crystal structure (Watanabe et al., 1999).
There is an emerging literature on the effects of NPs for fish and aquatic invertebrates. NPs have previously been shown to accumulate in cells such as macrophages and hepatocytes (Witsap et al., 2009; Johnston et al., 2010). Moreover, they are taken up into aquatic organisms such as fısh, mollusks, crustaceans, and artemia (Ward and Kach, 2009; Kashiwada, 2006; Tao et al., 2009; Lee et al., 2007; Ates et al., 2013). Fish are excellent sentinels of environmental health as they are sensitive to a wide range of xenobiotic chemicals. Their position in the aquatic food chain means assessment of the populations and health of fish can give an indication of the health of other lower levels of the food chain. Understanding the effects of NPs on fish is therefore an important aspect when considering the effects of NPs on the aquatic environment as a whole. Potential routes of uptake for NPs in fish include absorption via the gill epithelia, via the intestine epithelia as a result of dietary exposure and drinking or via the skin (Handy et al., 2008).
The purpose of this exposure study was to determine sub-acute toxicity, accumulation and tissue distribution of engineered TiO2 NPs in goldfish (Carassius auratus). Due to the importance of their size and aggregation behavior (Limbach et al., 2005; Fujiwara et al., 2008; Carlson et al., 2008), the NPs were characterized by transmission electron microscopy (TEM) and the size distribution of NPs was measured by dynamic light scattering (DSL). Fish tissues were used as in vitro model to determine the possible uptake of TiO2 NPs into gill, intestine, muscle, and brain. Total Ti accumulation in each tissue was determined by inductively coupled plasma mass spectrometry (ICP-MS). In addition, MDA was determined as a cause of systemic oxidative stress.
MATERIALS AND METHODS
Test organism and experimental condition
A group of healthy goldfish (Carassius auratus) was purchased from a local pet shop. The initial body weight and length of the fish were measured as 4.53±0.06 g and 5.5±0.7 cm respectively. All fish were maintained in 30-L glass aquarium supplied via a flow-through system with dechlorinated tap water, enriched with oxygen at a temperature of 23±2°C and pH of 6.8. Fish were fed daily with commercially available fish feed flakes (TetraFin Goldfish flakes, Germany) at the amount of 0.5% of mean body weight of the fish. The goldfish were anesthetized using 3-aminobenzoic acid ethylester (MS-222; Aqua Life, Syndel Laboratories Ltd., Vancouver, BC, Canada) at a lethal dose for dissection (excess of 200 mg/L) and a lower dose was used for all handling procedures (150 mg/L).
Reagents and chemical
TiO2 NPs (TiO2 10–30 nm NPs, 99.5% pure) were purchased, as uncoated nanomaterials, from Skyspring Nanomaterials Inc., in Houston, TX USA. TEM image of NPs was spherical with an average particle size (D50) of 10–30 nm and approximate surface area of 50 m2/g. The morphology of the NPs was rutile with pale yellow color, which is the most widely found polymorph of TiO2 in nature and in high pressure metamorphic rocks. The TiO2 NPs were stored at room temperature in the laboratory until the implementation of the experimental studies.
Deionized water produced by Barnstead E-pure system with the resistivity of 18.0 MΩ cm was used to prepare the exposure medium and experimental solutions. Trace metal grade nitric acid (HNO3, Fisher Scientific) and hydrofluoric acid (HF, 99.99%, Sigma Aldrich) used for dissolution of the goldfish were collected after the exposure to determine the total uptake levels. Stock titanium standard solution (1000 μg mL−1) was purchased from SCP Science (Champlain, NY). Calibration standards for ICP-MS analysis were prepared within a range from 0 to 500 μg L−1 from the stock Ti solution in 5% HNO3. Carbon coated Cu TEM grids (300 mesh) were purchased from Electron Microscopy Sciences (EMS), Hatfield, PA.
Characterization of nanoparticles
For preparation of exposure medium, TiO2 NPs were weighed in polypropylene tubes and dispersed in deionized water. To achieve maximum dispersion, the suspension was homogenized using a vortex (Daigger Vortex-Genie 2, Model G560) equipped with a titanium probe. Each suspension was exposed to the mixture for about 2 minutes and then immediately transferred into the exposure glass tanks. The characterizations of the TiO2 NP suspension were performed using TEM and DLS techniques. Size measurements in dried suspension were made by TEM while DLS provided the size distribution for the hydrated forms of the NPs. In addition, Zeta potential measurements were conducted using the DLS instruments to elucidate the surface charges of the suspensions in the exposure medium.
For TEM measurements, a drop (ca. 8 μL) of solution was allowed to dry on a carbon-coated copper grid overnight (CF300 Cu). The TEM grids were purchased from Electron Microscopy Sciences (EMS). Images were recorded by using a JEOL-1011 TEM instrument with 0.2 nm lattice resolution and magnification power up to 106 under the accelerating voltage of 40 to 100 kV. Captured images were analyzed using ImageJ software. For DLS measurement, the protocol followed the standards of the Nanotechnology Characterization Laboratory (Clogston and Patri, 2011). A stock solution (10mg/100ml) was prepared with dionized water and diluted to a final concentration of 10 μg/mL. Samples were then analyzed with Malvern Zetasizer Model Nano ZS according to manufacturer’s protocols. Samples were read in disposable plastic Malvern Cells.
Experiment design
NP exposure was conducted to assess acute toxicity and associated behavioral changes on goldfish by exposing the fish to two different doses, 10 and 100 mg/L, of the TiO2 NPs, using five day static tests according to OECD 203 testing guidelines (OECD 1992). A control group was also setup without the test compound. Studies were carried out in an aquarium (30 L inner volume). The volume for 10 L level was marked and filled with freshwater followed by addition of the NP suspensions prepared as described above. Sight aeration was provided by a line extending to the bottom of the aquarium. Details of the experimental conditions are summarized in Table 1. Each scheme (control or treatments) was conducted in duplicate with 5 healthy goldfish. Individual length and weight of the fish were measured at the beginning and end of the experiment. The data obtained enabled calculation of the live weight and lengthwise increases, and survival rates upon completion of the experiment.
Table 1.
Expanded design summary of goldfish (Carassius auratus)
| Parameter | Control | Group A | Group B |
|---|---|---|---|
| Volume of water in aquarium (L) | 10 | 10 | 10 |
| TiO2 NPs concentrations (mg/L) | 0 | 10 | 100 |
| Duration of exposure (day) | 5 | 5 | 5 |
| Water temperature (ºC) | 23 ± 2 | 23 ± 2 | 23 ± 2 |
| Oxygen (ppm) | 6 ± 1 | 6 ± 1 | 6 ± 1 |
| pH value of water (Start-End) | 6.30 - 6.05 | 6.03 – 6.15 | 6.63 - 6.45 |
| Number of fish within the aquarium | 5 | 5 | 5 |
| Number of replications | 2 | 2 | 2 |
Instrumental analysis
At the end of the exposure, fish tissues were sampled for analysis. About 150 mg of wet tissue was weighed and digested in Teflon vessels in 2 mL concentrated HNO3 and 0.5 mL HF for two hours using digestion a block (DigiPrep MS, SCP Science) at 160 °C according to protocols described elsewhere (Arslan et al., 2011). At the end, the contents were visually inspected for complete dissolution of TiO2 NPs (e.g., clear solution without any turbidity) and were diluted to 10 mL with dionized water. The sample solutions were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) using a Varian 820MS ICP-MS instrument (Varian, Australia). ICP-MS is a powerful multi-element technique for analysis of fish and other aquatic organisms for toxic metals even at sub-parts per billion levels because of its high sensitivity (Arslan, 2005). Titanium content of the solutions was measured to determine the accumulation pattern of the NPs across the dose of exposure. Total Ti concentration detected was then translated into corresponding TiO2 NP concentration.
Oxidative stress parameter analysis
The experiment was designed to allow for sub-lethal physiological effects over the exposure period. The five day exposure time was chosen to enable some physiological or biochemical responses to the test organism. Five fish per treatment were collected from each tank at the end of the experiment for biochemical analysis. The extent of lipid peroxidation in the tissues was determined by measuring the quantity of malondialdehyde (MDA) (Gerard-Monnier et al., 1998). Quantification of MDA was done following the methods described by Maness et al., (1999), Esterbauer and Cheeseman, (1990). The method is based on the formation of pink MDA-Thiobarbituric acid (TBA) adduct which has maximum absorption in acidic solution at 532 nm. Briefly, the liver and muscle tissues were removed separately and immediately frozen in liquid nitrogen and stored at −20°C until needed. The frozen tissues were rinsed in 9-fold chilled 100 mmol/L, pH 7.8 sodium phosphate buffer solution and homogenized by a hand-driven glass homogenizer. Approximately 150 mg muscle of tissue and 10 μL BHT reagent were immediately transferred into 500 ml cold water in tube and then the sample was homogenized using a sonicator (Sonic Dismembrator Model 100, Fisher Scientific). The samples were sonicated on ice by ultrasounds for 2 min at 80% power. All samples and standards were incubated at 90 °C for one hour, then centrifuged at 12 000 rpm for 15 minutes to separate the suspending tissue. The absorbance of the supernatant (reaction mixture) was measured at 532 nm with HP 8452A model dioarray spectrophotometer. The concentration of the MDA formed was calculated based on a standard curve for the MDA (Sigma Chemical Co.) complex with TBA. The extent of lipid peroxidation was expressed in nanomoles (or micromoles) of MDA.
Statistical analysis
All experiments were repeated twice independently and data were recorded as the mean with standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons was used to detect significant differences among groups. Student’s t-test was used for paired comparisons of two groups. In all data analyses, a p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Characterization of NPs by TEM
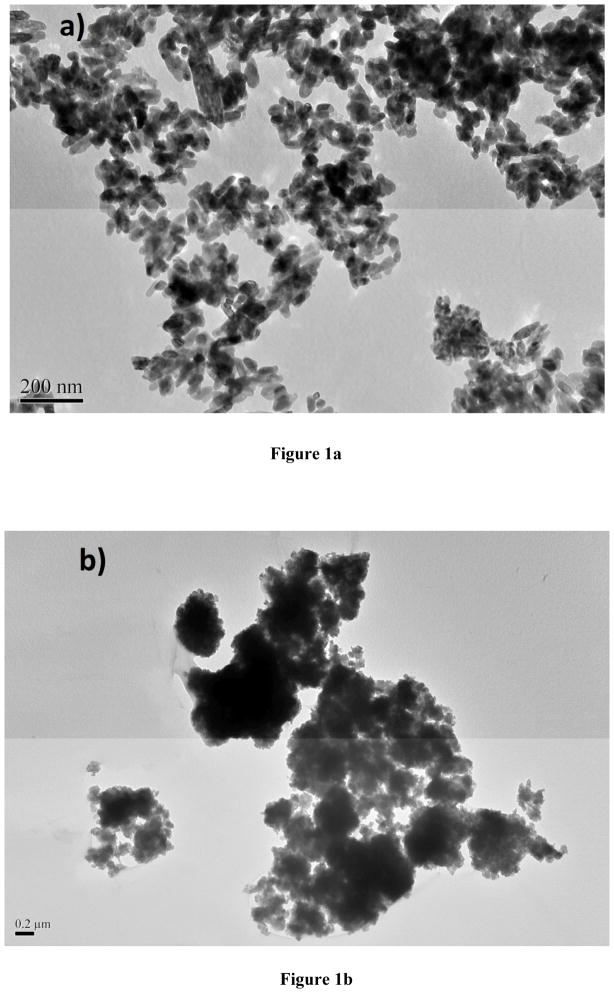
TiO2 NPs are highly hydrophobic, therefore aggregate substantially in aqueous solutions (Adams et al., 2006; Xiong et al., 2011; Zhu et al., 2008). The stability and aggregation behavior of NPs within an aquatic medium are determined by both the physicochemical properties of the liquid, and the charge on the surface of the NPs. The degree of aggregation of NPs has been shown in some cases to affect toxicity in vitro and aggregation of the NPs when suspended in water is a known issue for TiO2 NPs (Adams et al., 2006; Zhu et al., 2010). In this study, the water visibility decreased substantially with increasing concentration of TiO2 NPs and at 100g/L level, the solution was cloudy. Similar aggregation phenomenon has been reported in many NP studies including TiO2 that aggregates of NPs can sink out of the solution very quickly (Chen et al., 2011). The TEM images collected from the dried suspensions of stock solution and exposure medium are illustrated in Fig 1. The TiO2 NPs aggregated significantly in water yielding large aggregates ranging from around 100 to as high as 1.0 μm in size. Although aeration assisted in maintaining the homogeneity of the suspensions, aggregation could not be avoided at any concentration of TiO2 NPs.
Fig. 1.
TEM images for TiO2 NPs from stock solution (a) and the exposure medium (b).
Size distribution and surface charge measurement of NPs
Metal oxide particles tend to aggregate to various extents in water. The size distribution of the NPs is of interest in this study, since TiO2 NPs are highly hydrophobic the particles size distribution in water was measured to determine the effect of the stability. The mean size distribution of TiO2 NP in water was calculated as 432 ± 32 nm for 10 μg/mL NP suspension. Zeta potentials for the TiO2 NPs in aqueous suspensions were obtained using the Henry Equation. A viscosity of 0.8872 cP, a dielectric constant of 78.5, and Henry function of 1.330 were used for the calculations. The mean zeta potential was calculated as −31.6 ± 4.5 mV at a pH of 7.23 in 10 μg/mL aqueous suspensions of NP.
Accumulation in organs of goldfish
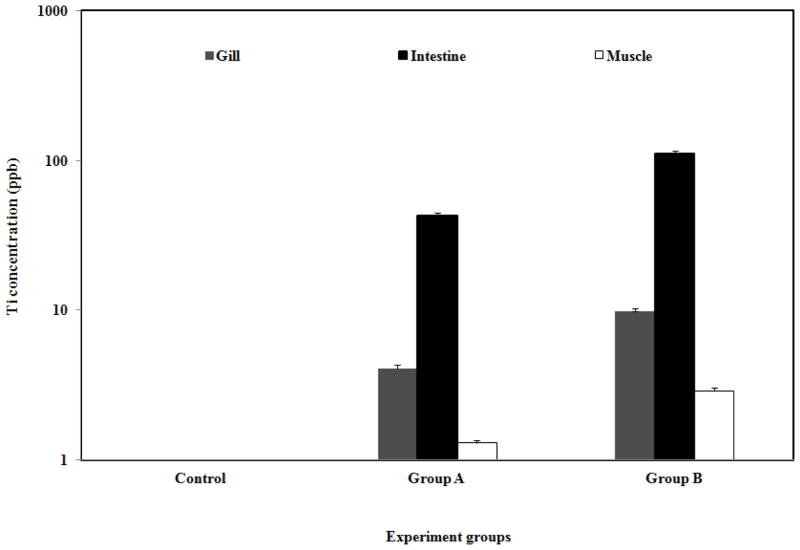
Uptake of nanomaterials by fish and other aquatic species has also been reported previously (Kashiwada, 2006; Moore, 2006). This study determined the accumulation of the unmodified TiO2 NPs in fish tissues following exposure via the water column without the use of a solvent vehicle or prior modification of the NP surface. The chemical fate of the metal oxide NPs in the aquatic environment was determined through a comprehensive evaluation of uptake in fish with full characterization of the NPs in low and high exposure conditions. The gill, intestine, muscle, and brain of the goldfish were used as in vitro model to determine the possible uptake of TiO2 NPs into tissues. For 10 mg/L and for 100 mg/L TiO2 exposure mediums, uptakes of TiO2 NPs in intestine were measured as 42.71 and 110.68 ppb, and in gills as 4.10 and 9.86 ppb, respectively. ICP-MS analysis showed a very small amount of Ti accumulation in the muscle and no accumulation in brain tissues of the goldfish (Fig. 2.). A study by Moger et al., (2008) however used coherent anti-Stokes Raman Scattering (CARS) to examine the gills of rainbow trout exposed to 5000 μg L−1 TiO2 NPs and confirmed the presence of small numbers of particle aggregates within the gill tissue.
Fig. 2.
Titanium (Ti) levels in gill, intestine, and muscle of the goldfish at the end of the exposure experiments (Group A and Group B exposed to 10 ppm and 100 ppm TiO2 NPs, respectively)
Oxidative stress
Lipid peroxidation generates a group of products among which are reactive electrophiles such as epoxides and aldehydes (Esterbauer and Cheeseman, 1990; Janero, 1990; Marnett, 2002). Malondialdehyde (MDA) is a major product of lipid peroxidation in aqueous solution. In this study, to elucidate possible role of oxidative stress in the effects observed by TiO2 NPs exposure, MDA content were assayed in the liver and muscle of each experimental group. The analysis for MDA content of goldfish liver showed lipid peroxidation in the controls and exposure groups. The mean MDA levels in the liver of the fish were 4.1±0.5, 7.6±1.1 and 11.3±0.9 nmol/gr for control, low and high dose exposure groups, respectively. No MDA level was measured in the muscles of the control and the exposure groups. Xiong et al., (2011) were also studied TiO2 NPs but on zebrafish and reported similar results as this study that oxidative effects were more severe in the livers of zebrafish exposed to 50 mg/L TiO2 NPs.
Aqueous exposure to low and high dose of TiO2 NP suspension did not cause any fish mortality during the experimental period (96 hr). Data is in agreement with the literature in, indicating low acute toxicity of TiO2 NPs to fish survival. Similarly, Warheit et al., (2007) reported the Daphnia magna 48 hr EC50 values and rainbow trout (Oncorhynchus mykiss) 96 hr LC50 values for fine TiO2 particles and ultrafine TiO2 particles based on nominal concentrations were >100 mg/L and the LC50 for TiO2 NPs was also found over 500 mg L−1 in fathead minnow Pimephales promelas (Hall et al., 2009). Furthermore, Zhu et al., (2008) showed that exposure to TiO2 NPs at the concentrations up to 500 mg/L for 96 hr did not affect hatching rate and did not cause deformity in embryonic zebrafish.
Effect of NPs on growth performance
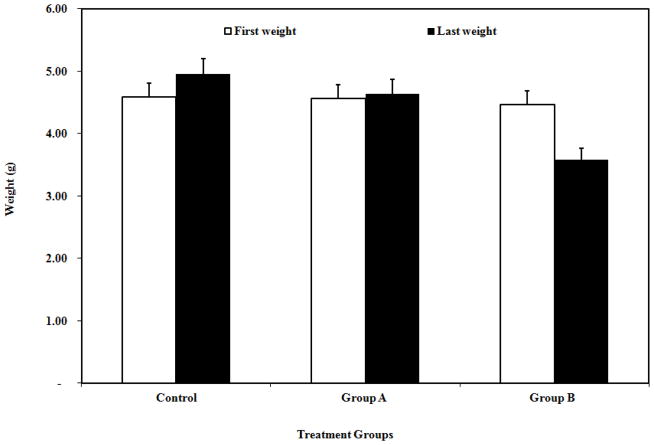
Out of growth performance parameters for trial groups of goldfish, the best weight-wise growth as of the completion of the trial period was attained in the control group, where NPs were not exposed. The growth of the fish was inhibited with increasing exposure dose of TiO2 NPs. While the controls showed about 8.1 % increase in body weight, those exposed to 10 mg/L NPs showed a small increase (1.8%) in body weight, and those exposed to 100 mg/L showed about 19.7% decrease in body weight at the end of the experiments (Fig. 3.).
Fig. 3.
Average live weights (g) for the goldfish at the beginning and at the end of the experiments (Group A and Group B exposed to 10 and 100 ppm TiO2 NPs, respectively).
CONCLUSIONS
A short period of exposure of the waterborne levels of 10 mg kg−1 TiO2 NP or 100 mg kg−1 TiO2 NPs is not lethal to goldfish. Abnormal physiological and behavioral changes of the goldfish occurred under the higher concentrations during the experimental period. Since TiO2 NPs could cause oxidative stress and the decreases in the growth rate on fish, the release of TiO2 NPs into the aqueous environment may pose potential risks to aquatic organisms. This needs to be considered against the context of a general lack of knowledge of the fate, behavior and bioavailability of these types of particles in natural systems and suggests a need for longer-term and more environmentally realistic NP exposure regimes to fully determine the transport capabilities of NPs in the aquatic environment. Although data on the behavior and effects of NPs in the environmental food-chain would be of primary importance for understanding their overall potential hazard for ecosystems, very few studies in fish have examined the uptake and partitioning of TiO2 NPs, probably due to the difficulties involved in measuring low levels of TiO2 and limitations in analytical equipment.
Acknowledgments
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS); (Contract #W912HZ-10-2-0045). The views expressed herein are those of authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors thank Jackson State University, Biostatistical Support Unit for assistance in statistical analysis.
References
- Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Arslan Z. Analysis of fish otoliths by electrothermal vaporization inductively coupled plasma mass spectrometry: aspects of precipitating otolith calcium with hydrofluoric acid for trace element determination. Talanta. 2005;65:1326–1334. doi: 10.1016/j.talanta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Arslan Z, Ates M, McDuffy W, Agachan MS, Farah IOWW, Yu, Bednar AJ. Probing metabolic stability of CdSe nanoparticles: alkaline extraction of free cadmium from liver and kidney samples of rats exposed to CdSe nanoparticles. J Hazard Mater. 2011;192(1):192–196. doi: 10.1016/j.jhazmat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess. 2013;185:3339–3348. doi: 10.1007/s10661-012-2794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalousha M, Manciulea A, Cumberland S, Kendall K, Lead J. Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter. Environ Toxicol Chem. 2008;27:1875–1882. doi: 10.1897/07-559.1. [DOI] [PubMed] [Google Scholar]
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- Chen CY, Lin TH, Tseng MC. Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio) Marine Poll Bull. 2011;63:303–308. doi: 10.1016/j.marpolbul.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol. 2011;697:63–70. doi: 10.1007/978-1-60327-198-1_6. [DOI] [PubMed] [Google Scholar]
- Crane M, Handy RD. Report for Defra. London, UK: 2007. An assessment of regulatory testing strategies and methods for characterizing the ecotoxicological hazards of nanomaterials. [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malondealdehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84:415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Suematsu H, Kiyomiya E, Aoki M, Sato M, Moritoki N. Size-ependent toxicity of silica nano-particles to Chlorella kessleri. J Environ Sci Health, Part A. 2008;43:1167–1173. doi: 10.1080/10934520802171675. [DOI] [PubMed] [Google Scholar]
- Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1176–1183. doi: 10.1021/tx9701790. [DOI] [PubMed] [Google Scholar]
- Hall S, Bradley T, Moore JT, Kuykindall T, Minella L. Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology. 2009;3(2):91–97. [Google Scholar]
- Handy RD, von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Society. 2007;9:125–144. [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acidreactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Semmler-Behnke M, Brown DM, Kreyling W, Tran L, Stone V. Evaluating the uptake and intracellular fate of polystyrene nanoparticles by primary and hepatocyte cell lines in vitro. Toxicol Appl Pharmacol. 2010;242:66–78. doi: 10.1016/j.taap.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Kashiwada S. Distribution of nanoparticles in the see-through Medaka (Oryzias latipes) Environ Health Perspect. 2006;114:1697–1702. doi: 10.1289/ehp.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1(2):133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach KL, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. Oxide nanoparticle uptake in human lung fibroblast: effects of particle size, agglomeration, and diffusion at low concentrations. Environ Sci Technol. 39:9370–9376. doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- Maness PC, Smolinski S, Blake Z, Huang DM, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65(9):4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–220. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- Medina-Valtierra J, Frausto-Reyes C, Ramirez-Ortiz J, Camarillo-Martinez G. Selfcleanin test of doped TiO2-coated glass plates under solar exposure. Ind Eng Chem Res. 2009;48(2):598–606. [Google Scholar]
- Moger J, Johnston BD, Tyler CR. Imaging metal oxide nanoparticles in biological structures with CARS microscopy. Opt Express. 2008;16(5):3408–19. doi: 10.1364/oe.16.003408. [DOI] [PubMed] [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey go on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37(3):251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- OECD. Organisation for Economic Co-operation and Development (OECD) Guideline for the Testing of Chemicals: (Part 203) 1992 [Google Scholar]
- Owen R, Handy RD. Formulating the problems for environmental risk assessment of nanomaterials. Environ Sci Techno. 2007;41(16):5582–5588. doi: 10.1021/es072598h. [DOI] [PubMed] [Google Scholar]
- Tao X, Fortner JD, Zhang B, He Y, Chen Y, Huges JB. Effects of aqueous stable fullerene nanochrystals (nC60) on Daphnia magna: Evaluation of sub-lethal reproductive responses and accumulation. Chemosphere. 2009;77:1482–1487. doi: 10.1016/j.chemosphere.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Ward JE, Kach DJ. Marine aggregates faciliate ingestion of nanoparticles by suspension-feeding bivalves. Mar Environ Res. 2009;68:137–142. doi: 10.1016/j.marenvres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakajima A, Wang R, Minabe M, Koizumi S, Fujishima A, Hashimoto K. Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass. Thin Solid Films. 1999;351:260–263. [Google Scholar]
- Witsap E, Kupferschmidt N, Bengtsson L, Hultenby K, Smedman C, Paulie S, Garcia AP, Bennett Fadeel B. Efficient internalization of mesoporous silica particles of different sizes by primary human macrophages without impairment of macrophage clearance of apoptotic or antibody-opsonized target cells. Toxicol Appl Pharmacol. 2009;239:306–319. doi: 10.1016/j.taap.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409(8):1444–1452. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang WX, Masciangioli T. Environmental technologies at the nanoscales. Environ Sci Techno. 2003;37:102–108. doi: 10.1021/es0323998. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhu L, Li Y, Qi R, Duan Z, Lang YP. Comparative toxicity of several metal oxide nano-particle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. J Environ Sci Health, Part A. 2008;43:278–284. doi: 10.1080/10934520701792779. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chang Y, Chen Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere. 2010;78:209–215. doi: 10.1016/j.chemosphere.2009.11.013. [DOI] [PubMed] [Google Scholar]