Abstract
Although several cancer immunotherapy strategies are currently based on the use of analog peptides and on the modulation of the TCR affinity of adoptively transferred T cells, it remains unclear whether tumor specific T cell activation by strong and weak TCR stimuli evoke different Ca2+ signatures from the Ca2+ intracellular stores and whether the amplitude of Ca2+ release from the ER can be further modulated by co-receptor binding to peptide/MHC complexes (pMHC). We here combined functional, structural and kinetic measurements to correlate the intensity of Ca2+ signals triggered by the stimulation of the 1G4 T cell clone specific to the tumor epitope NY-ESO-1157–165. Two analogs of the NY-ESO-1157–165 peptide, having similar affinity to HLA-A2 molecules, but a six-fold difference in binding affinity for the 1G4 TCR, resulted in different Ca2+ signals and T cell activation. 1G4 stimulation by the stronger stimulus emptied the ER of stored Ca2+, even in the absence of CD8 binding, resulting in sustained Ca2+ influx. In contrast, the weaker stimulus induced only partial emptying of stored Ca2+, resulting in significantly diminished and oscillatory Ca2+ signals, which was enhanced by CD8 binding. Our data define the range of TCR/pMHC affinities required to induce depletion of Ca2+ from intracellular stores and provide insights into the ability of T cells to tailor the use of the CD8 co-receptor to enhance Ca2+ release from the ER. This in turn modulates Ca2+ influx from the extracellular environment, ultimately controlling T cell activation.
Keywords: T cells, antigens/peptides/epitopes, signal transduction
Introduction
An important question for the optimization of vaccination strategies concerns the nature of the developmental programs, and consequent functional profiles, invoked by TCR triggering by agonists, super-agonists and weak peptide agonists. Since natural tumor antigens elicit relatively weak T cell responses, two approaches currently being investigated are the optimization of the MHC class I anchor residues in tumor epitopes, to enhance binding of the peptide to the MHC class I molecule (1-3), and site directed mutagenesis of TCRs to enhance T cell effector function of adoptively transfered T cells (4). Although in vitro and in vivo data with peptide analogs and mutagenized TCR showed larger antigen-specific CTL expansions than T cell proliferation obtained with wild-type peptides (5) (6), or wild type TCRs (7), the use of peptide agonists and mutated TCR in clinical applications needs to be carefully monitored, as such strategies may result in loss of antigen specificity due to the enhanced T cell reactivity, as reviewed by Iero et al. (8). Recent results have further extended this notion by demonstrating antigen cross-reactivity when CD8+ T cells were transfected with a higher affinity variant of the NY-ESO157–165 A2 restricted 1G4 TCR (7), while the same soluble higher affinity 1G4 TCR was capable of specifically recognizing NY-ESO157–165 pulsed target cells (9).
The manner in which the intensity of TCR triggering is translated functionally is an active area of T cell research. In the last few years it has become known that elevation of intracellular calcium (Ca2+) – a crucial early step in T cell activation – occurs within milliseconds of TCR engagement by peptide-MHC (pMHC) complexes (10, 11). Binding of the TCR to the pMHC results in egress of Ca2+ from the endoplasmic reticulum (ER) into the cytosol (12), which in turn initiates Ca2+ influx into the cytosol from the extracellular environment, via the opening of calcium release-activated calcium (CRAC)/Orai1 channels located at the cell surface (13, 14). The amplitude and duration of the increase in Ca2+ influx modulates both the strength and fitness of the T cell response, and must be sustained for a prolonged period before gene expression and lymphokine production begin (15). It is known that the predominant route of Ca2+ influx into T cells is the store-operated Ca2+ entry (SOCE) pathway, which is regulated by the filling state of the ER (16). The use of altered peptide ligands has shown that agonistic peptides stimulate a cytosolic Ca2+ response composed of an initial small sinusoidal peak followed by a high response, whereas the signal evoked by weaker agonist peptides leads to an oscillatory response (17, 18). The strength of the TCR–pMHC complex also affects the speed of the Ca2+ responses, contributing to the extent of subsequent T cell proliferation (19). Although the SOCE model predicts that the greater the store release (and hence emptying), the greater the Ca2+ influx, to date the relationship between the amount of Ca2+ influx and the release of Ca2+ from intracellular stores has not been addressed with antigen-specific T cell clones activated by peptide ligands with different TCR affinities.
In addition to the affinity of the TCR–pMHC interaction, binding of the CD4 and CD8 co-receptors to MHC class II or class I molecules, respectively (20-22) can influence the overall avidity of TCR–pMHC association (11, 22-25). Moreover, the CD4 and CD8 co-receptors are important for signal initiation, as they associate to the tyrosine kinase Lck (26), which is required for critical early events in TCR signaling (27). It has been shown that accumulation of the tyrosine kinase Lck at the immunological synapse is modulated by the strength of TCR binding to pMHC (28). Lck phosphorylates ITAMs (immunoreceptor tyrosine-based activation motifs), linking the antigen receptor to the downstream signaling machinery of the TCR CD3 molecules (29) and the tyrosine residues on the Syk family kinase ZAP70 (30, 31), thereby increasing the enzymatic activity of ZAP70 (32). Thus the co-receptor plays an important role in coupling ligand binding with the initiation of signal transduction.
Despite the large body of knowledge on Ca2+ influx in T cells, it remains unknown whether the release of Ca2+ from the intracellular stores, which are localized mainly in the ER (16), is modulated by the avidity of the TCR–pMHC interaction. To address this issue, we compared the release of Ca2+ from the ER and the subsequent influx of Ca2+ into the cytosol from the extracellular milieu after stimulation of a defined antigen specific T cell clone (1G4 CTL) (33) by two HLA-A2 (A2) bound peptide analogs, derived from the tumor antigen NY-ESO-1157–165 peptide (SLLMWITQC) (2). Our data indicate that the strength of TCR binding to pMHC results in different degrees of Ca2+ release from the intracellular stores, which in turn, drives a proportional Ca2+ influx. This effect is modulated by the binding of the CD8 co-receptor to MHC class I molecules, which can significantly enhance the amount of Ca2+ release from the intracellular stores triggered by both peptides and in particular by the weaker NY-ESO-1157–165 peptide agonist.
Materials and Methods
Synthetic peptides
Peptides were synthesized by standard solid-phase chemistry on a multiple peptide synthesizer (Genosys, The Woodlands, Texas, USA) by using F-moc for transient N-terminal protection. All peptides were 99% pure as determined by analytical HPLC and mass spectrometry. Lyophilized NY-ESO-1157–165 wild type peptide (SLLMWITQC) and analog peptides containing a substitution of cysteine at position 165 of the NY-ESO-1157–165 to either valine (ESO 9V) or leucine (ESO 9L) were diluted in DMSO and stored at −20°C.
Immunoprecipitation
Aliquots of 14×107 T2 cells were labeled with 74 MBq [35S] methionine for 23 min, washed twice with cold PBS and resuspended in lysis buffer containing protease inhibitors (150 mM NaCl, 50 mM TrisHCl pH 7.5, 5 mM EDTA, 1% NP40, 2 mM phenylmethylsulfonyl fluoride, 5 mM iodoacetamide). After a 30 min peptide pulse at 4°C, the nuclei were removed and the supernatant were precleared overnight at 4°C with 100 μl washed 10% (w/v) Staphylococcus A. BB7.2 antibody (15 μg/tube) was added for 90 min on ice, followed by 150 μl of 5% (w/v) protein A-Sepharose (Sigma). The tubes were rotated for 45 min and then the beads washed four times with lysis buffer. The samples were eluted and analyzed on 12% polyacrylamide gels.
Flow cytometry
T2 cells (3×104) were pulsed with different concentrations of peptides for 2 h at 37°C. Cells were incubated with 40 μg/ml biotinylated F(ab′)2 antibodies for 30 min at 4°C and visualized by Streptavidin conjugated R-PE (Sigma-Aldrich) for 20 min at room temperature.
pMHC production
Residues 1–278 of the A2 heavy chain with the COOH-terminal BirA tag were expressed in Escherichia coli as inclusion bodies, and refolded and purified with the NY–ESO-1 peptides SLLMWITQV or SLLMWITQL and β2M as described previously (33).
Expression and purification of the 1G4 NY–ESO-1 TCR
The 1G4 TCR was refolded and purified from E. coli derived inclusion bodies as described previously (33).
Surface plasmon resonance
Surface plasmon resonance (SPR) experiments were performed by using a Biacore3000 (Biacore). Biotinylated soluble HLA (ligand) was immobilized on Streptavidin-coated CM5 chip (Biacore) at the level of 1,000 RU (response units) per flow cell. Equilibrium binding was measured at the flow rate of 5 μl/min, starting from the lowest analyte concentration. Binding responses were determined by subtracting responses obtained in reference flow cells with irrelevant pMHC immobilized. KD values were calculated by fitting the 1:1 Langmuir binding isotherm to the data using Origi. Kinetics of TCR–pMHC interactions were measured at 30 μl/min, and the curves fitted by global fitting of the standard 1:1 Langmuir binding model to the data (BIAevaluation software; Biacore).
Crystallization and x-ray diffraction data collection
All crystallizations were performed by the sitting drop vapor diffusion technique. Crystals of A2–ESO 9L complexes were grown to dimensions of 130 μm × 80 μm × 70 μm at room temperature (21°C) from 2 μl protein (at 10 mg/ml) + 2 μl mother liquor (14% PEG 8000, 50 mM MES, pH 6.5) drops. Crystals were cryoprotected by sequential transfer into reservoir solutions containing 10 and 20% glycerol and were then flash-cooled, and maintained at 100 K, using a cryostream (Oxford Cryosystems). High-resolution data for the A2–ESO 9L complex were collected at station ID14 EH2 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) with an Area Detector Systems Corporation Quantum 4 CCD detector. Datasets were auto-indexed and integrated with the program DENZO (34) followed by scaling with the program SCALEPACK (34), the results are summarized in Supplemental Table S1.
Crystal structure determination, refinement, and analysis
The HLA-A*0201-SLLMWITQL crystal structure (consisting of two A2–ESO 9L complexes per crystallographic asymmetric unit) was determined by molecular replacement using the CCP4 program Phaser with a search model comprising a representative HLA-A*0201 complex crystal structure (PDB accession code 2V2X). Using the refinement algorithms in REFMAC5 (35) the two A2–ESO 9L complexes were subjected to several rounds of rigid body refinement of individual domains. Then using the restrained TLS refinement algorithms in REFMAC5, crystallographic conjugate gradient minimization refinement was performed and manual refitting of the models was carried out using COOT (36). In the final stages of refinement water picking was carried out with ARPw/ARP (37). All regions of the A2-ESO 9L complex were visible as clear electron density and were included in the final models.
1G4:target cell conjugate measurements
T2 target cells were pulsed with 1 μM, 100 nM, 10 nM or 5 nM of ESO 9V or ESO 9L peptide for 1 h at 37 °C and washed twice in PBS. 1G4 CTL ad T2 cells were washed in PBS and each cell pellet was resuspended to a final concentration of 5 × 105 cells/ml in RPMI. 1G4 CTL and target were mixed 1: 1, left for 5 min in suspension, and then plated on glass multiwell slides and incubated for 30 min at 37 °C. Cells were fixed in 100% methanol precooled to −20°C washed in PBS, and blocked in PBS, 2% BSA. The slides were mounted in PBS containing 90% glycerol and 2.5% DABCO. Samples were examined using a Bio-Rad Radiance 2000 MP laser scanning microscope and the conjugation rate and granule polarization were quantified using an Axioplan 2 epifluorescent microscope (Zeiss).
Live cell video microscopy
2 × 104 1G4 CTL were incubated with 60 nM lysotracker green DND-26 (Molecular Probes) for 1 h at 37 °C in RPMI without phenol red, 5% human serum, 1mM Hepes. T2 target cells were pulsed with 1 μM of either ESO 9V peptide or ESO 9L peptide for 1 h at 37 °C and washed twice in PBS. 2 × 104 target cells were allowed to adhere on a glass coverslip mounted in a temperature-controlled chamber for 10 min at 37 °C in RPMI without serum, without phenol red plus Hepes. Lysotracker green DND-26-labelled 1G4 NY-ESO-1157–165 CTL were added to the T2 cells in the chamber. Sequential confocal images were acquired every 20 s. A Nikon TE300 microscope attached to a Bio-Rad Radiance2000 MP laser scanning microscope was used, with a 488 nm and a 543 nm laser for epifluorescence and Nomarski differential interference contrast for the transmitted light. The images were processed using MetaMorph version 4.5 software.
ELISA assays
1G4 CTL and peptide pulsed target cells (A2 C1R or A2 227/8KA C1R) were incubated at 37°C for 4 h at an effector: target ratio of 1:1, and the supernatant was harvested and assayed for MIP-1β and IFN-γ by ELISA (R&D Systems). Data shown are standard deviation from the mean of two duplicate assays. For the detection of folded HLA-A2 tetramers (Supplemental Fig. S4), HLA-A2 tetramers were immobilized by binding to streptavidin coated plates (Sigma M5432): 100 μl of serial threefold dilutions of pMHC (concentration 100 μg/ml to 500 pg/ml in blocking buffer; 10% FCS in PBS) were added/well and plates were incubated for 1.5 h at 4°C. Plates were washed four times with PBS/0.5% Tween, incubated for 4 h at 37°C and washed four times with wash buffer. Antibody BB7.2 (4°C/well, concentration 10μg/ml in blocking buffer) was added and the plates incubated overnight at 4°C. Following four washes in cold wash buffer 100μl of a 1:1000 dilution (in blocking buffer) of goat anti-mouse IgG (Dako) was added. Plates were incubated for 1 h at 4°C, washed six times and 100μl TMB was added per well. The reaction was stopped with 50μl 0.5 M H2SO4 and signals were quantitated using an ELISA plate reader.
Chromium51 release assay
ESO peptide recognition was assessed using target cells (A2 C1R or A2 227/8KA C1R) labeled with 51Cr for 90 min at 37°C and washed three times. Labeled target cells (5000 cells/100 μl) were then added to U-bottom microwells in the presence or absence of peptides at different concentrations. 51Cr release was measured after incubation for 4 h at 37°C. Percentage specific lysis was calculated as: 100 × [(experimental − spontaneous release)/(total − spontaneous release)].
Stimulation of 1G4 CTL for subsequent immunoblotting
1G4 CTL were washed twice in RPMI and incubated overnight in RPMI containing 10% fetal calf serum (FCS). FCS was then washed off by two changes of RPMI and 1 × 106 1G4 CTL were resuspended in 10 μl RPMI. After 10 min at 37°C and 5% CO2, 1G4 CTL were stimulated by incubation with 1 μg/ml HLA A2 tetramer loaded with either ESO 9V, ESO 9C or ESO 9L peptides for 3 min. The reaction was stopped by washing once with 0.5 ml of ice-cold PBS, and re-suspending the pellet in cold lysis buffer (140 mm NaCl, 20 mm Tris, pH 8.0, 10 mM sodium fluoride, 2 mM EDTA, 20% glycerol, 1% IGEPAL, 1 mM Na3VO4,10 μg/ml aprotinin, 10 μg/ml leupeptin) at 5 × 107 cells/ml.
Antiphosphotyrosine immunoblotting
1G4 CTL were lysed on ice for 30 min and the nuclear fraction pelleted by centrifugation at 16,000 × g for 15 min. The remaining lysate was aspirated and added to an equal volume of SDS loading buffer (350 mm Tris, pH 6.8, 350 mm SDS, 30% glycerol, 600 mm dithiothreitol, 175 μm bromophenol blue). After boiling for 6 min with agitation the sample was separated by electrophoresis (100 V for 16 h) on a 12% SDS/PAGE gel. Proteins were transferred from the gel by electrophoresis at 25 V for 50 min. After transfer, protein bands on the nitrocellulose membrane were visualized by staining (0.1% (w/v) Ponceau S (Sigma) in 5% (v/v) glacial acetic acid (Sigma) for 60 sec with agitation, and incubated with double-distilled water for 60 sec with agitation and drained. The membrane was incubated with sheep anti-mouse peroxidase-linked secondary antibody (Amersham Biosciences, 1:2000 in wash buffer, 2.5% milk powder) for 1.5 h. After three further washes the blot was developed using chemiluminescent substrate Supersignal Pico (Perbio). All washes and incubations with antibody were performed at 4°C.
Intracellular Ca2+ concentration measurements in 1G4 CTL clone
[Ca2+] was monitored in the 1G4 CTL clone by fluorescence microscopy. 1G4 cells adhering to poly-lysine-coated glass coverslips were loaded with Ca2+-sensitive fluorescent dye by incubating cells with RPMI containing 5 μM fluo-3/AM plus 0.03% (w/v) Pluronic F127 for 30 min at room temperature, and mounted on the stage of a Zeiss LSM510 Meta confocal laser scanning microscope (excitation 488 nm, emission >505 nm) equipped with a 63× objective. Target cells (wild type C1R-A2 or mutant C1R-A2 227/8KA) were pulsed with 5 or 100 nM peptide (ESO 9V or ESO 9L) for 90 min at 37°C, washed twice with FCS-free RPMI medium (Sigma) and then added to the cell chamber. Experiments were conducted at room temperature with an image collected every 5 s.
Further validation of our [Ca2+] recordings was obtained by repeating several experiments at 37°C and with the ratiometric dye, fura-2. 1G4 CTL were loaded with 5 μM fura-2/AM (plus 0.03% Pluronic F127) at room temperature for 30 min. Loaded 1G4 cells were then washed with RPMI pre-warmed to 37 °C and mounted on the heated stage of an Olympus IX71 microscope equipped with a 40 × UApo/340 objective (1.35 NA) and a 12-bit Photometrics Coolsnap HQ2 CCD camera. Cells were then excited alternately by 340 nm and 380 nm light using a Cairn monochromator and emission data were collected at 480–540 nm using a bandpass filter. Data are expressed as the 340/380 ratio which is proportional to the intracellular [Ca2+] By analogy with the analysis of the fluo-3 traces, the maximum amplitude and mean elevated ratio (340/380) were calculated on a single-cell basis.
Results
Kinetic analyses of the ESO 9V and ESO 9L binding to A2 molecules and the 1G4 TCR
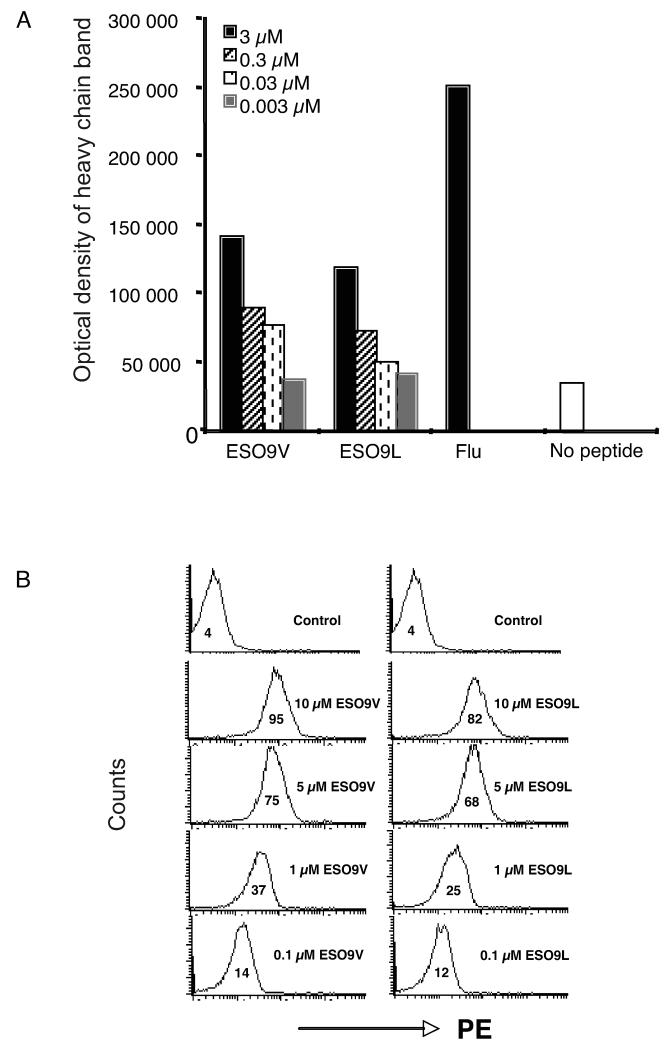
The binding affinity of the ESO 9V and ESO 9L peptides, containing a substitution of cysteine at position 165 of the NY-ESO-1157–165 to either valine (ESO 9V) or leucine (ESO 9L), to A2 molecules was initially assessed by measuring peptide binding to metabolically labeled A2 molecules in T2 cells. Optical density measurements of metabolically labeled A2 eluted from SDS containing polyacrylamide gels showed that ESO 9V and ESO 9L peptides stabilized A2 molecules with a comparable efficiency (Fig. 1A). To further validate these results, experiments were performed using the 3M4E5 F(ab′)2 antibody specific to NY-ESO-1157–165–A2 complexes (33). Initial Biacore measurements showed that the 3M4E5 F(ab′)2 antibody recognized A2 molecules loaded with either ESO 9V or ESO 9L peptide with a similar affinity (Kd) of ~60 nM (data not shown). FACS staining of T2 cells pulsed with different concentrations of the ESO 9V and ESO 9L peptides with the 3M4E5 antibody confirmed that the ESO 9V and ESO 9L peptides bind to A2 molecules equally well (Fig. 1B).
FIGURE 1. Binding measurements of peptide to A2 molecules.
(A) T2 cells were pulsed with either ESO 9V or ESO 9L peptide at the concentrations indicated and the HLA-A2–peptide complexes were precipitated. HLA-A2 molecules from T2 cells pulsed with either 3 μM influenza virus matrix 58–66 peptide or in the absence of peptide were used as positive and negative controls, respectively. (B) T2 cells were stained with the A2–ESO 9C-specific F(ab′)2 antibody 3M4E5. T2 cells were pulsed with either ESO 9V (left) or ESO 9L (right) peptide at the concentrations indicated and then stained with the A2-ESO 9C-specific F(ab′)2. Negative control cells were pulsed with the influenza virus matrix peptide. Data are mean channel fluorescence.
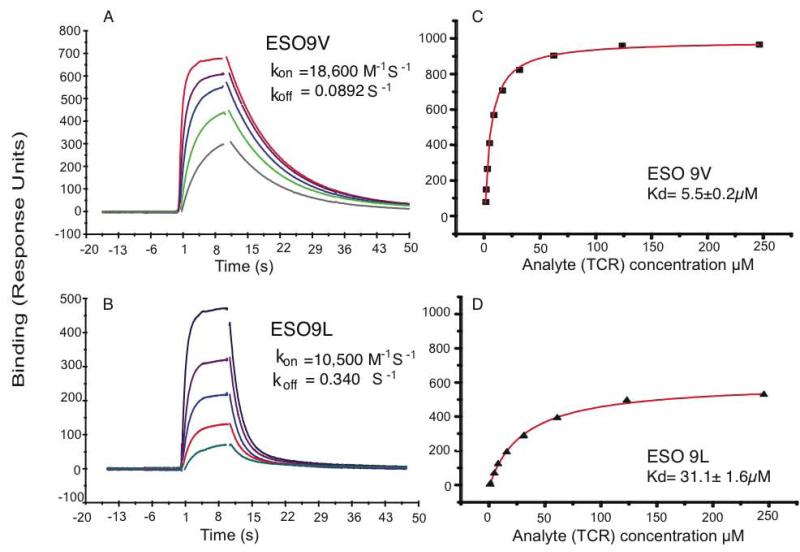
In contrast, binding of the 1G4 TCR to A2 molecules loaded with either the ESO 9V or the ESO 9L peptide demonstrated that the binding affinity of the 1G4 TCR for the A2–ESO 9V complex is six times higher than that for the A2–ESO 9L complex (Fig. 2). The equilibrium binding constants (Kd) at 25°C for the soluble 1G4 TCR binding to A2 loaded with the ESO 9V or ESO 9L peptide were determined to be 5.5 μM and 31.1 μM, respectively (Fig. 2C and D). Kinetic measurements demonstrated that the 1G4 TCR–A2–ESO 9V complex has slower ligand dissociation (t1/2=8.77 s-1) than the 1G4 TCR–A2–ESO 9L complex (t1/2=1.69 s-1) (Fig. 2A and B). Analysis of the binding curves of conformation-specific anti-A2–peptide and anti-β2M antibodies confirmed that equivalent amounts of correctly refolded A2–peptide complexes were immobilized to the Biacore chips (Supplemental Fig. S1).
FIGURE 2. Binding of TCR to the pMHC complex.
For kinetic measurements (A, C) TCR was injected at five concentrations (24 μM, 12 μM, 6 μM, 3 μM) over the indicted immobilized peptide–A2 complex. The binding responses for all TCR concentrations are overlaid. The indicated rate constants were determined by global fitting of the 1:1 Langmuir binding model to these data. For affinity measurements (B, D) TCR was injected over immobilized peptide–A2 complex and the response was allowed to reach equilibrium. The binding response at equilibrium is plotted against concentration. The 1:1 Langmuir binding model was fitted to the data (solid line) to determine the indicated Kd (mean ± SD of fit).
Crystal structure of A2–ESO 9L and A2–ESO 9V indicates alterations in the surface presented for TCR recognition
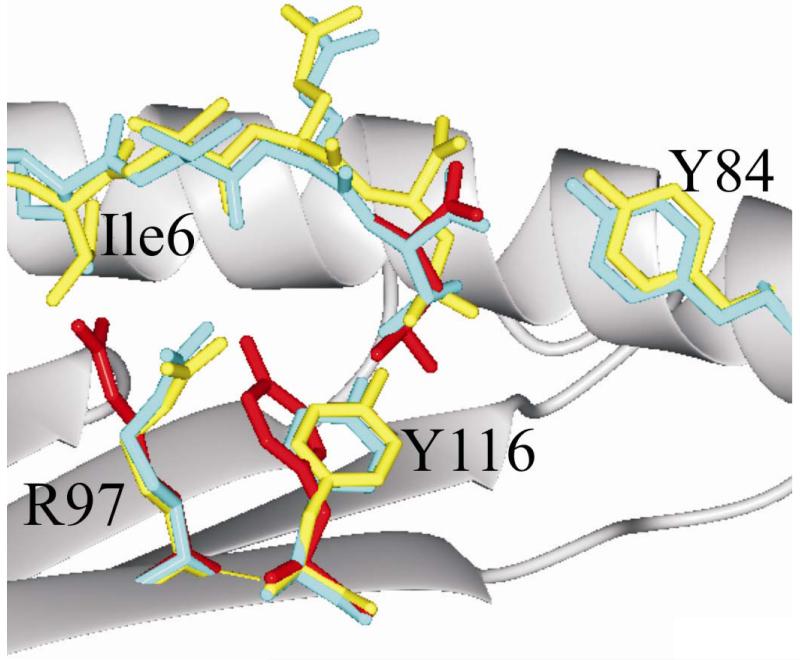
To explore the molecular basis for the similar affinity of ESO 9L and ESO 9V to A2 molecules and to gain insights into the mechanisms controlling the different affinity of binding to the 1G4 TCR, we determined the crystal structure of the A2-ESO 9L complex at 1.7 Å resolution. Comparison of this high resolution structure with those previously reported for A2–ESO 9C (38) and the 1G4 TCR–A2–ESO 9V complex (33) revealed changes in the surface presented for TCR recognition that were the indirect result of the substitution of the P9 anchor side chain. These changes are triggered by the larger size of the leucine side chain compared to those of cysteine and valine. The F pocket in A2 can accommodate valine or leucine, but in previously reported structures the latter choice of anchor residue triggers a local rearrangement of the binding groove residues, expanding the volume of the F pocket to match the longer side chain (Fig. 3). This rearrangement requires a concerted switch in side chain conformation by A2 binding groove residue Y116, which directly abuts the F pocket, and its neighboring residue R97. As first observed by Madden et al. (39) the Y116-R97 conformational switch fine-tunes the capacity of the F pocket to the size of the P9 side chain. However, the overall capacity of the A2 binding groove remains constant and thus this rearrangement can occur only if there is spare capacity in the central region of the binding groove. In the A2–ESO 9C and 1G4 TCR–A2–ESO 9V complexes peptide residue I6 acts as a secondary anchor, its side chain bound in the center of the A2 groove. Our crystallographic structure determination reveals that the use of this secondary anchor is maintained in A2–ESO 9L, and consequently the Y116–R97 conformational switch is blocked, precluding any expansion of the F pocket. The longer side chain of leucine cannot be accommodated fully within the F pocket, necessitating an overall upward displacement of the P9 residue relative to its position in other A2–ESO complexes, a change which propagates through the peptide to give a distinct shift in main chain position for the entire region between P4 and P9 and hence to an altered surface presented for TCR recognition.
FIGURE 3. Crystal structure of A2-ESO 9L reveals changes in peptide position on substitution of the P9 anchor.
The binding groove of A2 is shown schematically as a main chain ribbon (grey) with selected side chains in stick forma. The α2 helix is omitted for clarity and only the structures containing the peptide residues I-T-Q-C (P6-P9) are shown. The ESO 9L peptide is shown in yellow and the ESO 9V peptide from the 1G4 TCR–A2–ESO9V complex (PDB code 2BNQ) in cyan. The P9 anchor residue and selected A2 side chains from the A2–SLFNTVATL complex (PDB code 2V2X a representative P9 leucine anchor peptide–A2 structure) are shown in red, and are shown positioned on the A2–ESO 9L structure in accordance with structural super-positions of the A2 binding grooves.
ESO 9V is more efficient in inducing conjugate formation and lytic granule polarization than ESO 9L
Formation of conjugates between 1G4 CTL and A2 C1R target cells pulsed with either the ESO 9V or the ESO 9L peptide was captured by videomicroscopy. Supplemental Movie S1 and S2 show that for ESO 9V pulsed target cells (Movie S1) conjugate formation was both faster and more frequent than for ESO 9L pulsed target cells (Movie S2).
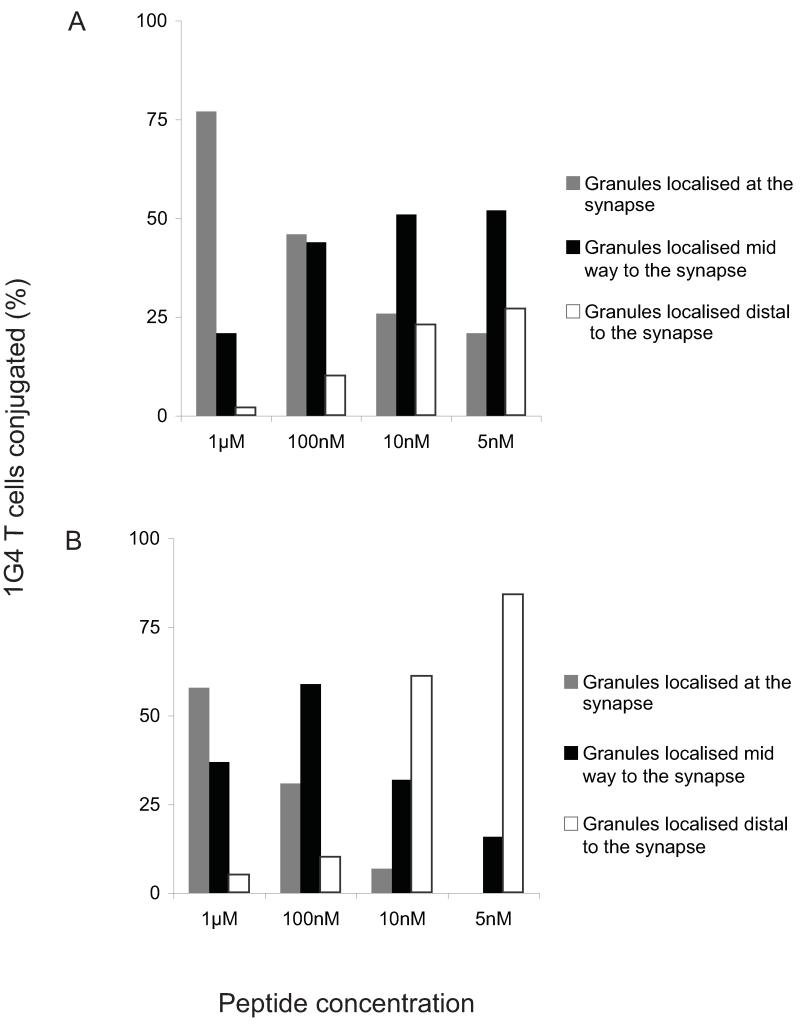
To assess the rate of polarization of cytotoxic granules after stimulation of NY-ESO-1157–165 specific 1G4 T cells with either the ESO 9V or ESO 9L peptide, 1G4 T cells were incubated for 30 min at 37 °C with T2 target cells pulsed with different concentrations of the two peptides and then fixed. Anti-cathepsin D antibodies were used to visualize the lytic granules which were then quantified according to whether the granules and Golgi were localised at the synapse, distal to the synapse or mid-way. T2 cells pulsed with ESO 9V peptide were much more efficient in inducing polarization of granules to the synapse in conjugated 1G4 CTL than T2 cells pulsed with the weaker ESO 9L agonist (Fig. 4A and B). For example, whereas 10 nM ESO 9V resulted in approximately 25% of granules being located at the synapse (Fig. 4A), 100 nM of the ESO 9L peptide was required to achieve approximately the same effect (Fig. 4B). The more efficient lytic granule polarization with the stronger ESO 9V peptide is reflected in the higher percentage of target cell killing seen with ESO 9V as compared with ESO 9L (see Supplemental Fig. S2).
FIGURE 4. Quantitative analysis of granule polarization in the 1G4 CTL clone recognizing targets pulsed with ESO 9V (A) or ESO 9L peptides (B).
Cell conjugates with granules and Golgi localized at the synapse (dark gray bars), distal to the synapse (open bars) or mid-way (black bars) were quantified by using a fluorescent microscope.
Examination of the tyrosine phosphorylation patterns induced in the 1G4 CTL clone by stimulation with either HLA-A2 tetramers or cell surface presentation of the ESO 9L, ESO 9V and ESO 9C peptides showed that the main difference in the early membrane proximal signaling induced by the three peptide agonists were associated with the TCR zeta chain. As shown in Supplemental Fig. S3, ESO 9V is very efficient in inducing phosphorylation of TCR zeta both when loaded onto HLA-A2 tetramers and when presented at the target cell surface, and was the only one of the peptide agonists to induce appreciable amounts of LAT (p36). ESO 9L induced zeta phosphorylation only poorly. Supplemental Fig. S4 confirms that HLA A2 tetramers used in Supplemental Figure S3B were equally folded, as defined by their ability to be recognized by the HLA-A2 conformational specific antibody BB7.2
ESO 9L elicits a lower cytokine response than ESO 9V in 1G4 T cells, which is significantly enhanced by CD8 binding
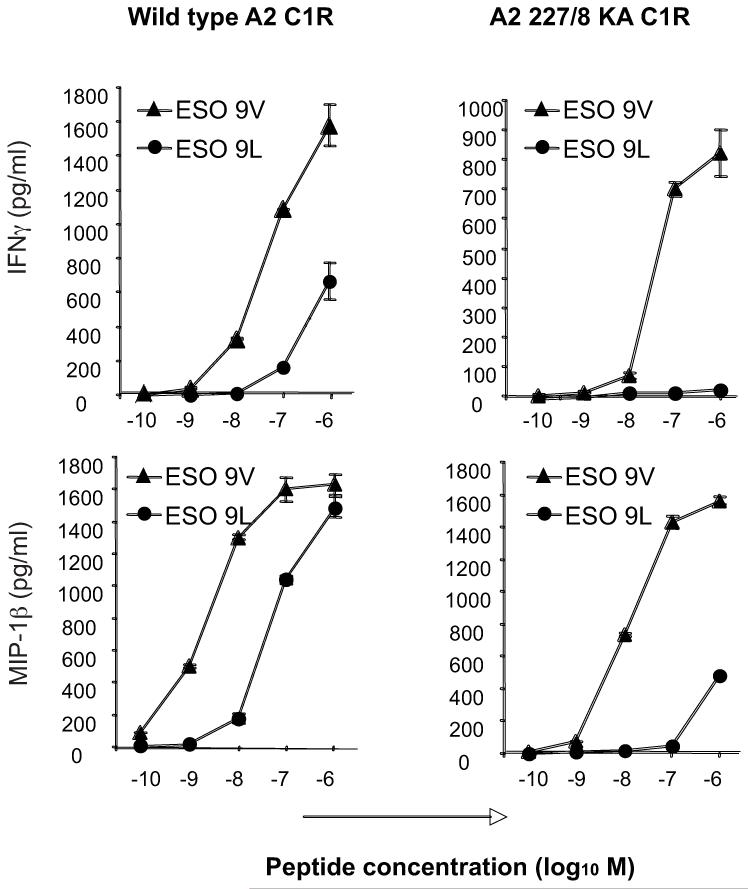
To examine whether a six-fold difference in the affinity of 1G4 TCR binding to NY-ESO-1157–165 analogs could affect activation of the 1G4 T cell clone, we measured secretion of IFNγ and MIP-1β by the 1G4 CTL clone stimulated with different concentrations of the ESO 9L and ESO 9V peptides, which were loaded onto wild-type A2 C1R target cells (Fig. 5, left panels). Consistent with the differences in 1G4 TCR affinities measured by SPR, the level of cytokines secreted by the 1G4 CTL clone sensitized with the ESO 9V peptide was ~50-fold higher than the cytokines secreted after stimulation with the ESO 9L peptide.
FIGURE 5. Role of CD8 in the recognition of the ESO 9V and ESO 9L peptides.
C1R B cells transfected with either A2 (wild type, having a normal interaction with CD8) or A2 227/8 KA (mutant, showing no interaction with CD8) were pulsed with either the ESO 9V or ESO 9L peptide for 90 min at 37°C. 5×104 CTL were incubated with 5×104 pulsed target cells in a total volume of 200 μl in a 96 well plate. Supernatants were harvested and used for ELISA for MIP-1β or IFN-γ.
To assess the requirement of CD8 binding in the activation of the 1G4 CTL clone by the ESO 9V and ESO 9L peptides, we compared 1G4 activation by C1R cells expressing either the wild type or CD8-null A2 molecules (i.e. A2 227/228 KA C1R cells) (Fig. 5, right panels). Recognition of the ESO 9V peptide is much less dependent on CD8 than recognition of the ESO 9L peptide, as 1G4 T cell activation by the ESO 9L was much more efficient in the presence of A2 molecules bearing a CD8 binding site than the activation of the 1G4 T cells by the ESO 9V peptide. FACS staining of A2 C1R and A2 227/228 KA C1R cells pulsed with different concentrations of the ESO 9V and ESO 9L peptides with the 3M4E5 antibody confirmed that the ESO 9V and ESO 9L peptides bind to A2 and A2 227/228 KA molecules equally well (data not shown).
These results confirm the SPR measurements and indicate that, in the presence of equal A2 loading by the ESO 9L and ESO 9V peptides, the six-fold difference in binding affinity of the 1G4 TCR to the A2/ESO 9V and A2/ESO 9L complexes results in 50-100 difference in the activation of the 1G4 T cell clone.
Different Ca2+ influx signatures induced by the ESO 9V and ESO 9L peptides
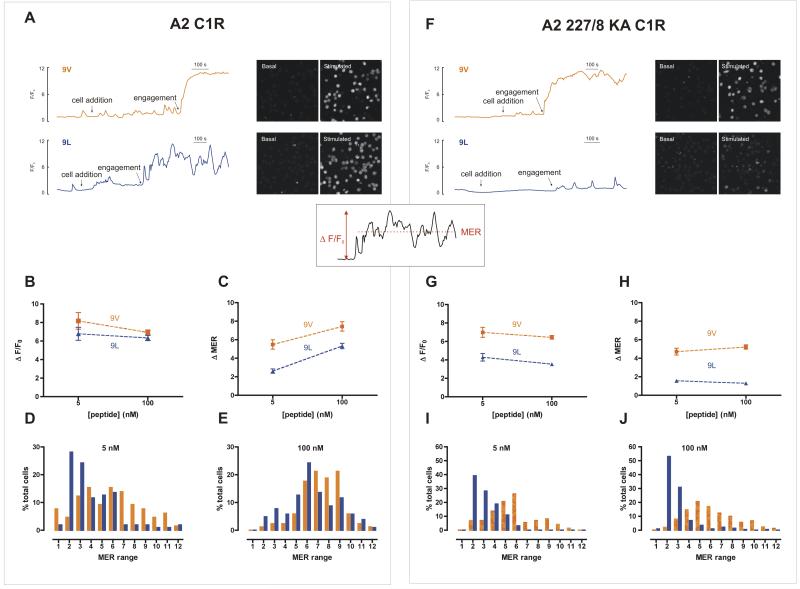
Having defined the threshold of 1G4 T cell activation, we assessed Ca2+ responses at the single cell level by confocal microscopy upon stimulation by C1R-A2 cells pulsed with different concentrations of either the ESO 9V or ESO 9L peptides. C1R-A2 cells pulsed with the influenza matrix58-66 peptide served as a negative control. Using either 5 nM or 100 nM ESO 9V peptide resulted in a robust and sustained Ca2+ increase (Fig. 6A, upper trace and data not shown), whereas recognition of the ESO 9L peptide resulted in a less sustained and more oscillatory response (Fig. 6A, lower trace). The height of the maximum peak Ca2+ response (F/F0) was not significantly different between the two peptides, irrespective of the peptide concentration (P>0.05, Fig. 6B). However, the change in the mean fluorescence (Δ mean elevated ratio, MER) (an indicator of how sustained the Ca2 influx remains throughout the post-engagement period) was consistently higher with ESO 9V than with ESO 9L, at both 5nM and 100nM peptide concentrations (Fig. 6C). These findings can be accounted for by the nonsustained, oscillatory nature of the ESO 9L-dependent response, which results in a lower mean Ca2+ concentration. In terms of population distribution, a large proportion of the 1G4 CTL responded weakly to 5 nM of the ESO 9L peptide, whereas a larger proportion of the 1G4 CTL responded more strongly to the same concentration of the ESO 9V peptide (Fig. 6D). These differences are preserved with 100 nM peptide, albeit they are less marked (Fig. 6E). No significant Ca2+ response was observed with influenza matrix58-66 peptide loaded target cells (data not shown).
FIGURE 6. Ca2+ signals in 1G4 CTL upon engagement with C1R cells loaded with ESO 9V and ESO 9L peptides.
[Ca2+]i changes were monitored in fluo-3-loaded 1G4 CTL using a confocal microscope. A2 (A–E) or CD8-null 227/8 KA A2 (F–J) C1R cells were loaded with ESO 9V or ESO 9L peptide (5 nM or 100 nM) and added to the chamber (‘cell addition’). (A, F) Uncalibrated [Ca2+]i traces are from representative single cells normalized to initial fluorescence (F/F0) using 100 nM of the indicated peptide and have been aligned to the time of engagement (TCR–pMHC interaction). Time on the x-axis is represented to the scale shown. Basal (initial) and stimulated (final) images of fluo-3 fluorescence of a field of cells depict basal and stimulated [Ca2+]I, and are aligned with the appropriate trace. (B and G) Maximal peak fluorescence changes were determined as the difference between the basal and the maximum fluorescence, ΔF/F0 (calculated as shown in the inset schematic). (C and H) The mean elevated ratio (MER) is the mean value of fluorescence throughout the post-engagement period, as normalized to F0. (D, E, I, J) % of total 1G4 T cells stimulated with either the ESO 9V or ESO 9L peptide with the shown MER value. The MER range is incremented with the maximum value for each range indicated on the x axis. Data are plotted as the mean ± SEM of 59–104 cells (A2 C1R cells) or 54–260 cells (A2 227/8 AK C1R cells).
To address the contribution of CD8 binding to the overall Ca2+ signatures, we then tested the CD8-null C1R A2 227/8KA cells pulsed with either ESO 9V or ESO 9L peptide. Recognition of ESO 9V elicited large and sustained Ca2+ responses, similar to the responses observed with C1R-A2 cells (Fig. 6F upper trace). However, in contrast with the Ca2+ responses seen with C1R cells expressing wild type A2 molecules, C1R A2 227/8KA cells pulsed with ESO 9L peptide evoked only small, oscillatory signals (Fig. 6F lower trace), even at the higher peptide concentration (Fig. 6G and H). Indeed, the majority of 1G4 CTL responded weakly to the ESO 9L peptide, in contrast with the stronger response triggered by the ESO 9V peptide stimulation (Fig. 6I and J and supplemental Movie S3, Movie S4, Movie S5 and Movie S6). These functional data demonstrate the importance of the co-receptor CD8 in amplifying the Ca2+ signal driven by the weaker ESO 9L peptide, enabling compensation for the lower TCR–pMHC affinity (Fig. 6B and C). In conclusion, these results highlight the ability of individual 1G4 T cells to integrate the affinity of TCR binding and tailor the use of the CD8 co-receptor to the avidity of TCR binding.
We further confirmed the differences in [Ca2+] response to stimulation by the ESO 9V and ESO 9L peptides and also by the unmodified ESO 9C peptide by using the ratiometric dye fura-2 at 37° C on a widefield inverted microscope. As shown in Supplemental Fig. S5 the robust and sustained Ca2+ release invoked by ESO 9V was apparent in both A2 C1R and the CD8-null A2 227/228 KA C1R cells, whereas the response to the ESO 9L peptide was confirmed to be dependent on CD8 expression. Furthermore, the use of this protocol allowed us to rule out the possibility that Ca2+ oscillations seen in individual cells when stimulated with the weaker ESO 9L agonist, were due to cell movement and were not methodological artifacts, but variation in the Ca2+ concentration (see Supplemental Fig. S6).
Differences in the Ca2+ release from the ER evoked by the ESO 9V and ESO 9L peptides
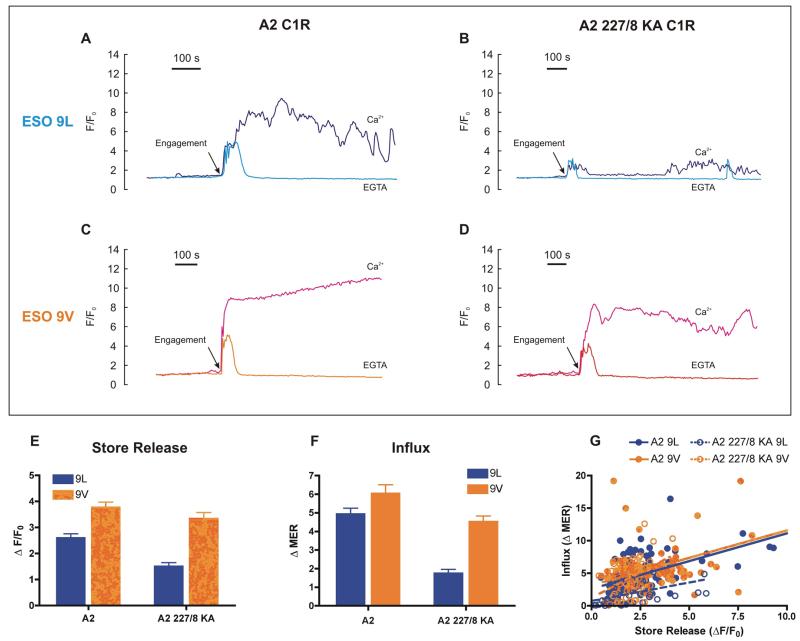
There are two sources of Ca2+ that can be utilized by T cells: intracellular Ca2+ stores (Ca2+ release) and the extracellular reservoir (Ca2+ influx) (16). In order to dissect their relative contributions to the antigen-induced T cell responses, we performed experiments in Ca2+-free medium (Fig. 7A–D), which abolishes Ca2+ influx and isolates the intracellular Ca2+ release phase (16). In control (Ca2+-containing) medium, Ca2+ increased in two phases: an immediate smaller spike, which is followed by a slower, sustained rise (or Ca2+ oscillations) (Fig. 7A–D). As expected, removing extracellular Ca2+ with EGTA confirmed that the first transient peak represents Ca2+ release from intracellular stores (Fig. 7A–D); clearly, the subsequent response was predominantly Ca2+ influx since Ca2+-free medium abolished this phase in all cases (Fig. 7A–D). Therefore, the major difference between stimuli lies in their ability to promote this Ca2+ influx (Fig. 7F).
FIGURE 7. The relative contribution of intracellular Ca2+ release and Ca2+ influx to the 1G4 CTL response.
1G4 CTL [Ca2+]i responses were monitored with fluo-3 as described previously. Before cell addition (not shown), C1R cells and 1G4 CTL were washed in Ca2+-free medium supplemented with 1 mM EGTA to remove all traces of extracellular Ca2+ and experiments conducted in the same medium where indicated (EGTA). In parallel, control cells were maintained throughout in Ca2+-containing medium (0.5 mM, as for RPMI, Ca2+). (A–D) Traces from representative single cells normalized to initial fluorescence (F/F0) using 100 nM of the indicated peptide under either condition (Ca2+ or EGTA), and manually aligned to the time of engagement (TCR–pMHC interaction). Time on the x-axis is represented to the scale shown. (E and F) Summary of the responses in EGTA containing medium. (E) Amplitude of the initial Ca2+ release from intracellular stores. (F) Subsequent Ca2+ influx quantified as the total post-engagement ΔMER. Data are mean intracellular Ca2+ signal in the presence of EGTA expressed as a percentage of the parallel control in the presence of extracellular Ca2+. Data in E and F are expressed as the mean ± SEM of n single cells (n = 102 (A2, ESO9L); 113 (A2, ESO9V); 68 (A2 227/8, ESO9L); 63 (A2 227/8, ESO9V). (G) Correlation analysis in which data are fitted by linear regression. A significant correlation was observed for all data sets (P<0.001) with r2 values of 0.40 (A2, ESO9L); 0.41 (A2, ESO9V); 0.22 (A2 227/8, ESO9L); 0.20 (A2 227/8, ESO9V).
The predominant route of Ca2+ influx into T cells is the SOCE pathway, which is regulated by the filling state of the intracellular Ca2+ stores via a process involving the recently discovered STIM1 and Orai1 (40, 41). Our data might be explained by the differential emptying of the internal stores, which, in turn, drives a proportional Ca2+ influx. Several lines of evidence support such a hypothesis. First, Ca2+ release (Fig. 7E) and Ca2+ influx (Fig. 7F) appear to mirror each other e.g. they are greater for the stronger ESO 9V as compared to the weaker ESO 9L stimulus, and this difference is more pronounced in the absence of CD8. Moreover, these pooled mean data are a reflection of the underlying single cell Ca2+ signals, which show a significant correlation (P<0.001) between the magnitude of the Ca2+ release from stores and the subsequent Ca2+ influx for all conditions (Fig. 7G). This proportionality of Ca2+ store release and Ca2+ influx is a hallmark of the SOCE model. Taken together, it emerges that each stimulus evokes a Ca2+ release from the ER proportional to the stimulus intensity, which in turn drives a proportional Ca2+ influx.
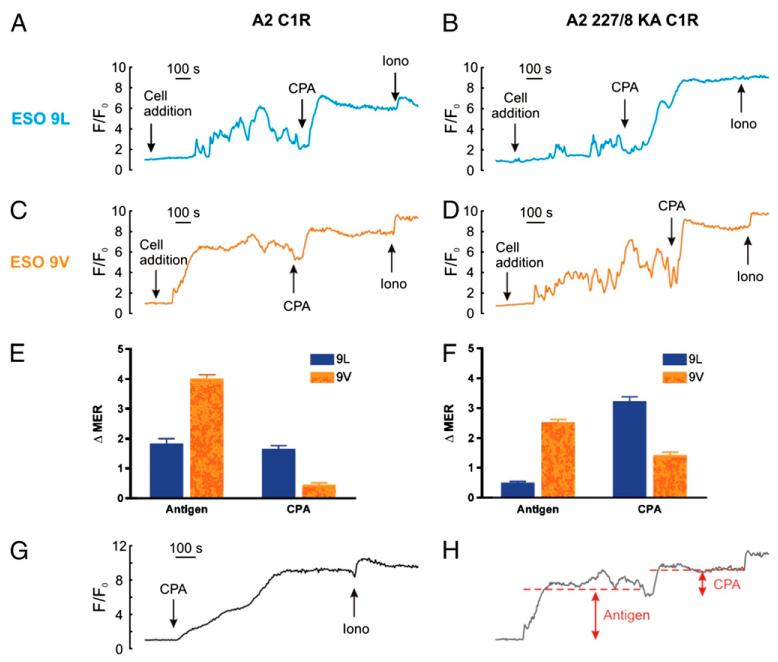
To further strengthen the interpretation of our results indicating differential Ca2+ release from stores upon T cell stimulation with the different peptides, we used a complementary approach to probe the filling state of the ER by emptying the ER pharmacologically using cyclopiazonic acid (CPA), which inhibits the ER Ca2+ pump, resulting in Ca2+ leakage from the ER (42). Diagnostic for SOCE, CPA alone empties the ER and activates Ca2+ entry (43). To confirm that the ER was fully emptied by CPA, the Ca2+ ionophore, ionomycin, was subsequently applied, demonstrating little further effect, as shown in (Fig. 8G). We then went on to test the ER Ca2+ store size using CPA after stimulating the 1G4 T cell clone with either the ESO 9V or ESO 9L peptide, in the presence or absence of CD8 binding (the mean Ca2+ responses were quantified as schematically depicted in Fig. 8H). We observed that 1G4 T cells stimulated by the stronger ESO 9V peptide agonist emptied the intracellular stores almost completely, since CPA and ionomycin had little further effect on Ca2+ release (Fig. 8C and E). In contrast, the weaker ESO 9L peptide agonist failed to empty the stores completely, as shown by the larger increase in Ca2+ release after the addition of CPA and ionomycin (Fig. 8A and E). Consistent with the results shown in Fig. 6, the absence of CD8 binding to the A2 227/8KA cells compromised the Ca2+ release from stores after stimulation with the weaker ESO 9L and a large residual Ca2+ content was revealed by CPA (Fig. 8B and F). In contrast, Ca2+ store depletion was still large with the ESO 9V peptide even in the absence of CD8 co-receptors as evidenced by the small response to CPA and ionomycin (Fig. 8D and F). The reciprocal relationship between the responses to antigen and to CPA is consistent with a common pathway and further reinforces our SOCE hypothesis.
FIGURE 8. Probing the Ca2+ store filling status with the Ca2+ pump inhibitor, cyclopiazonic acid.
1G4 CTL were stimulated with C1R A2 and C1R A2 227/8 KA cells pulsed with 100 nM ESO 9V or ESO 9L peptide as described previously, and subsequently treated with 30 μM cyclopiazonic acid (CPA) and 5 μM ionomycin as indicated (A–D). Control cells were treated with CPA and ionomycin only (G). (H) Schematic illustrating how the traces were measured, with the dotted line showing the MER compared to F/F0 immediately prior to the additions. Data were quantified by measuring the change in the mean Ca2+ response as shown in (E and F), and expressed as the mean ± SEM of n single cells (n= 71 (A2, ESO9L); 105 (A2, ESO9V); 79 (A2 227/8, ESO9L); 88 (A2 227/8, ESO9V). Time on the x-axis is represented to the scale shown.
Taken together these results further support the conclusion that the strength of TCR affinity stimulates differential egress of Ca2+ release from intracellular stores, which, in turn drives a proportional Ca2+ influx.
Discussion
Our results demonstrate that the amount of Ca2+ released from intracellular stores is dependent on the strength of TCR stimulation and can be modulated by CD8 binding to pMHC complexes. While strong agonist peptides are capable of depleting the ER of Ca2+, even in the absence of CD8 binding to pMHC, T cell activation by weaker peptide agonists requires CD8 binding to pMHC to enhance the amount of Ca2+ released from the ER, resulting in greater Ca2+ influx from the extracellular environment and significantly more efficient T cell activation.
By applying a combination of structural, kinetic and functional analyses, we characterized two peptide analogs of the wild type NY-ESO-1157–165 tumor antigen peptide—the ESO 9V and ESO 9L peptides—that have a very similar affinity for A2 molecules and differ by only six-fold in their affinity to the 1G4 TCR. This model provided us with the opportunity to study the activation of the 1G4 T cell clone by the strong (ESO 9V) or weak (ESO 9L) peptide, and interpret its differential activation as a direct effect of the two peptides’ affinity of binding to the 1G4 TCR, which is currently being used by several groups to study the relationship between TCR mutagenesis, affinity to pMHC and T cell activation (7, 9, 33, 44, 45).
A high resolution structure (1.65 Å) of unliganded A2 molecules loaded with the ESO 9L peptide demonstrated that the overall structure of the ESO 9L epitope in A2 is similar to the structure of the 1G4 liganded A2–ESO 9V (33) and unliganded A2–ESO 9C complexes (38). The analysis of the A2–ESO 9L crystal structure revealed an overall upward displacement of the P9 residue relative to its position in the ESO 9V peptide, due to the blocked Y116-R97 conformational switch, which precludes any expansion of the F pocket. It is likely that such subtle, indirectly triggered, changes in the pMHC surface may account for the six-fold variation in binding affinity between the 1G4 TCR and the ESO 9V and ESO 9L peptides, as similar changes in the pMHC surface have been shown to alter TCR binding properties in a number of pMHC-TCR systems (46).
Analysis of cytokine secretion released by the 1G4 T clone stimulated by either the ESO 9V or ESO 9L peptide in the presence or absence of CD8 binding allowed us to define the threshold of activation of the 1G4 T cell clone by either strong or weak stimuli, and to confirm that T cell stimulation by strong stimuli is less dependent on the use of the co-receptor CD8.
After characterizing the threshold of 1G4 activation by strong and weak stimuli, we then analyzed the pattern of Ca2+ signals evoked by two different concentrations (5 nM and 100 nM) of ESO 9L and ESO 9V peptides in the presence or absence of CD8 binding. The results of these experiments confirmed that the pattern of the Ca2+ signals can be modulated by both the strength of TCR stimulation and by the binding of the co-receptor CD8 to MHC class I molecules. Our data unequivocally demonstrate that the Ca2+ signals elicited by the ESO 9V peptide are more robust and sustained than those evoked with ESO 9L. The increase of TCR dependent cytosolic Ca2+ in T cells serves a number of functions in both the short and long term (47-49)—Ca2+ signals are associated with motility, T cell proliferation, changes in T cell gene expression and secretion of cytokines, and the pattern of these signals determines T cell polarization, fitness and life span. For example, more sustained Ca2+ responses favor activation of NF-AT, c-JNK and IL-2 expression, whereas lower Ca2+ signals promote NF-κB activation (15, 50, 51).
By investigating what underlies these Ca2+ signatures, we propose a model whereby the strength of the pMHC–TCR interaction dictates the degree of ER Ca2+ store depletion which, in turn, drives a proportional activation of SOCE. Such a model is upheld by various aspects of our data: first, the abolition of Ca2+ influx (by using Ca2+-free medium) confirmed that the initial Ca2+ phase was derived from intracellular stores; second, this Ca2+ release represented only a minor (but crucial) contributor to the Ca2+ signature which was otherwise dominated by Ca2+ influx; third, this Ca2+ influx was proportional to the degree of store emptying (as assessed by two independent means). Overall, the stronger stimuli evoked a substantial depletion of stores and a marked Ca2+ influx, whilst for the weaker stimuli, both phases were proportionally smaller.
In terms of the underlying cell signalling, it has been suggested that TCR stimulation leads to production of various Ca2+-mobilizing second messengers, inositol 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (52). Each messenger can evoke Ca2+ release from internal stores by activating its own unique cognate receptor—IP3 receptors, ryanodine receptors and two-pore channels, NAADP receptors respectively (53). While the role of IP3 receptors in TCR mediated responses has previously been shown (16, 54), it is still unclear whether the other second messengers (cADPR and NAADP) are involved in the response of T cell clones by cognate peptide agonists, since only Jurkat T cells activated by anti-CD3 antibody treatment have thus far been tested (55-57).
Following this first phase, Ca2+ influx ensues as a result of the recruitment of the SOCE pathway, operated via the opening of calcium release-activated channels (CRAC)/Orai1 located at the T cell surface (13, 14), which are linked to ER Ca2+ store emptying, through the ER-resident Ca2+ sensor STIM1 (14, 47, 48). We contend that the Orai/STIM1 complex is differentially activated by the different peptides we have used, but this will require experimental confirmation. Whilst we acknowledge that other factors can influence the Ca2+ influx phase such as plasma membrane Ca2+ pumps (58) or changes in membrane potential (47, 59), the fact that CPA responses reach a similar plateau after any antigen would tend to discount them as the major influence. We therefore conclude that different antigens evoke different Ca2+ influx signals as a consequence of different Ca2+ release from internal stores.
We also showed that the CD8 co-receptor influences the release of Ca2+ from the ER and the overall pattern of Ca2+ influx. While for strong stimuli, the presence or absence of CD8 binding did not significantly alter the release of Ca2+ from the stores, the amount of Ca2+ released from the stores in response to weaker stimuli was enhanced by CD8 binding to MHC class I molecules. It is known that CD8 binding to MHC class I molecules is important for stabilizing the interaction between low affinity TCR and MHC class I molecules (60, 61) and for controlling the dynamics of CTL activation and immunological synapse formation (62). In addition, it has been shown that lymphokines can regulate the level of CD8 co-receptor expression (63). Our observation that the CD8 co-receptor influences the release of Ca2+ from the ER and the overall pattern of Ca2+ influx provides further evidence that T cells are able to integrate the strength of TCR signals and tailor the use of co-receptors, to increase the avidity of the TCR–pMHC interaction for a weaker ligand so that the T cell activation threshold is exceeded. This latter finding is consistent with the results of a recently published report indicating that T cells can ‘sense’ the affinity of cognate peptide by fine tuning the duration of T cell interaction with target cells, depending on the dose of peptides presented on the surface of target cells (64) and of integrating TCR dependent signals by tailoring the use of the co-receptor CD8 to reach a set threshold of activation.
In conclusion, our data highlight the correlation between TCR affinity and the release of Ca2+ from intracellular stores. This effect is further enhanced by the binding of the co-receptor CD8 to MHC class I molecules, indicating that T cells are capable of ‘sensing’ the affinity of peptide agonists, and determining their requirement for a co-receptor such as CD8 in the context of weak peptide agonists, to ensure T cell activation. Together, these data provide important insights into the mechanisms controlling the activation of melanoma specific CD8+ T cells, underscoring the ability of T cells to recruit the CD8 co-receptor to enhance Ca2+ release from the ER in the context of weak peptide agonists, thus ensuring an appropriate T cell response. This ability to modulate the strength of T cell interaction by the recruitment of co-receptors ensures that individual T cells can recognize a broader pool of self and non-self peptides. This not only has implications for the use of altered peptide ligands and mutagenized TCRs in adoptively transferred tumor specific CD4+ and CD8+ T cells, it may also be important for the modulation of the immune response to self-antigens in autoimmune disorders, and for cross-reactivity between virus serotypes, such as in the recognition of rapidly mutating HIV-1 escape variants.
Supplementary Material
Acknowledgments
We wish to thank Oreste Acuto for critically reading the manuscript and Moira Johnson for editing the manuscript.
This work was funded by Cancer Research UK (C399/A2291), European Grant FP6 Cancer Immuntherapy and the UK Medical Research Council. E.Y.J. is a CR-UK Principal Research Fellow. AJM and AG are funded by the Wellcome Trust.
Footnotes
Coordinates for the ESO-9L A2 crystal structure have been deposited in the RCSB Protein Data Bank (www.rcsb.org/pdb/home/home.do) with the accession number 3KLA. We thank the staff of the European Synchrotron Radiation Facility and the EMBL outstation at Grenoble for assistance with data collection. Crystallization trials were carried out using facilities provided by the Oxford Protein Production Facility and the European Commission Integrated Programme SPINE (QLG2-CT-2002-00988).
References
- 1.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JL, Dunbar PR, Gileadi U, Jager E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A, Old LJ, Cerundolo V. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 3.Romero P, Dutoit V, Rubio-Godoy V, Lienard D, Speiser D, Guillaume P, Servis K, Rimoldi D, Cerottini JC, Valmori D. CD8+ T-cell response to NY-ESO-1: relative antigenicity and in vitro immunogenicity of natural and analogue sequences. Clin Cancer Res. 2001;7:766s–772s. [PubMed] [Google Scholar]
- 4.Schumacher TN, Restifo NP. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2009;21:187–189. doi: 10.1016/j.coi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 6.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 7.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, Bennett AD, Dunn SM, Mahon TM, Jakobsen BK, Rosenberg SA. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iero M, Filipazzi P, Castelli C, Belli F, Valdagni R, Parmiani G, Patuzzo R, Santinami M, Rivoltini L. Modified peptides in anti-cancer vaccines: are we eventually improving anti-tumour immunity? Cancer Immunol Immunother. 2009;58:1159–1167. doi: 10.1007/s00262-008-0610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purbhoo MA, Sutton DH, Brewer JE, Mullings RE, Hill ME, Mahon TM, Karbach J, Jager E, Cameron BJ, Lissin N, Vyas P, Chen JL, Cerundolo V, Jakobsen BK. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol. 2006;176:7308–7316. doi: 10.4049/jimmunol.176.12.7308. [DOI] [PubMed] [Google Scholar]
- 10.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 11.Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, Davis MM. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. J Biol Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- 13.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 15.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 16.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 17.Chen YZ, Lai ZF, Nishi K, Nishimura Y. Modulation of calcium responses by altered peptide ligands in a human T cell clone. Eur J Immunol. 1998;28:3929–3939. doi: 10.1002/(SICI)1521-4141(199812)28:12<3929::AID-IMMU3929>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Sloan-Lancaster J, Steinberg TH, Allen PM. Selective activation of the calcium signaling pathway by altered peptide ligands. J Exp Med. 1996;184:1525–1530. doi: 10.1084/jem.184.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig R, Huang L, Germain R. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–8. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 21.Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TP, Clayberger C, Krensky AM, Norment AM, Littman DR, Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990;345:41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- 22.Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988;336:79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- 23.Cole DK, Dunn SM, Sami M, Boulter JM, Jakobsen BK, Sewell AK. T cell receptor engagement of peptide-major histocompatibility complex class I does not modify CD8 binding. Mol Immunol. 2008;45:2700–2709. doi: 10.1016/j.molimm.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher PF, Fazekas de St Groth B, Miller JF. CD4 and CD8 molecules can physically associate with the same T-cell receptor. Proc Natl Acad Sci U S A. 1989;86:10044–10048. doi: 10.1073/pnas.86.24.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitenberg D, Boutin Y, Constant S, Bottomly K. CD4 regulation of TCR signaling and T cell differentiation following stimulation with peptides of different affinities for the TCR. J Immunol. 1998;161:1194–1203. [PubMed] [Google Scholar]
- 26.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 27.Zamoyska R. CD4 and CD8: modulators of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82–87. doi: 10.1016/s0952-7915(98)80036-8. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich LI, Ebert PJ, Krummel MF, Weiss A, Davis MM. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- 29.Lewis LA, Chung CD, Chen J, Parnes JR, Moran M, Patel VP, Miceli MC. The Lck SH2 phosphotyrosine binding site is critical for efficient TCR-induced processive tyrosine phosphorylation of the zeta-chain and IL-2 production. J Immunol. 1997;159:2292–2300. [PubMed] [Google Scholar]
- 30.van Oers NSC, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 32.LoGrasso PV, Hawkins J, Frank LJ, Wisniewski D, Marcy A. Mechanism of activation for Zap-70 catalytic activity. Proc Natl Acad Sci U S A. 1996;93:12165–12170. doi: 10.1073/pnas.93.22.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JL, Stewart-Jones G, Bossi G, Lissin NM, Wooldridge L, Choi EM, Held G, Dunbar PR, Esnouf RM, Sami M, Boulter JM, Rizkallah P, Renner C, Sewell A, van der Merwe PA, Jakobsen BK, Griffiths G, Jones EY, Cerundolo V. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J Exp Med. 2005;201:1243–1255. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otwinowski ZM, Wladek M. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 35.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 36.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 37.Morris RJ, Perrakis A, Lamzin VS. ARP/wARP’s model-building algorithms. I. The main chain. Acta Crystallogr D Biol Crystallogr. 2002;58:968–975. doi: 10.1107/s0907444902005462. [DOI] [PubMed] [Google Scholar]
- 38.Webb AI, Dunstone MA, Chen W, Aguilar MI, Chen Q, Jackson H, Chang L, Kjer-Nielsen L, Beddoe T, McCluskey J, Rossjohn J, Purcell AW. Functional and structural characteristics of NY-ESO-1-related HLA A2-restricted epitopes and the design of a novel immunogenic analogue. J Biol Chem. 2004;279:23438–23446. doi: 10.1074/jbc.M314066200. [DOI] [PubMed] [Google Scholar]
- 39.Madden DR, Garboczi DN, Wiley DC. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- 40.Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 42.Morgan AJ, Jacob R. Differential modulation of the phases of a Ca2+ spike by the store Ca2+-ATPase in human umbilical vein endothelial cells. J Physiol. 1998;513(Pt 1):83–101. doi: 10.1111/j.1469-7793.1998.083by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, Rosenberg SA, Morgan RA. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luz JG, Huang M, Garcia KC, Rudolph MG, Apostolopoulos V, Teyton L, Wilson IA. Structural comparison of allogeneic and syngeneic T cell receptor-peptide-major histocompatibility complex complexes: a buried alloreactive mutation subtly alters peptide presentation substantially increasing V(beta) Interactions. J Exp Med. 2002;195:1175–1186. doi: 10.1084/jem.20011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 50.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 51.Zhong F, Davis MC, McColl KS, Distelhorst CW. Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J Cell Biol. 2006;172:127–137. doi: 10.1083/jcb.200506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan AJ, Churchill GC, Masgrau R, Ruas M, Davis LC, Billington RA, Patel S, Yamasaki M, Thomas JM, Genazzani AA, Galione A. Methods in cADPR and NAADP research. In: Putney JW Jr., editor. Methods in Calcium Signalling. CRC Press; Boca Raton: 2006. pp. 265–334. [Google Scholar]
- 53.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Treves S, Di Virgilio F, Cerundolo V, Zanovello P, Collavo D, Pozzan T. Calcium and inositolphosphates in the activation of T cell-mediated cytotoxicity. J Exp Med. 1987;166:33–42. doi: 10.1084/jem.166.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarzmann N, Kunerth S, Weber K, Mayr GW, Guse AH. Knock-down of the type 3 ryanodine receptor impairs sustained Ca2+ signaling via the T cell receptor/CD3 complex. J Biol Chem. 2002;277:50636–50642. doi: 10.1074/jbc.M209061200. [DOI] [PubMed] [Google Scholar]
- 56.Steen M, Kirchberger T, Guse AH. NAADP mobilizes calcium from the endoplasmic reticular Ca(2+) store in T-lymphocytes. J Biol Chem. 2007;282:18864–18871. doi: 10.1074/jbc.M610925200. [DOI] [PubMed] [Google Scholar]
- 57.Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P, Hohenegger M, Ashamu GA, Schulze-Koops H, Potter BV, Mayr GW. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 58.Madge L, Marshall IC, Taylor CW. Delayed autoregulation of the Ca2+ signals resulting from capacitative Ca2+ entry in bovine pulmonary artery endothelial cells. J Physiol. 1997;498(Pt 2):351–369. doi: 10.1113/jphysiol.1997.sp021863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donnadieu E, Bismuth G, Trautmann A. Calcium fluxes in T lymphocytes. J Biol Chem. 1992;267:25864–25872. [PubMed] [Google Scholar]
- 60.Choi EM, Chen JL, Wooldridge L, Salio M, Lissina A, Lissin N, Hermans IF, Silk JD, Mirza F, Palmowski MJ, Dunbar PR, Jakobsen BK, Sewell AK, Cerundolo V. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J Immunol. 2003;171:5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 61.Laugel B, Price DA, Milicic A, Sewell AK. CD8 exerts differential effects on the deployment of cytotoxic T lymphocyte effector functions. Eur J Immunol. 2007;37:905–913. doi: 10.1002/eji.200636718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 63.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 64.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008 doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.