Abstract
Great uncertainty exists in the global exchange of carbon between the atmosphere and the terrestrial biosphere. An important source of this uncertainty lies in the dependency of photosynthesis on the maximum rate of carboxylation (Vcmax) and the maximum rate of electron transport (Jmax). Understanding and making accurate prediction of C fluxes thus requires accurate characterization of these rates and their relationship with plant nutrient status over large geographic scales. Plant nutrient status is indicated by the traits: leaf nitrogen (N), leaf phosphorus (P), and specific leaf area (SLA). Correlations between Vcmax and Jmax and leaf nitrogen (N) are typically derived from local to global scales, while correlations with leaf phosphorus (P) and specific leaf area (SLA) have typically been derived at a local scale. Thus, there is no global-scale relationship between Vcmax and Jmax and P or SLA limiting the ability of global-scale carbon flux models do not account for P or SLA. We gathered published data from 24 studies to reveal global relationships of Vcmax and Jmax with leaf N, P, and SLA. Vcmax was strongly related to leaf N, and increasing leaf P substantially increased the sensitivity of Vcmax to leaf N. Jmax was strongly related to Vcmax, and neither leaf N, P, or SLA had a substantial impact on the relationship. Although more data are needed to expand the applicability of the relationship, we show leaf P is a globally important determinant of photosynthetic rates. In a model of photosynthesis, we showed that at high leaf N (3 gm−2), increasing leaf P from 0.05 to 0.22 gm−2 nearly doubled assimilation rates. Finally, we show that plants may employ a conservative strategy of Jmax to Vcmax coordination that restricts photoinhibition when carboxylation is limiting at the expense of maximizing photosynthetic rates when light is limiting.
Keywords: Carbon assimilation, carbon cycle, carboxylation, DGVM, electron transport, Farquhar model, land surface model, meta-analysis, mixed-effect multiple regression, noncarbon photosynthesis, TBM
Introduction
Photosynthesis is the proximal driver of the carbon cycle (Canadell et al. 2007; Cadule et al. 2010) and is thus a core driver of carbon flux and central to carbon cycle models (e.g., Woodward et al. 1995; Cox 2001; Sitch et al. 2003; Zaehle and Friend 2010; Bonan et al. 2011). Enzyme kinetic models of leaf photosynthesis (Farquhar et al. 1980; described below) are typically embedded in global carbon cycle models to mechanistically reflect plant physiological responses to atmospheric CO2. The Farquhar et al. (1980) photosynthetic submodel and its subsequent variants (Von Caemmerer and Farquhar 1981; Farquhar and Wong 1984; Collatz et al. 1991; Harley et al. 1992) are at the heart of almost all land surface models of carbon flux, several ecosystem dynamic models, and dynamic global vegetation models. We hereafter refer to these global land surface, ecosystem, and vegetation models as terrestrial biosphere models (TBMs).
Simulated photosynthetic rates in TBMs are highly sensitive to Vcmax and Jmax (Zaehle et al. 2005; Bonan et al. 2011; Verheijen et al. 2012), the maximum rate parameters of enzyme kinetic processes driving photosynthesis. Accuracy in these parameters is central to an effective photosynthetic submodel in the TBMs. Theory and empirical data suggest that these photosynthetic rates scale with leaf nitrogen (N) via the large amount of leaf N invested in the ribulose 1-5-bisphosphate oxygenase/carboxylase (RuBisCO) protein, and phosphorus (P) availability influences many aspects of plant physiology central to photosynthesis, including membrane solubility, ATP, and NADPH production (Marschner 1995; Taiz and Zeiger 2010). Vcmax and Jmax have also been linked to structural leaf traits via specific leaf area (SLA). Theory and data (Kattge et al. 2009; Domingues et al. 2010; Cernusak et al. 2011) clearly suggest mechanistic links between Vcmax, Jmax, and several functional plant traits that correlate with photosynthetic biochemistry.
Accurate simulation of plant physiological responses to atmospheric CO2 in TBMs thus requires data on how Vcmax and Jmax scale with plant traits N, P, and SLA accounting for the immense species-specific and regional variation in availability of N and P and subsequent variation in leaf N, P, and SLA.
Here, we provide a global assessment of the relationship between Vcmax and Jmax and leaf N, P, and SLA, drawing on estimates made on 356 species around the world.
When do Vcmax and Jmax variation matter?
TBMs typically assign a single, fixed Vcmax or Jmax parameter value (Rogers 2014) to each plant functional type (PFT). Scaling from plant to ecosystem or globe is achieved via PFT distribution maps. Recently, however, the predictive performance of such models has improved by allowing parameter values to vary. For example, at sites of the FLUXNET network where high-resolution data exist on all parameters and rates, predictive performance improved when Vcmax and Jmax were allowed to vary interannually (Groenendijk et al. 2011). Additionally, some TBMs improve prediction by simulating leaf nitrogen as part of the model and specify a linear relationship between Vcmax and leaf N (e.g., Woodward et al. 1995), defined for each PFT (Kattge et al. 2009). Finally, Mercado et al. (2011) demonstrated considerable improvements to model predictions of carbon fluxes in the Amazon when leaf P was taken into account.
Empirically, there is also a strong relationship between Jmax and Vcmax (Wullschleger 1993; Beerling and Quick 1995), and most TBMs simulate Jmax as a linear function of Vcmax. However, this assumption could be erroneous because the correlation between Jmax and Vcmax is likely to be influenced by leaf N, P, and SLA. The coordination hypothesis of photosynthetic resource allocation (Chen et al. 1993) states that the Calvin–Benson cycle limited rate of assimilation (Wc, see below) equals the electron transport-limited rate of assimilation (Wj). The relationship between Jmax and Vcmax affects the relationship between Wc and Wj and may reflect coordination of these two rate-limiting biochemical cycles. When carboxylation is limiting photosynthesis, high investment in Jmax relative to Vcmax would lead to electron transport not used in photosynthesis requiring dissipation of that energy to avoid photoinhibition (Powles 1984; Krause et al. 2012). However, when light is limiting photosynthesis, high investment in Jmax relative to Vcmax would maximize photosynthetic rates. Therefore, a trade-off exists in high investment in Jmax relative to Vcmax whereby the marginal benefit to photosynthetic rates when light is limiting is offset by the cost of energy dissipation when carboxylation is limiting.
Moving forward: global variation in Vcmax and Jmax as a function of N, P, and SLA
As noted above, we make here a global assessment of the relationship between Vcmax and Jmax and leaf N, P, and SLA, drawing on estimates made on 356 species by treatment combinations around the world from 24 different studies. We used these data to test several hypotheses. First, we hypothesized that leaf P will modify the relationship of Vcmax to leaf N. Second, we hypothesized that leaf P will modify the relationship of Jmax to Vcmax. Third, drawing on the coordination hypothesis of photosynthetic resource allocation, we predict that the relationship between Jmax and Vcmax results from efficient resource investment in Jmax reflecting the trade-off between photosynthetic gain and costs of energy dissipation.
To test our hypotheses, we combine a global meta-analysis of the relationships of Vcmax and Jmax with N, P, and SLA and then examine the consequences of these patterns in a leaf photosynthesis model. Combined, our effort offers a global-scale definition of Vcmax and Jmax variation in relation to leaf-trait variation and provides an empirical alternative to single value PFT scaling or the type of tuned relationships presented above in global TBMs. Our empirical representation of Vcmax and Jmax should lead to improved simulation of carbon fluxes across multiple scales.
Materials and Methods
Literature review & data collection
In September 2012, we searched the Thompson Reuters Web of Science database for “photosynthesis” or “carboxylation” and either “N,” “P,” or “SLA” and similar related search terms. The aim was to find papers that had simultaneously measured as many of the following leaf traits: Vcmax, Jmax, leaf N, leaf P, and specific leaf area (SLA) or leaf mass-to-area ratio (LMA). Data were copied from tables or digitized from graphics using Grab It! (Datatrend Software 2008). Minimum requirements for inclusion in this study were that either Vcmax or Jmax were calculated from A/Ci curves along with two of the other three leaf traits, yielding data from 24 papers and 135 species x location combinations, distributed globally (Tables 1 and S1). Some of these data were collected on plants in their natural environment and subject to natural environmental variation, while other data were collected on laboratory-grown plants (mostly tree species) subjected to experimental treatments. The majority of the species used in the greenhouses and laboratories were native to the area of the research center. Either species means or treatment means were collected leading to a dataset of 356 species/treatment combinations. The data can be downloaded from the ORNL DAAC (http://dx.doi.org/10.3334/ORNLDAAC/1224).
Table 1.
Sources of data collected for the meta-analysis and associated information including location, number of species and any experimental treatment
| Reference | Number of species | PFT* | Longitude (°E) | Latitude (°N) | Elevation (m) | Location | Country | Experiment | N | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Aranda et al. (2005) | 1 | Temp Ev Bl | −3.43 | 39.23 | 650 | Alburquerque | Spain | Light*water | Y | N |
| Bauer et al. (2001) | 6 | Temp Dc Bl and Ev Nl | −71.03 | 42.21 | 40 | Havard forest | USA | CO2*N | Y | N |
| Bown et al. (2007) | 1 | Temp Ev Nl | 176.13 | −38.26 | 600 | Purokohukohu Experimental Basin | NZ | N*P | Y | Y |
| Brück and Guo (2006) | 1 | Temp legume crop | 10.08 | 54.19 | 40 | Kiel | Germany | NH4 vs. NO3 | Y | N |
| Calfapietra (2005) | 1 | Temp Dc Bl | 11.48 | 42.22 | 150 | Viterbo | Italy | CO2*N canopy level | Y | N |
| Carswell et al. (2005) | 4 | Temp Dc Bl and Ev Nl | 170.3 | −43.2 | 90 | Okarito | NZ | N*P | Y | Y |
| Cernusak et al. (2011) | 2 | Trop Ev Bl | 139.56 | −22.59 | 150 | Boulia | Australia | None | Y | Y |
| Cernusak et al. (2011) | 2 | “ | 133.19 | −17.07 | 230 | Sturt plains | Australia | None | Y | Y |
| Cernusak et al. (2011) | 2 | “ | 132.22 | −15.15 | 170 | Dry creek | Australia | None | Y | Y |
| Cernusak et al. (2011) | 2 | “ | 131.23 | −14.09 | 70 | Daly river | Australia | None | Y | Y |
| Cernusak et al. (2011) | 2 | “ | 131.07 | −13.04 | 80 | Adelaide river | Australia | None | Y | Y |
| Cernusak et al. (2011) | 2 | “ | 131.08 | −12.29 | 40 | Howard springs | Australia | None | Y | Y |
| Deng (2004) | 2 | Sub-trop forb | 113.17 | 23.08 | 10 | Guanzhou | China | None | Y | N |
| Domingues et al. (2010) | 3 | Trop Dc Bl | −1.5 | 15.34 | 280–300 | Hombori | Mali | None | Y | Y |
| Domingues et al. (2010) | 7 | “ | −1.17 | 12.73 | 250 | Bissiga | Burkina Faso | None | Y | Y |
| Domingues et al. (2010) | 8 | “ | −3.15 | 10.94 | 300 | Dano | Burkina Faso | None | Y | Y |
| Domingues et al. (2010) | 5 | “ | −1.86 | 9.3 | 370 | Mole | Ghana | None | Y | Y |
| Domingues et al. (2010) | 8 | “ | −1.18 | 7.3 | 170 | Kogye | Ghana | None | Y | Y |
| Domingues et al. (2010) | 21 | Trop Dc Bl and Ev Bl | −1.7 | 7.72 | 200 | Boabeng Fiame | Ghana | None | Y | Y |
| Domingues et al. (2010) | 4 | “ | −2.45 | 7.14 | 25 | Asukese | Ghana | None | Y | Y |
| Grassi (2002) | 1 | Sub-trop Ev Bl | 149.07 | −35.18 | 600 | Canberra | Australia | N | Y | N |
| Han et al. (2008) | 1 | Temp Ev Nl | 138.8 | 35.45 | 1030 | Canberra | Australia | N | Y | N |
| Katahata et al. (2007) | 1 | Ev shrub | 138.4 | 36.51 | 900 | Niigata | Japan | Light*leaf age | Y | N |
| Kubiske (2002) | 2 | Temp Bl Dc | −84.04 | 45.33 | 215 | Pellston | USA | N* CO2*light | Y | N |
| Manter (2005) | 1 | Temp Ev Nl | −122.4 | 45.31 | 75 | Portland | USA | N | Y | N |
| Merilo et al. (2006) | 2 | Temp Ev Nl | 26.55 | 58.42 | 65 | Saare | Estonia | Light | Y | N |
| Midgley et al. (1999) | 4 | Temp Ev shrub | 20 | −34.5 | 120 | Cape Agulhas | SA | CO2*N&P | Y | N |
| Porte and Lousteau (1998) | 1 | Temp Ev Nl | −0.46 | 44.42 | 60 | Bordeaux | France | Leaf age*canopy level | Y | Y |
| Rodriguez-Calcerrada et al. (2008) | 2 | Temp Dc Bl | −3.3 | 41.07 | 50 | Madrid | Spain | Light | Y | N |
| Sholtis (2004) | 1 | Temp Dc Bl | −84.2 | 35.54 | 230 | Oak Ridge | USA | CO2*canopy level | Y | N |
| Tissue et al. (2005) | 3 | Temp Ev Nl and Bl Dc | 170.3 | −43.2 | 50 | Okarito forest south Westland | NZ | Canopy level | Y | Y |
| Turnbull et al. (2007) | 1 | Temp Ev Bl | 142.05 | −37.03 | 470 | Ballarat | Australia | Defoliation | Y | Y |
| Warren (2004) | 1 | Temp Ev Bl | 143.53 | −37.25 | 450 | Creswick | Australia | N | Y | N |
| Watanabe et al. (2011) | 1 | Temp Dc Nl | 141 | 43 | 180 | Asapporo | Japan | CO2*N | Y | Y |
| Wohlfahrt et al. (1999a) | 28 | Temp C3 grass and forb | 11.01 | 46.01 | 1540–1900 | Monte Bondone | Estern Alps | None | Y | N |
| Zhang and Dang (2006) | 1 | Temp Dc Bl | 89.14 | 48.22 | 200 | Ontario | Canada | CO2*age | N | Y |
| Additional datasets | ||||||||||
| TRY – Kattge et al. (2011) | 1048 | |||||||||
| Wullschleger (1993) | 110 | |||||||||
PFT abbreviations: Temp, temperate; Trop, tropical; Ev, evergreen; Dc, deciduous; Nl, needleleaf tree; Bl, broadleaf tree.
Vcmax and Jmax are calculated by fitting equations 1 and 2, or 1,3, and 4 to sections of the A/Ci curve (Von Caemmerer and Farquhar 1981; Sharkey et al. 2007), and these calculations are sensitive to the kinetic parameters, Kc and Ko and to Γ*, used in the fitting process (Medlyn et al. 2002). Using a method (detailed in Appendix S1) similar to Kattge and Knorr (2007), we removed the variation in Vcmax and Jmax across studies caused by different parametric assumptions by standardizing Vcmax and Jmax to a common set of kinetic parameters (derived by Bernacchi et al. 2001). We also corrected Vcmax and Jmax to a common measurement temperature of 25°C and to the O2 partial pressure at the measurement elevation. Errors introduced by the standardization were well within the measurement error of A/Ci curves (Appendix S2). Standardizing for the kinetic parameters had a substantial impact on Vcmax and to a lesser extent Jmax (Figure S1), as observed by Kattge et al. (2009). Standardization for O2 partial pressure decreases with altitude had a small impact on values taken from plants growing at altitudes up to 2000 m (Figure S2).
We related Jmax and Vcmax such that:
| (1) |
where bjv is the slope of the relationship and ajv the intercept. Gu et al. (2010) demonstrated a method-specific bias on bjv (on non-log-transformed variables) caused by predetermination of the limitation state of points on the A/Ci. However, most authors in this meta-analysis used a fitting procedure which removed points that were potentially either limitation state (Wullschleger 1993; Sharkey et al. 2007) which minimizes potential biases in bjv.
Where LMA was reported, we converted to SLA by taking the reciprocal of LMA. While this introduced some error (the reciprocal of the mean of a set of values does not equal the mean of the reciprocals of that set), the error was distributed across the whole range of SLA so was unlikely to have biased the effect of SLA. To compare the Jmax to Vcmax relationship from our dataset, we also used Vcmax and Jmax data from Wullschleger (1993) and the TRY database (Kattge et al. 2011; data from Atkin et al. 1997; Kattge et al. 2009). Vcmax and Jmax are measured on a leaf area basis, and in models of photosynthesis, area-based measurement integrates these parameters with light capture. Therefore, we restricted our analysis to leaf-area-based measurements.
Statistical analysis
To assess the importance of P and SLA as covariates with leaf N in determining Vcmax and Jmax, we developed multiple regressions of Vcmax or Jmax as the dependent variable and leaf N, leaf P, and SLA as the independent variables. To increase sample size and increase the range of each variable, we also developed multiple regressions of Vcmax or Jmax against leaf N and either SLA or leaf P. In the analysis of Jmax, we also included Vcmax as an independent variable based on our hypothesis that Wc and Wj are coordinated via the Jmax to Vcmax relationship. We used linear mixed-model regression framework with leaf traits as fixed effects and the author of the paper from which the data were collected as the random effect (Ordonez et al. 2009). Including the study author as a random effect in the regression model accounted for the nonindependence of data within a study. We were unable to account for differential accuracy between studies, often measured by sampling variance or sample size in meta-analysis, and therefore did not weight the data. All variables were natural-log-transformed to ensure normality of residuals.
Similar to all meta-analyses (Gurevitch and Hedges 1999), there is likely to be some error introduced by the different methods used by the different research groups, but the standardization method and the mixed-model analysis with study group as the random effect will have minimized this error.
All statistical analyses were carried out using the open-source software package R, version 2.13.0 (R Core Development Team 2011). We employed a backward, stepwise, AIC-based model simplification process. Our maximal models contained 3-way interactions for Vcmax (and all 2-way interactions in the models with two independent variables) and Jmax and were fit with the “lme” function of the “nlme” library (Pinheiro et al. 2011). Models were then simplified using the “dropterm” function of the “MASS” library to conserve marginality (see Venables and Ripley 2002). Model selection aimed to find the minimum adequate model – the model explaining the most variation in the dependent variable with minimum necessary parameters. Model selection was based on the model with the lowest corrected Akaike information criterion (AICc) and with a significance level of each model term of P < 0.1, subject to conservation of marginality. The AIC is a relative measure of competing models' likelihood penalized by the number of parameters fit by the model, and the AICc is the AIC when corrected for finite sample size (Burnham and Anderson 2002). Given a set of competing models, the model with the lowest AICc can be considered the preferred model (the minimum adequate model).
We report the likelihood ratio test (LRT) statistic between a model and an intercept only (i.e., only random effects) null model and calculated model significance P-values using the chi-square distribution. As there is no mixed-model method to estimate variance in the dependent variable explained by the model, we report the proportional decrease in the residual variance in the minimum adequate model compared with the null, random effects only, model as a metric of explained variance (Xu 2003).
Models were checked for violation of the assumptions of mixed-model linear regression (homoscedasticity of residuals; normal distribution of residuals within the random groups and that observed values of the dependent variable bore a linear relationship to model fitted values), and all minimum adequate models satisfied these checks (a comparison of model assumptions when using nontransformed and transformed data are presented in Appendix S3).
Modeling carbon assimilation
After Medlyn et al. (2002) and Kattge and Knorr (2007), carbon assimilation was modeled using the Farquhar et al. (1980) biochemical model for perfectly coupled electron transport and the Calvin–Benson cycle, as reported in Medlyn et al. (2002). Enzyme kinetic models of photosynthesis (Farquhar et al. 1980) simulate net CO2 assimilation (A) as the minimum of the RuBisCO-limited gross carboxylation rate (Wc) and the electron transport-limited gross carboxylation rate (Wj), scaled to account for photorespiration, minus mitochondrial (dark) respiration (Rd). The net assimilation function takes the form:
| (2) |
where Γ* is the CO2 compensation point (Pa), the Ci at which the carboxylation rate is balanced by CO2 release from oxygenation. Both Wc and Wj are modeled as functions of the intercellular CO2 partial pressure (Ci − Pa). Wc follows a Michaelis–Menten function of Ci in which Vcmax (μmol CO2 m−2·s−1) determines the asymptote:
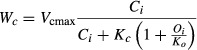 |
(3) |
where Oi is the intercellular O2 partial pressure (kPa); Kc and Ko are the Michaelis–Menten constants of RuBisCO for CO2 (Pa) and for O2 (kPa). The light-limited gross carboxylation rate (Wj) is a function of the electron transport rate (J - μmol·e·m−2·s−1) following a similar function of Ci where the asymptote is proportional to J:
J is a function of incident photosynthetically active radiation (I – μmol photons m−2·s−1) that saturates at the maximum rate of electron transport (Jmax), formulated by Harley et al. (1992) following Smith (1937), though other formulations exist:
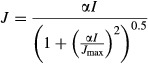 |
where α is the apparent quantum yield of electron transport (assumed to be 0.24 mol electrons mol−1 photons by Harley et al. (1992) although α is not invariant in nature) and is the result of multiplying the true quantum yield and light absorption by the leaf. By determining the asymptotes of the two rate-limiting cycles of photosynthesis, it is clear from the above set of equations that carbon assimilation is highly sensitive to Vcmax and Jmax.
Temperature sensitivities of Vcmax and Jmax were simulated using the modified Arrhenius equation of Johnson et al. (1942), see Medlyn et al. (2002). For consistency with the temperature sensitivity functions of Vcmax and Jmax (see Medlyn et al. 2002), the temperature sensitivities of the kinetic properties of RuBisCO and the CO2 compensation point in the absence of dark respiration were modeled after Bernacchi et al. (2001). See Appendix S4 for further details.
Coefficients of the equations relating Vcmax to leaf N and Jmax to Vcmax were taken from the models presented in Table 3. The impact of P and SLA on assimilation was simulated by predicting Vcmax using the 5th and 95th percentile of either P (0.05 and 0.22 mg·g−1) or SLA (adjusted to provide realistic combinations of SLA and leaf N 0.01 m2·g−1 and 0.025 m2·g−1) from our database. The biophysical space over which carbon assimilation was simulated was PAR ranging from 0 to 1500 μmol·m−2·s−1, internal CO2 partial pressure of 30 Pa, at two levels of leaf N (0.5 and 3 g·m−2) and at a temperature of 25°C.
Table 3.
Details of the recommended minimum adequate models (MAM) explaining Vcmax and Jmax. All traits were expressed on an area basis and were natural-log-transformed. The LRT was the likelihood ratio test statistic of the model against the null (intercept only) model, and the residual variance reduction was the proportional reduction in residual variance when compared to the null model. A colon represents the interaction between two variables. Using the example of model 1, the equation describing Vcmax would take the form: ln(Vcmax) = 1.993 + 2.555ln(N) − 0.372ln(SLA) + 0.422ln(N)ln(SLA)
| Response trait | Explanatory traits of the maximal model1 | Explanatory variables of the MAM | Coefficient | SE | df | Student's t-test | P | N obs | N groups | Residual variance reduction (%) | LRT | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Vcmax | N, P | Intercept | 3.946 | 0.229 | 99 | 17.26 | <0.001 | 110 | 8 | 19.5 | 25.5 | <0.001 |
| N | 0.921 | 0.301 | 99 | 3.06 | 0.003 | ||||||||
| P | 0.121 | 0.085 | 99 | 1.42 | 0.156 | ||||||||
| N:P | 0.282 | 0.145 | 99 | 1.95 | 0.054 | ||||||||
| Model 2 | Vcmax | N, SLA | Intercept | 1.993 | 0.410 | 237 | 4.86 | <0.001 | 260 | 20 | 36.6 | 99.1 | <0.001 |
| N | 2.555 | 0.522 | 237 | 4.89 | <0.001 | ||||||||
| SLA | -0.372 | 0.093 | 237 | -4.00 | <0.001 | ||||||||
| N:SLA | 0.422 | 0.115 | 237 | 3.67 | <0.001 | ||||||||
| Model 3 | Jmax | Vcmax, N, P | Intercept | 1.246 | 0.233 | 96 | 5.33 | <0.001 | 105 | 7 | 83.5 | 189.1 | <0.001 |
| Vcmax | 0.886 | 0.043 | 96 | 20.60 | <0.001 | ||||||||
| P | 0.089 | 0.041 | 96 | 2.20 | 0.033 | ||||||||
| Model 4 | Jmax | Vcmax, N, SLA | Intercept | 1.197 | 0.115 | 215 | 10.45 | <0.001 | 235 | 17 | 84.2 | 416.1 | <0.001 |
| Vcmax | 0.847 | 0.025 | 215 | 34.23 | <0.001 |
Including all combinations of interactions between each trait.
To simulate the sensitivity of carbon assimilation to the Jmax to Vcmax slope, the model was driven with a full range of photosynthetically active radiation (PAR, 0–1500 μmol·m−2·s−1) and three levels of Vcmax (25, 50 & 90 μmol·m−2·s−1). For simplicity, we only simulated the sensitivity at 25°C, acknowledging that temperature is also an important factor determining the sensitivity of assimilation to the Jmax to Vcmax slope.
Results
Vcmax and Jmax in relation to leaf N, leaf P, and SLA
The most likely model, that is, the minimum adequate model, when Vcmax was regressed on all three leaf traits together (leaf N, P, and SLA) was the model with SLA as the only explanatory variable (see Table S2). However, there were less data available for this analysis (n = 90, over 50% of which came from a single study), and as a consequence, the range of leaf N and SLA values were restricted compared with their range in the trivariate models discussed below. For this reason, we present no further discussion of Vcmax regressed on leaf N, leaf P, and SLA. For Jmax regressed on Vcmax, leaf N, leaf P, and SLA, the minimum adequate model was of Jmax regressed only on Vcmax and leaf P with no interaction (see Table S2). With increased range in the explanatory variables, we focus on the models with one less explanatory variable.
For Vcmax regressed against leaf N and either leaf P or SLA, the minimum adequate models were also the maximal models – those with both traits and their interaction (Table 2; models 1 and 2). Models of Vcmax regressed on leaf N and either SLA or leaf P were both highly significantly different from the null (intercept and random effects only) model (P < 0.001).
Table 2.
Model selection table for multiple regressions of Vcmax and Jmax regressed against leaf N, or leaf N and Vcmax respectively, and in combination with either leaf P or SLA. The minimum adequate model (MAM) was the model with the lowest AICc. All traits were expressed on a leaf area basis and were natural-log-transformed
| Response trait | Model | Model explanatory variables1 | Residual variance reduction (%) | AICc |
|---|---|---|---|---|
| Vcmax | Maximal model, MAM – Model 1 | N, P, N:P | 19.5 | 44.2 |
| N, P | 16.6 | 45.8 | ||
| N | 13.5 | 47.7 | ||
| P | 6.5 | 56.9 | ||
| Vcmax | Maximal model, MAM – Model 2 | N, SLA, N:SLA | 36.6 | 174.6 |
| N, SLA | 32.5 | 185.7 | ||
| N | 30.2 | 187.8 | ||
| SLA | 12.3 | 248.4 | ||
| Jmax | Maximal model | Vcmax, N, P, all 2-way interactions, 3-way interaction | 83.6 | −115.6 |
| Vcmax, N, P, all 2-way interactions | 83.6 | −117.9 | ||
| Vcmax, N, P, Vcmax:N, N:P | 83.4 | −118.9 | ||
| Vcmax, N, P, Vcmax:N | 83.4 | −120.1 | ||
| Vcmax, N, P | 83.4 | −121.4 | ||
| MAM – Model 3 | Vcmax, P | 83.5 | −123.2 | |
| Vcmax, P, Vcmax:P | 83.5 | −121.2 | ||
| Vcmax | 82.9 | −120.8 | ||
| N | 10.4 | 49.3 | ||
| P | 12.5 | 46.2 | ||
| Jmax | Maximal model | Vcmax, N, SLA, all 2-way interactions, 3-way interaction | 85.1 | −196.2 |
| Vcmax, N, SLA, all 2-way interactions | 84.7 | −193.7 | ||
| Vcmax, N, SLA, Vcmax:N, Vcmax:SLA | 84.7 | −195.3 | ||
| Vcmax, N, SLA, Vcmax:SLA | 84.6 | −194.5 | ||
| Vcmax, SLA, Vcmax:SLA | 84.5 | −196.4 | ||
| Vcmax, SLA | 84.3 | −196.4 | ||
| MAM – Model 4 | Vcmax | 84.2 | −196.0 | |
| Vcmax, N, Vcmax:N | 84.2 | −193.0 | ||
| Vcmax, N | 84.2 | −194.0 |
All models include an intercept term.
For Vcmax against leaf N and P (model 1), leaf N was a significant explanatory variable (P = 0.003), as was the interaction between leaf P and leaf N (P = 0.054), although just outside the 95% confidence level (Table 3). The AICc model selection procedure indicates that the P x N interaction was important and the response surface of Vcmax to leaf N and leaf P (Fig. 1) also shows the importance of leaf P in determining Vcmax. Leaf P modified the relationship of Vcmax to leaf N such that as leaf P increased, the sensitivity of Vcmax to leaf N increased (Fig. 1), that is, the coefficient of the interaction term was positive (Table 3). The term for leaf P alone was not significant, but was retained in the minimum adequate model to preserve marginality (see Venables and Ripley 2002).
Figure 1.
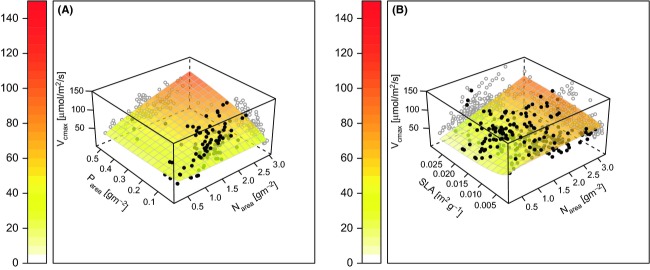
The derived relationships between Vcmax and leaf nitrogen (Table 3), as modified by leaf P (A – Table 3, model 1) and SLA (B – Table 3, model 2).
For Vcmax against leaf N and P (model 2), increasing SLA increased the sensitivity of Vcmax to leaf N; however, the magnitude of the effect was smaller than the effect of increasing leaf P (Fig. 1). In contrast to the effect of leaf P, the effect of SLA alone was significant and was contradictory to its effect in interaction – increasing SLA decreased Vcmax although this effect was only clearly visible at low levels of SLA and leaf N (Fig. 1B). There were few data points at low SLA and low leaf N because as SLA decreases, leaf N concentrations would have to be extremely low to allow low values of leaf N when expressed on an area basis, again suggesting that the effect of SLA on Vcmax was not substantial.
Leaf P had a larger effect on the Vcmax to leaf N relationship than did SLA (compare Fig. 1A and B), by contrast SLA was more significant in model 2 than was leaf P in model 1. The contrast arises from the reduced sample size of the leaf P regressions (110 observations in eight groups) compared with the SLA regressions (260 in 20 groups). While the effect of leaf P was greater, statistical confidence in the effect was lower and more data are needed to improve our confidence in the statistical model.
For the multiple regressions of Jmax against Vcmax, N, and P, the minimum adequate model was that of Vcmax and P, with no interaction term, explaining 84% of the residual variance compared with the null model (Table 2; model 3). For Jmax regressed against Vcmax, N, and SLA, the minimum adequate model was that with Vcmax alone, explaining 84% of the residual variance when compared to the null model (Table 2; model 4). Both models were highly significantly different from the null model (P < 0.001 – Table 3). While model 4 had a slightly higher AICc than the model with Vcmax, SLA and their interaction as model terms (Table 2), SLA and the Vcmax x SLA interaction were not significant model terms (P > 0.1; results not shown). This was also the case for the model with Vcmax and SLA and this led to the selection of model 4 (Jmax against Vcmax alone; Tables 2 and 3) as the minimum adequate model. The inclusion of Vcmax in the regressions of Jmax meant that the traits leaf N, leaf P, and SLA were tested for their effect on Jmax that were orthogonal to their effect already implicitly considered via their effect on Vcmax. The leaf traits were considered as modifiers of the Jmax to Vcmax relationship, not as direct determinates of Jmax.
The effect of leaf P was significant in model 3; however, variation in leaf P had little effect on calculated values of Jmax (Fig. 2). The effect of Vcmax was the most important in determining Jmax demonstrating the tight coupling between the two maximum rate parameters. A regression of Jmax on Vcmax alone yielded 301 observations, with a bjv of 0.89 ± 0.02 (Table 4). In the first analysis to our knowledge of the in vivo relationship between Jmax and Vcmax, Wullschleger (1993) described a slope coefficient (bjv) of 1.64 for untransformed data. For comparison with our dataset, we natural-log-transformed Jmax and Vcmax from the Wullschleger (1993) dataset and re-analyzed them with a linear regression. Regression assumptions were not violated by the transformation and bjv was 0.84 with an R2 of 0.87 (Table 4). In an analysis of natural-log-transformed Jmax against Vcmax from the TRY database (Kattge et al. 2011), Jmax scaled against Vcmax with a bjv of 0.75 (and R2 of 0.79). All three datasets have similar slope parameters for the log-transformed relationship ranging from 0.75 for the TRY data to 0.89 for our dataset (Fig. 3).
Figure 2.
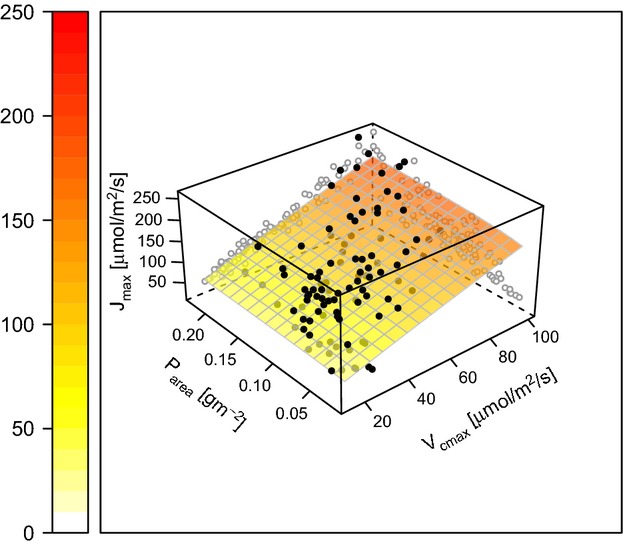
The relationship between Jmax and Vcmax as modified by leaf P (Table 3, model 3).
Table 4.
Slope coefficients from linear regressions of log-transformed Jmax on Vcmax from the data collected in this study, from the TRY database and from Wullschleger (1993). The data collected in this study were analyzed using a mixed-effects model with the author as the random effect, while data from the other two studies were analyzed using a fixed-effects model
| N | Model term | Coefficient | SE | Reduction in residual variance (%) | P-value* | |
|---|---|---|---|---|---|---|
| This study | 301 | Intercept | 1.010 | 0.097 | 86.7 | <0.001 |
| Slope | 0.890 | 0.021 | ||||
| TRY/Kattge | 1048 | Intercept | 1.668 | 0.048 | 78.9 | <0.001 |
| Slope | 0.750 | 0.012 | ||||
| Wullschleger | 110 | Intercept | 1.425 | 0.128 | 87.2 | <0.001 |
| Slope | 0.837 | 0.031 |
For this study's dataset, the P-value is based on the LRT statistic, and for Kattge and Wullschleger, it is based on the F statistic.
Figure 3.
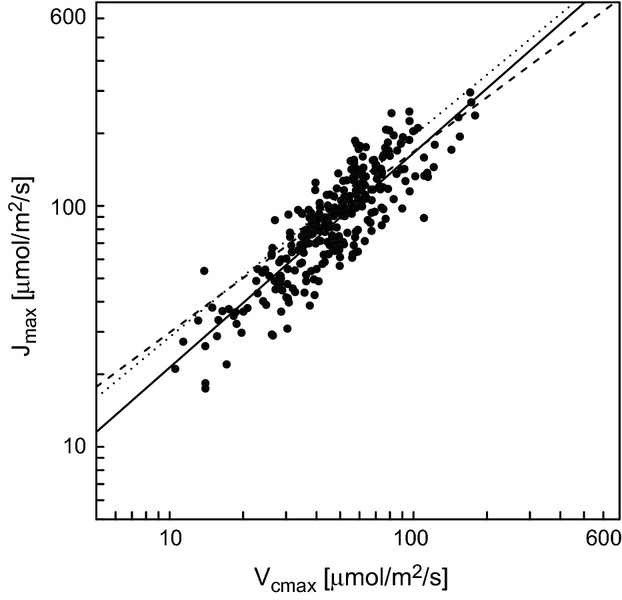
The relationship between Jmax and Vcmax collected in this study (black circles and solid line) and compared against the regressions based on the Kattge et al. (2009) dataset (dotted line) and the Wullschleger (1993) dataset (dashed line). Log-scaled axes.
Variation in carboxylation rates caused by variation in P and SLA
The sensitivity of simulated carboxylation rates to variation in Vcmax and Jmax caused by variation in leaf P or SLA (based on the minimum adequate models presented in Table 3) is shown in Fig. 4). At high leaf N (3 gm−2), increasing leaf P from the 5th to the 95th percentile (0.05 gm−2 to 0.22 gm−2) almost doubled carboxylation rates at high PAR (Fig. 4), while at low leaf N (0.5 gm−2), assimilation was little affected by changes in leaf P. The increase in assimilation caused by increased leaf P at moderate-to-high leaf N, but not at low N, was because leaf P was important only in interaction with N. At low leaf P (0.05 gm−2), increasing leaf N from 0.5 to 3 gm−2 resulted in a slight increase in carboxylation rates (compare solid lines in Fig. 4A and B). The effect of leaf P on Jmax was so small (Table 3 and Fig. 2) in comparison with the effect of Vcmax that there was very little effect on carboxylation rates caused by variation in Jmax resulting from variation in leaf P (results not shown).
Figure 4.
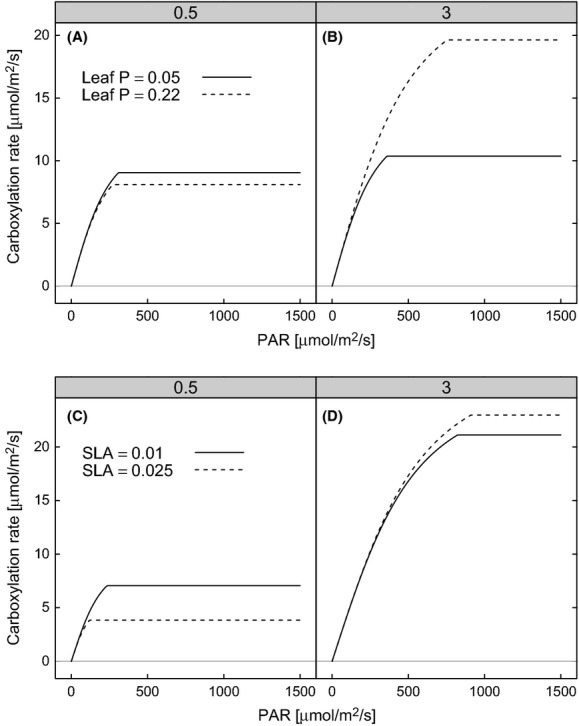
Simulated variation in gross carboxylation light-response curves as a result of variation in leaf P (A–B) or SLA (C–D) used in the minimum adequate models presented in Table 3. Light responses were simulated at two levels of leaf N, 0.5 gm−2 (A & C) and 3 gm−2 (B & D).
At high leaf N, increasing SLA from 0.01 m2·g−1 to 0.025 m2·g−1 had little effect on simulated carboxylation rates. At low leaf N (0.5 gm−2), carboxylation rates were decreased as SLA increased. Assimilation was reduced at low leaf N because the effect of SLA alone (which has a negative relationship to Vcmax) was larger than the effect of SLA in interaction with low levels of leaf N. At higher leaf N, the effect of SLA alone was canceled by the effect of SLA in interaction with leaf N, and therefore, there was little overall effect of SLA on Vcmax and hence carboxylation rates (Fig. 4).
The consequence of variation in bjv on carbon assimilation
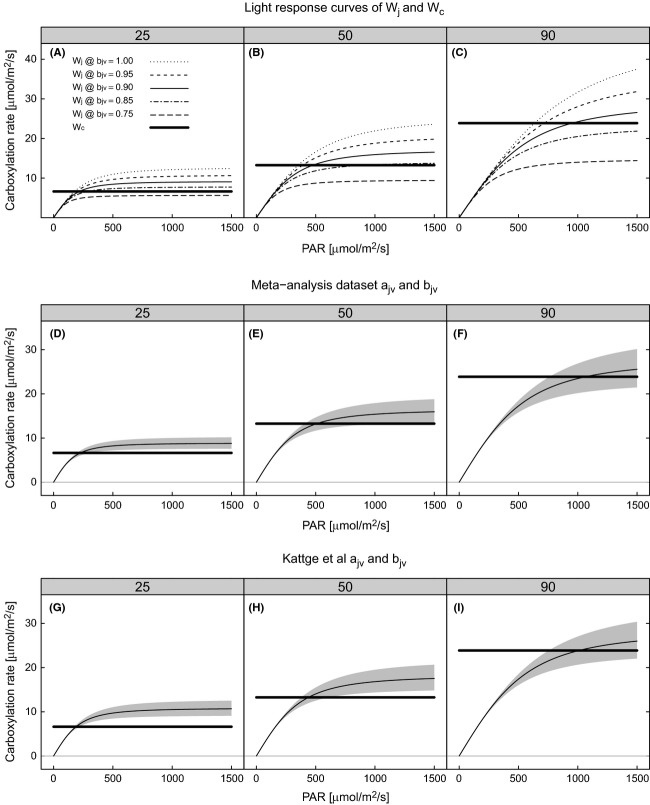
To analyze the relationship of Jmax to Vcmax in more depth, we investigated the effect of the slope parameter (bjv) on the modeled light response of carbon assimilation at three levels of Vcmax (25, 50, and 90 μmol·m−2·s−1). Figure 5A–C shows the light-response curves of the Wc and Wj gross carboxylation rates. Obviously, Wc is insensitive to variation in irradiance, and Wj shows the typical saturating response at high light. Increasing bjv increases the asymptote of Wj, which affects the transition point between Wc and Wj limitation. The light level at the transition where Wc and Wj are colimiting increases as bjv decreases (Fig. 5A–C).
Figure 5.
Simulated light-response curves of Wj and Wc in response to bjv variation (A–C), using ajv and bjv calculated from the dataset compiled in this study (D–F) and using ajv and bjv calculated from the dataset of Kattge et al. (2009) (G–I). All curves calculated at three levels of Vcmax 25 (A, D & G), 50 (B, E & H), and 90 (C, F, & I) μmol·m−2·s−1. On panels D–I, the black line within the gray-shaded area represents Wj using the calculated coefficients and the gray-shaded area 95% confidence interval of Wj.
The relationship of the colimiting light level to bjv allows us to categorize values of bjv into two types: (1) intermediate values of bjv where the point of colimitation occurs between the linear phase and the asymptote of the light response; and (2) low values at which there is no colimitation point, that is, electron transport is always limiting. Within the first category, the light level of colimitation is highly sensitive to bjv. At the upper end of these intermediate bjv values, the colimitation point occurs while assimilation is still in the linear phase of the light response and thus maximizes quantum yield (the differential of the curve), while Wj limits photosynthesis (Fig. 5A–C). At levels of irradiance above the colimitation point, high values of bjv cause Wj to be substantially higher than Wc representing “spare” electron transport capacity. As bjv increases, quantum yield decreases and the Wj asymptote approaches the Wc rate of carboxylation. In the second category of bjv values, the light–response curve asymptotes below the value of Wc, that is, assimilation is light limited at all light levels, there is no colimitation, and quantum yield is very low (see Fig. 5c). It is also possible at high values of bjv for the colimitation point to occur at a fixed level of irradiance, independent of bjv although these are at values of bjv > 1 (see Fig. 5A), substantially higher than observed (Table 4).
The Jmax to Vcmax relationship of the data collected in this study, and those from the TRY database (Table 4), both have values of bjv within the first category (Fig. 5D–I). The transition is highly dependent on bjv, and the Wc rate of assimilation is generally within the uncertainty of the potential Wj carboxylation rate at saturating light. For the coefficients derived from the data collected in this study, quantum yield is not maximized, that is, the colimitation point is never in the linear phase of the light response. When Vcmax was 50 μmol·m−2·s−1 and over, at light levels above those at the colimitation point, Wj was similar but slightly higher than Wc. At low photosynthetic capacity (i.e., Vcmax = 25 μmol·m−2·s−1) across the whole range of uncertainty, electron transport capacity above that necessary for carboxylation is apparent when Wc is limiting (Fig. 5D and G).
Discussion
Our goal in this study was to derive relationships of Vcmax and Jmax in relation to leaf N, P, and SLA. Using a meta-analytic approach to assess patterns among 356 species drawn from 24 different studies around the world, in agreement with many previous studies, we found that Vcmax increased in relation to leaf N (Wohlfahrt et al. 1999b; Aranda et al. 2006; Bown et al. 2007; Kattge et al. 2009; Domingues et al. 2010) and that both leaf P and SLA increased the sensitivity of Vcmax to leaf N. We also found that the relationship between Jmax and Vcmax was not substantially affected by leaf N, leaf P, or SLA (Table 2). Our efforts and in particular the statistical models provide a formal template on which to improve the parameterization of terrestrial ecosystem and biosphere models (TBMs; Tables 3 and 4). We demonstrated the impact of these variable rate parameters in a simple model of photosynthesis.
Evaluating the three hypotheses
In analyzing the data, we had three a priori hypotheses: (1) leaf P will modify the relationship of Vcmax to leaf N, (2) leaf P will modify the relationship of Jmax to Vcmax, (3) the relationship between Jmax and Vcmax results from a trade-off between photosynthetic gain and costs of energy dissipation.
In support of our first hypothesis, we found that leaf P was an important factor modifying the Vcmax to leaf N relationship. For Vcmax, we recommend the use in TBMs of coefficients and terms of model 1 and model 2 presented in Table 3. For those models, such as CABLE and CLM-CNP, that prognostically simulate, or explicitly parameterize leaf N and leaf P, we recommend the use of model 1 to simulate Vcmax (Table 3) and we suggest that incorporation of variation in leaf P is necessary for accurate scaling of Vcmax. Many models do not prognostically simulate SLA, and we have demonstrated that while significant, the effect size of SLA on Vcmax was small and we suggest it is not a priority for inclusion in TBMs for accurate parameterization of Vcmax. However, depending on model structure, SLA is indirectly important for scaling leaf N concentrations to area-based values of leaf N.
In contrast, and with reference to hypothesis two, we find that leaf P had little effect on the Jmax to Vcmax relationship. For Jmax, we recommend the use in TBMs and related tools of the model presented in Table 4 of Jmax regressed on Vcmax alone. Although the minimum adequate model of Jmax regressed on Vcmax, leaf N and P included leaf P as an explanatory variable, the small coefficients (Table 3) suggested that the additional impact of leaf P on Jmax was minimal as demonstrated in Fig. 3.
The observed relationship between Jmax and Vcmax run through a chloroplast-level photosynthesis model showed that the Wc rate of assimilation is generally within the uncertainty of the potential Wj carboxylation rate at saturating light and that quantum yield is not maximized. In terms of hypothesis three, the results suggest that the costs of energy dissipation and potential for photoinhibition outweigh the marginal benefits to photosynthetic gain.
The impact of leaf P
The empirical functions we present can be applied in TBMs with a phosphorus cycle and would allow scaling of Vcmax and Jmax that will be more in tune with nutrient cycling than using a single parameter value for a particular plant functional type (PFT). The use of the empirical function we developed (model 1, Table 3) will reduce simulated carbon assimilation and productivity by TBMs in regions where leaf P is low and leaf N is high, and should help to improve these simulations (Mercado et al. 2011; Yang et al. 2013). Our finding for leaf P was similar to that of Reich et al. (2009) who found that, in a global analysis, increased leaf P increased the sensitivity of Amax to leaf N. Reich et al. (2009) showed this modification of the relationship between Amax and leaf N by leaf P to hold true across biomes with different N/P ratios.
The analysis of Vcmax and Jmax by Domingues et al. (2010) concluded that leaf N and leaf P were best considered in terms of limiting factors, that is, that Vcmax was determined by either leaf N or leaf P, as often the interaction term between leaf N and P was not significant. Although within the mixed-model framework we were not able to test the limiting factor hypothesis of Domingues et al. (2010), our results suggest that aggregated across diverse sites and species, there is likely to be some colimitation between N and P.
We also aimed to ascertain whether the effect of leaf P held true across multiple biomes and whether this may be a reason for the different Vcmax to N sensitivities. There was some suggestion that there was an interaction of biome with the Vcmax relationship to N and P (results not shown), but the majority of leaf P data were gathered from within the tropical zone (Table 2) and the datasets when divided by biome were dominated by individual studies, reducing the power of the meta-analysis. In data gathered primarily within tropical latitudes, we have shown that leaf P substantially impacts the Vcmax to leaf N relationship.
Kattge et al. (2009) demonstrated variability in the Vcmax to leaf N relationship across biomes, indicating that in tropical biomes where P was expected to be more limiting, Vcmax was less sensitive to leaf N. Our analysis shows that across a range of predominantly tropical biomes, the sensitivity of Vcmax to N was reduced by low leaf P and the derived relationship may help to move forward from PFT-/biome-based parameterizations in TBMs toward a trait correlation approach.
We demonstrated that variation in Vcmax related to variation in leaf P had a large impact on carboxylation rates. Increasing leaf P from 0.05 gm−2 to 0.22 gm−2 approximately doubled modeled gross carboxylation rates under high N levels (Fig. 4). Some of the latest generation of TBMs now includes a P cycle (Wang et al. 2010; Goll et al. 2012; Yang et al. 2013), and Mercado et al. (2011) demonstrated the importance of considering P when simulating carbon fluxes in the Amazon. In addition, anthropogenic N and P pollution has had profound effects on global ecosystems (Penuelas et al. 2012). Evidence suggests that N is more limiting than P in temperate and boreal zones (Elser et al. 2007), which may preclude the measurement of P in these zones or that studies measured P but the effects were not significant so were left out of publications. Despite a comprehensive survey of the literature, assessment of the variation in Vcmax in relation to the leaf N, leaf P, and SLA remains data limited. To fully quantify the effect of leaf P on the Vcmax to N relationship, we need more data from all ecosystems, but especially temperate and boreal ecosystems. We appeal to the leaf gas exchange research community to measure leaf P in conjunction with leaf gas exchange across all biomes.
The impact of SLA
Our results show that the relationship of Vcmax to leaf N was affected by SLA, albeit a small effect, at low values of leaf N (Fig. 1). Both similar and contrasting effects (Wright et al. 2004; Aranda et al. 2006) in the literature suggest that the effect of SLA on Vcmax is complex. SLA responds to multiple environmental and ecological factors and leaf density and leaf thickness strongly correlate with leaf N (Niinemets 1999; Poorter et al. 2009). In a previous meta-analysis, the components of SLA – leaf thickness and leaf density – showed different relationships to Amax (Niinemets 1999), indicating that SLA may not have a consistent effect on photosynthesis. For example, leaf thickness and leaf density are likely to have different effects on internal CO2 conductance (gi) and the N allocation ratio between RuBisCO and leaf structural components (Poorter et al. 2009). Unfortunately, with this dataset, we were unable to assess the effect of mesophyll conductance (gi) on the Vcmax to N relationship. SLA is likely to affect gi (Flexas et al. 2008), and the effects of SLA on the Vcmax to N relationship will be best assessed once when variation in gi can be accounted for.
Resource allocation between Jmax and Vcmax
The Jmax and Vcmax relationship represents resource allocation between the two photosynthetic cycles – electron transport and the Calvin–Benson cycle. Coordination of resource investment in photosynthetic capacity is reflected by the strong relationship between Vcmax and Jmax. Given the tight coupling of Jmax with Vcmax across growth environments and species (Fig. 5), we suggest, as noted in many previous studies (Wullschleger 1993; Beerling and Quick 1995; Harley and Baldocchi 1995; Leuning 1997; Medlyn et al. 2002; Kattge and Knorr 2007), that their coupling may be a fundamental feature of plant photosynthetic trait relationships.
Traditionally, Jmax has been related to Vcmax based on the assumption that optimization of resource allocation to photosynthesis would maintain a close relationship between these two parameters, an assumption verified by analysis of empirical data (e.g., Wullschleger 1993; Beerling and Quick 1995). The similarity in the regression model parameters between our dataset, the TRY dataset, and that of Wullschleger (1993) was remarkable considering the differences between these datasets (Table 4 & Fig. 3). The Wullschleger (1993) dataset comprised mainly grass and crop species as well as some temperate trees, while our dataset predominantly consists of tropical and temperate tree species.
While the general relationship between Jmax and Vcmax is preserved across datasets (Fig. 3), there is substantial variation of individual species data from this relationship (Fig. 3). Some of this variation may arise due to the measurement error. Vcmax and Jmax are differentially sensitive to temperature (Medlyn et al. 2002; Kattge and Knorr 2007), and their temperature sensitivity varies across species (Wohlfahrt et al. 1999b). For most species, this temperature sensitivity is not known, and while necessary, the correction of Vcmax and Jmax to 25°C with non-species-specific sensitivity parameters may add variation into the Jmax to Vcmax relationship. Vcmax is more sensitive to mesophyll conductance than Jmax (Sun et al. 2013) and it may be that some of the variation in the relationship may be attributable to variation in gi; however, it was not possible to determine the effect of gi with this dataset. We present our results assuming infinite gi because assuming infinite gi is currently standard practice in TBMs and was the assumption made by most of the studies used in our meta-analysis. By analyzing the general relationship between Jmax and Vcmax, we aim to provide a framework that can be applied to explain Jmax to Vcmax relationships and consequences of variation in the relationship.
Maire et al. (2012) demonstrated that plants adjust leaf N investment to coordinate Wc and Wj (Chen et al. 1993) for environmental conditions over the previous month (the lifetime of RuBisCO). Scaling between Jmax and Vcmax, represented by the slope parameter bjv, affects the light (and CO2, Von Caemmerer and Farquhar 1981) transition point at which carbon assimilation switches between Wc and Wj, that is, the light level where Wc and Wj are colimiting. We hypothesized that bjv may also coordinate instantaneous Wc and Wj when Wc is limiting as investment in Jmax that would support rates of Wj higher than Wc, when Wc is limiting, represents investment in unused resources. At the assumed leaf absorptance and at 25°C, simulations show that potential Wj rates at high light and Wc rates are similar (Fig. 5D–I), when the probable range in bjv values from our dataset (Table 4) are used. Generally, quantum yield is not maximized. Synthesized across multiple species and environments, the presented relationship suggests that Jmax is related to Vcmax to coordinate Wj with Wc and hedge against photoinhibition, when RuBisCO carboxylation is limiting. Aggregated across the different species and environments, support for co-ordination at light saturation is a very general assertion. The degree of control that plants have over the relationship between Jmax and Vcmax needs to be tested in controlled environments at a range of temperature and light levels (Wohlfahrt et al. 1999a) and giving consideration to mesophyll conductance and leaf absorptance.
Maire et al. (2012) show that coordination occurs over monthly timescales, while our simulations (Fig. 5) are on instantaneous timescales. The timescale over which coordination is considered is important, and given the huge diurnal variability in incident light, Wc and Wj cannot always be coordinated on subdaily timescales. The relationship that we derived between Jmax and Vcmax appears to coordinate, within uncertainty bounds, the Wc and Wj rates of photosynthesis at high light levels (Fig. 5D–I). However, there is some variability and the derived relationship has high Wj at low photosynthetic capacity (Fig. 5D and G), and Wj higher than Wc when Wc is limiting indicates unused electron transport capacity at high light. Unused electron transport capacity could produce reducing power not used in carbon reduction and which could be used in biochemical pathways other than the Calvin–Benson cycle (Buckley and Adams 2011) such as the reduction of nitrite to ammonium that occurs in the chloroplast (Anderson and Done 1978; Searles and Bloom 2003) and the production of isoprene (Morfopoulos et al. 2013).
Conclusion
For the first time, we assess the sensitivity of carbon assimilation to the Jmax to Vcmax relationship, and results from the meta-analysis suggest that plants may employ a conservative strategy of Jmax to Vcmax coordination to avoid photoinhibition. Work is needed to extend this analysis with the consideration of mesophyll conductance and species-specific temperature effects.
We also present for the first time the significance of P and SLA on the relationship of Vcmax to nitrogen and of Jmax to Vcmax in a globally extensive meta-analysis. Modeling demonstrates that variation in leaf P has large consequences for carbon assimilation. The relationships presented in this study can be used to parameterize Vcmax and Jmax in a rigorous fashion based on data-derived relationships, moving parameterization away from methods with limited variation or limited grounding in the literature. To fully understand variability in the relationship of Vcmax and Jmax to leaf N, leaf P, and SLA, work is needed to extend the geographic range of data, particularly into temperate and boreal regions.
Acknowledgments
APW was funded by a Natural Environment Research Council PhD studentship awarded by the National Centre for Earth Observation. APW, SDW, and LG were supported by the US Department of Energy, Office of Science, Biological and Environmental Research Program. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the US Department of Energy under contract DE-AC05-00OR22725. Data were supplied by the TRY initiative on plant traits (http://www.try-db.org), which is supported by DIVERSITAS, IGBP, the Global Land Project, QUEST, and the French programs FRB and GIS Climat, Environnement et Société. We would like to thank Belinda Medlyn, Ebe Merilo, David Tissue, and Tarryn Turnbull for help with data to standardize Vcmax and Jmax calculations.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1
Table S1. Species that feature in the meta-analysis
Table S2. Model selection table for multiple regressions of Vcmax and Jmax regressed against leaf N, P and SLA, or leaf N, P, SLA and Vcmax respectively. The minimum adequate model (MAM) was the model with the lowest AICc. All traits were expressed on a leaf area basis and were natural log transformed.
Appendix S2. Standardisation of Vcmax and Jmax to common kinetic parameters and photosynthetic functions.
Figure S1. Original and standardised values of Vcmax and Jmax both expressed at 25°C.
Figure S2. Standardised values of Vcmax and Jmax (both expressed at 25°C) using Eq 9 to calculate Г* using Oi assumed by the authors of the original publication (20–21 kPa; x-axis) and an assumed reduction in Oi with altitude (y-axis).
Appendix S3. Model assumptions and selection
Table S3. Model selection table for multiple regressions of Vcmax and Jmax regressed against leaf N, P and SLA, or leaf N, P, SLA and Vcmax respectively.
Figure S3. Plots of the mixed-model regression assumptions for the un-transformed Jmax to Vcmax relationship for the data collected in this study.
Figure S4. Plots of the mixed-model regression assumptions for the transformed Jmax to Vcmax relationship for the data collected in this study.
Appendix 4. Modelling photosynthesis.
References
- Anderson JW, Done J. Light-dependent assimilation of nitrite by isolated Pea chloroplasts. Plant Physiol. 1978;61:692–697. doi: 10.1104/pp.61.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda X, Agusti C, Joffre R, Fleck I. Photosynthesis, growth and structural characteristics of holm oak resprouts originated from plants grown under elevated CO2. Physiol. Plant. 2006;128:302–312. [Google Scholar]
- Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons TL. Leaf respiration in light and darkness - A comparison of slow- and fast-growing Poa species. Plant Physiol. 1997;113:961–965. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer GA, Berntson GM, Bazzaz FA. Regenerating temperate forests under elevated CO2 and nitrogen deposition: comparing biochemical and stomatal limitation of photosynthesis. New Phytol. 2001;152:249–266. [Google Scholar]
- Beerling DJ, Quick WP. A new technique for estimating rates of carboxylation and electron-transport in leaves of C-3 plants for use in dynamic global vegetation models. Glob. Change Biol. 1995;1:289–294. [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell Environ. 2001;24:253–259. [Google Scholar]
- Bonan GB, Lawrence PJ, Oleson KW, Levis S, Jung M, Reichstein M, et al. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J. Geophys. Res. Biogeosci. 2011;116:G02014. doi: 10.1029/2010JG001593/abstract;jsessionid=5E06E595C92C1AEC027A49CA8BD11601.f03t0. [Google Scholar]
- Bown HE, Watt MS, Clinton PW, Mason EG, Richardson B. Partitioning concurrent influences of nitrogen and phosphorus supply on photosynthetic model parameters of Pinus radiata. Tree Physiol. 2007;27:335–344. doi: 10.1093/treephys/27.3.335. [DOI] [PubMed] [Google Scholar]
- Brück H, Guo S. Influence of N form on growth photosynthesis of Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 2006;169:849–856. [Google Scholar]
- Buckley TN, Adams MA. An analytical model of non-photorespiratory CO2 release in the light and dark in leaves of C-3 species based on stoichiometric flux balance. Plant, Cell Environ. 2011;34:89–112. doi: 10.1111/j.1365-3040.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson D. Model selection and multi-model inference. New York, Berlin, Heidelberg: Springer; 2002. [Google Scholar]
- Cadule P, Friedlingstein P, Bopp L, Sitch S, Jones CD, Ciais P, et al. Benchmarking coupled climate-carbon models against long-term atmospheric CO(2) measurements. Global Biogeochem. Cycles. 2010;24:GB2016. [Google Scholar]
- Calfapietra C, Tulva I, Eensalu E, Perez M, De Angelis P, Scarascia-Mugnozza G, et al. Canopy profiles of photosynthetic parameters under elevated CO2 and N fertilization in a poplar plantation. Environ. Pollut. 2005;137:525–535. doi: 10.1016/j.envpol.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Canadell JG, Le Quere C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl Acad. Sci. USA. 2007;104:18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell FE, Whitehead D, Rogers GND, Mcseveny TM. Plasticity in photosynthetic response to nutrient supply of seedlings from a mixed conifer-angiosperm forest. Austral Ecol. 2005;30:426–434. [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, Holtum JAM, Turner BL. Photosynthetic physiology of eucalypts along a sub-continental rainfall gradient in northern Australia. Agric. For. Meteorol. 2011;151:1462–1470. [Google Scholar]
- Chen JL, Reynolds JF, Harley PC, Tenhunen JD. Coordination theory of leaf nitrogen distribution in a canopy. Oecologia. 1993;93:63–69. doi: 10.1007/BF00321192. [DOI] [PubMed] [Google Scholar]
- Collatz GJ, Ball JT, Grivet C, Berry JA. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration - A model that includes a laminar boundary-layer. Agric. For. Meteorol. 1991;54:107–136. [Google Scholar]
- Cox P. Hadley centre technical note 24: description of the TRIFFID dynamic global vegetation model. Bracknell, Berks: Hadley Centre, Met Office; 2001. [Google Scholar]
- Deng X, Ye WH, Feng HL, Yang QH, Cao HL, Hui KY, et al. Gas exchange characteristics of the invasive species Mikania micrantha and its indigenous congener M. cordata (Asteraceae) in South China. Bot. Bull. Acad. Sinica. 2004;45:213–220. [Google Scholar]
- Domingues TF, Meir P, Feldpausch TR, Saiz G, Veenendaal EM, Schrodt F, et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant, Cell Environ. 2010;33:959–980. doi: 10.1111/j.1365-3040.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Wong S. An empirical model of stomatal conductance. Funct. Plant Biol. 1984;11:191–210. [Google Scholar]
- Farquhar GD, Caemmerer SV, Berry JA. A biochemical-model of photosynthetic CO2 assimilation in leaves of C-3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell Environ. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Goll DS, Brovkin V, Parida BR, Reick CH, Kattge J, Reich PB, et al. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences. 2012;9:3547–3569. [Google Scholar]
- Grassi G, Meir P, Cromer R, Tompkins D, Jarvis PG. Photosynthetic parameters in seedlings of Eucalyptus grandis as affected by rate of nitrogen supply. Plant, Cell Environ. 2002;25:1677–1688. [Google Scholar]
- Groenendijk M, Dolman AJ, van der Molen MK, Leuning R, Arneth A, Delpierre N, et al. Assessing parameter variability in a photosynthesis model within and between plant functional types using global Fluxnet eddy covariance data. Agric. For. Meteorol. 2011;151:22–38. [Google Scholar]
- Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD. Reliable estimation of biochemical parameters from C3 leaf photosynthesis–intercellular carbon dioxide response curves. Plant, Cell Environ. 2010;33:1852–1874. doi: 10.1111/j.1365-3040.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- Gurevitch J, Hedges LV. Statistical issues in ecological meta-analyses. Ecology. 1999;80:1142–1149. [Google Scholar]
- Han Q, Kawasaki T, Nakano T, Chiba Y. Leaf-age effects on seasonal variability in photosynthetic parameters and its relationships with leaf mass per area and leaf nitrogen concentration within a Pinus densiflora crown. Tree Physiol. 2008;28:551–558. doi: 10.1093/treephys/28.4.551. [DOI] [PubMed] [Google Scholar]
- Harley PC, Baldocchi DD. Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest. I. Leaf model parametrization. Plant, Cell Environ. 1995;18:1146–1156. [Google Scholar]
- Harley PC, Loreto F, Marco GD, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FH, Eyring H, Williams RW. The nature of enzyme inhibitions in bacterial luminescence - Sulfanilamide, urethane, temperature and pressure. J. Cell. Comp. Physiol. 1942;20:247–268. [Google Scholar]
- Katahata S-I, Naramoto M, Kakubari Y, Mukai Y. Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub Daphniphyllum humile along a natural light gradient. Tree Physiol. 2007;27:199–208. doi: 10.1093/treephys/27.2.199. [DOI] [PubMed] [Google Scholar]
- Kattge J, Knorr W. Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant, Cell Environ. 2007;30:1176–1190. doi: 10.1111/j.1365-3040.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 2009;15:976–991. [Google Scholar]
- Kattge J, Diaz S, Lavorel S, Prentice C, Leadley P, Boenisch G, et al. TRY - a global database of plant traits. Glob. Change Biol. 2011;17:2905–2935. [Google Scholar]
- Krause GH, Winter K, Matsubara S, Krause B, Jahns P, Virgo A, et al. Photosynthesis, photoprotection, and growth of shade-tolerant tropical tree seedlings under full sunlight. Photosynth. Res. 2012;113:273–285. doi: 10.1007/s11120-012-9731-z. [DOI] [PubMed] [Google Scholar]
- Kubiske ME, Zak DR, Pregitzer KS, Takeuchi Y. Photosynthetic acclimation of overstory Populus tremuloides and understory Acer saccharum to elevated atmospheric CO2 concentration: interactions with shade and soil nitrogen. Tree Physiol. 2002;22:321–329. doi: 10.1093/treephys/22.5.321. [DOI] [PubMed] [Google Scholar]
- Leuning R. Scaling to a common temperature improves the correlation between the photosynthesis parameters Jmax and Vcmax. J. Exp. Bot. 1997;48:345–347. [Google Scholar]
- Maire V, Martre P, Kattge J, Gastal F, Esser G, Fontaine S, et al. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE. 2012;7:e38345. doi: 10.1371/journal.pone.0038345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manter DK, Kavanagh KL, Rose CL. Growth response of Douglas-fir seedlings to nitrogen fertilization: importance of Rubisco activation state and respiration rates. Tree Physiol. 2005;25:1015–1021. doi: 10.1093/treephys/25.8.1015. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London; San Diego: Academic Press; 1995. [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell Environ. 2002;25:1167–1179. [Google Scholar]
- Mercado LM, Patino S, Domingues TF, Fyllas NM, Weedon GP, Sitch S, et al. Variations in Amazon forest productivity correlated with foliar nutrients and modelled rates of photosynthetic carbon supply. Philos. Trans. R. Soc. B. Biol. Sci. 2011;366:3316–3329. doi: 10.1098/rstb.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Heinsoo K, Kull O, Soderbergh I, Lundmark T, Koppel A. Leaf photosynthetic properties in a willow (Salix viminalis and Salix dasyclados) plantation in response to fertilization. Eur. J. Forest Res. 2006;125:93–100. [Google Scholar]
- Midgley GF, Wand SJE, Pammenter NW. Nutrient and genotypic effects on CO2-responsiveness: photosynthetic regulation in Leucadendron species of a nutrient-poor environment. J. Exp. Bot. 1999;50:533–542. [Google Scholar]
- Morfopoulos C, Prentice IC, Keenan TF, Friedlingstein P, Medlyn BE, Penuelas J, et al. A unifying conceptual model for the environmental responses of isoprene emissions from plants. Ann. Bot. 2013;112:1223–1238. doi: 10.1093/aob/mct206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U. Components of leaf dry mass per area - thickness and density - alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999;144:35–47. [Google Scholar]
- Ordonez JC, van Bodegom PM, Witte J-PM, Wright IJ, Reich PB, Aerts R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009;18:137–149. [Google Scholar]
- Penuelas J, Sardans J, Rivas-Ubach A, Janssens IA. The human-induced imbalance between C, N and P in Earth's life system. Glob. Change Biol. 2012;18:3–6. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D Team RDC. 2011. nlme: Linear and Nonlinear Mixed Effects Models.
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Porte A, Loustau D. Variability of the photosynthetic characteristics of mature needles within the crown of a 25-year-old Pinus pinaster. Tree Physiol. 1998;18:223–232. doi: 10.1093/treephys/18.4.223. [DOI] [PubMed] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984;35:15–44. [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Calcerrada J, Reich PB, Rosenqvist E, Pardos JA, Cano FJ, Aranda I. Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol. 2008;28:761–771. doi: 10.1093/treephys/28.5.761. [DOI] [PubMed] [Google Scholar]
- Rogers A. The use and misuse of Vcmax in earth system models. Photosynth. Res. 2014;119:15–29. doi: 10.1007/s11120-013-9818-1. [DOI] [PubMed] [Google Scholar]
- Searles PS, Bloom AJ. Nitrate photo-assimilation in tomato leaves under short-term exposure to elevated carbon dioxide and low oxygen. Plant, Cell Environ. 2003;26:1247–1255. [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell Environ. 2007;30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- Sholtis JD, Gunderson CA, Norby RJ, Tissue DT. Persistent stimulation of photosynthesis by elevated CO2 in a sweetgum (Liquidambar styraciflua) forest stand. New Phytol. 2004;162:343–354. [Google Scholar]
- Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W, et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Change Biol. 2003;9:161–185. [Google Scholar]
- Smith EL. The influence of light and carbon dioxide on photosynthesis. J. Gen. Physiol. 1937;20:807–830. doi: 10.1085/jgp.20.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Software D. Grab it! Raleigh, NC USA: Datatrend Software; 2008. [Google Scholar]
- Sun Y, Gu L, Dickinson RE, Pallardy SG, Baker J, Cao Y, et al. Asymmetrical effects of mesophyll conductance on fundamental photosynthetic parameters and their relationships estimated from leaf gas exchange measurements. Plant, Cell Environ. 2013:978–994. doi: 10.1111/pce.12213. [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. Sunderland, Mass: Sinauer Assoc; 2010. [Google Scholar]
- Tissue DT, Griffin KL, Turnbull MH, Whitehead D. Stomatal and non-stomatal limitations to photosynthesis in four tree species in a temperate rainforest dominated by Dacrydium cupressinum in New Zealand. Tree Physiol. 2005;25:447–456. doi: 10.1093/treephys/25.4.447. [DOI] [PubMed] [Google Scholar]
- Turnbull TL, Adams MA, Warren CR. Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus seedlings is not caused by increased leaf nitrogen. Tree Physiol. 2007;27:1481–1492. doi: 10.1093/treephys/27.10.1481. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer; 2002. [Google Scholar]
- Verheijen LM, Brovkin V, Aerts R, Bönisch G, Cornelissen JHC, Kattge J, et al. Impacts of trait variation through observed trait-climate relationships on performance of a representative earth system model: a conceptual analysis. Biogeosci. Discuss. 2012;9:18907–18950. [Google Scholar]
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wang YP, Law RM, Pak B. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences. 2010;7:2261–2282. [Google Scholar]
- Warren CR. The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J. Exp. Bot. 2004;55:2313–2321. doi: 10.1093/jxb/erh239. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Watanabe Y, Kitaoka S, Utsugi H, Kita K, Koike T. Growth and photosynthetic traits of hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) under elevated CO2 concentration with low nutrient availability. Tree Physiol. 2011;31:965–975. doi: 10.1093/treephys/tpr059. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt G, Bahn M, Cernusca A. The use of the ratio between the photosynthesis parameters Pml and Vcmax for scaling up photosynthesis of C3 plants from leaves to canopies: a critical examination of different modelling approaches. J. Theor. Biol. 1999a;200:163–181. doi: 10.1006/jtbi.1999.0985. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt G, Bahn M, Haubner E, Horak I, Michaeler W, Rottmar K, et al. Inter-specific variation of the biochemical limitation to photosynthesis and related leaf traits of 30 species from mountain grassland ecosystems under different land use. Plant, Cell Environ. 1999b;22:1281–1296. [Google Scholar]
- Woodward FI, Smith TM, Emanuel WR. A global land primary productivity and phytogeography model. Global Biogeochem. Cycles. 1995;9:471–490. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wullschleger SD. Biochemical limitations to carbon assimilation in C3 plants - a retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 1993;44:907–920. [Google Scholar]
- Xu R. Measuring explained variation in linear mixed effects models. Stat. Med. 2003;22:3527–3541. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- Yang X, Thornton PE, Ricciuto DM, Post WM. The role of phosphorus dynamics in tropical forests – a modeling study using CLM-CNP. Biogeosci. Discuss. 2013;10:14439–14473. [Google Scholar]
- Zaehle S, Friend AD. Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1. Model description, site-scale evaluation, and sensitivity to parameter estimates. Global Biogeochem. Cycles. 2010;24 GB1005. [Google Scholar]
- Zaehle S, Sitch S, Smith B, Hatterman F. Effects of parameter uncertainties on the modeling of terrestrial biosphere dynamics. Global Biogeochem. Cycles. 2005;19:GB3020. [Google Scholar]
- Zhang S, Dang Q-L. Effects of carbon dioxide concentration and nutrition on photosynthetic functions of white birch seedlings. Tree Physiol. 2006;26:1457–1467. doi: 10.1093/treephys/26.11.1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1. Species that feature in the meta-analysis
Table S2. Model selection table for multiple regressions of Vcmax and Jmax regressed against leaf N, P and SLA, or leaf N, P, SLA and Vcmax respectively. The minimum adequate model (MAM) was the model with the lowest AICc. All traits were expressed on a leaf area basis and were natural log transformed.
Appendix S2. Standardisation of Vcmax and Jmax to common kinetic parameters and photosynthetic functions.
Figure S1. Original and standardised values of Vcmax and Jmax both expressed at 25°C.
Figure S2. Standardised values of Vcmax and Jmax (both expressed at 25°C) using Eq 9 to calculate Г* using Oi assumed by the authors of the original publication (20–21 kPa; x-axis) and an assumed reduction in Oi with altitude (y-axis).
Appendix S3. Model assumptions and selection
Table S3. Model selection table for multiple regressions of Vcmax and Jmax regressed against leaf N, P and SLA, or leaf N, P, SLA and Vcmax respectively.
Figure S3. Plots of the mixed-model regression assumptions for the un-transformed Jmax to Vcmax relationship for the data collected in this study.
Figure S4. Plots of the mixed-model regression assumptions for the transformed Jmax to Vcmax relationship for the data collected in this study.
Appendix 4. Modelling photosynthesis.