Abstract
The University of Minnesota Transdisciplinary Tobacco Use Research Center has been examining the multiple dimensions and the scientific evidence required to determine the feasibility of tobacco harm reduction as a means to reduce tobacco-related mortality and morbidity. Because of the complexity associated with exploring this area, an interdisciplinary approach is necessary. The research components that have been of particular focus at our center include (a) developing and validating biomarkers of tobacco-related exposure and toxicity, (b) developing animal models and designing studies with humans to assess a variety of smoking reduction approaches and potential reduced exposure products, and (c) determining individual differences in response to these interventions and products. A description of the ongoing activities and challenges in these areas is provided, along with projected directions for the future.
Introduction
Use of reduced toxin exposure/reduced risk tobacco products has recently been actively debated among scientists, the tobacco control community, and even in U.S. congressional hearings. This area is controversial partly because of experience with the introduction of low-yield tobacco products, which initially seemed to hold promise for reducing tobacco harm but instead led to a negative public health impact (National Cancer Institute, 2001). Several reasons have been given for the importance of reexamining this approach to reduce tobacco-related mortality and morbidity. First, a substantial number of smokers continue to smoke. After the dramatic decreases observed in the prevalence of tobacco use over the past decades, the rate of quitting smoking has slowed, leading scientists to debate whether the remaining smokers tend to be hardened or hard-core smokers (Warner & Burns, 2003). Of the 22 million smokers who tried to quit, approximately 3%–5% are successful for at least 1 year (U.S. Department of Health and Human Services, 2000); and at any one time, only 4%–20% are seriously thinking of quitting smoking within the next month (Etter, Paernegger, & Ronchi, 1997; Wewers, Stillman, Hartman, & Shopland, 2003).
Second, studies suggest that the risk for tobacco-related diseases is related both to duration of tobacco use (Doll & Peto, 1978; Doll, Peto, Boreham, & Sutherland, 2004; Flanders, Lally, Zhu, Henley, & Thun, 2003) and to dose (measured in cigarettes smoked per day; Thun, Day-Lally, Calle, Flanders, & Heath, 1995). Dose-dependent relationships have been observed between number of cigarettes smoked and the risk of cancer, respiratory diseases including chronic obstructive pulmonary disease (COPD), cardiovascular and peripheral vascular disease, as well as pregnancy complications (e.g., Burns, 2003; Jimenez-Ruiz, Kunze, & Fagerstroöm, 1998; Menotti et al., 2001; Stratton, Shetty, Wallace, & Bondurant, 2001; Wynder & Stellman, 1979).
Similarly, based on data from the Cancer Prevention Study I of the American Cancer Society, Burns (1997) found that significant and sustained reduction in exposure to tobacco toxins could significantly lower the risk for premature mortality and that the magnitude of the benefit was related to the extent of cigarette reduction and age at reduction onset. Partly for these reasons, several reports were published in the mid-to-late 1990s, recommending that reduction of tobacco toxicant exposure should be considered as a possibility for some continuing smokers (Henningfield, 1995; Hughes, 1995; Shiffman, Mason, & Henningfield, 1998; U.K. Department of Health, 1998,UN Focal Point on Tobacco or Health, 1998). The roundtable organized by the UN Focal Point on Tobacco or Health concluded that “to attain a substantial reduction in tobacco-caused death and disease in existing smokers and in future generations, it is important to adopt a triadic approach: a) tobacco-use prevention, b) smoking cessation, and c) reduction of exposure to tobacco toxins in people who are unable or unwilling to completely abstain from tobacco.”
The need for research in this area has escalated because tobacco companies have marketed tobacco products that purportedly reduce exposure to tobacco toxicants and may potentially reduce disease risk. Pharmaceutical companies and independent scientists have renewed efforts to examine ways to reduce smoking, primarily through the use of medications. The expanding interest in this area led the U.S. Food and Drug Administration to commission the Institute of Medicine (IOM) to examine the science base for assessing tobacco harm reduction approaches (Stratton et al., 2001). One of the principle conclusions in the IOM report stated that, although reducing exposure to tobacco toxicants is feasible, “potential reduced exposure products (PREPs) … have not been evaluated comprehensively enough to provide a scientific basis for concluding that they are associated with reduced risk of disease compared to conventional products” (p. 5). It further stated that improving “the science base for a harm reduction strategy for tobacco products and [protecting] public health [requires] a substantial and sustained research program to address the critical unresolved issues.”
To develop scientific based information on these products or strategies and to determine the impact they may have on public health, several basic questions need to be addressed, including (a) What valid surrogate biomarkers can be used to determine exposure to tobacco toxicants? (b) What is the tobacco toxicant exposure when smokers use PREPs or reduction strategies? and (c) How does the use of these products affect motivation and achievement of abstinence? Without research relevant to evaluating these products, we may be faced with another public health challenge. To date, prevention of tobacco uptake and tobacco cessation are the only known ways to significantly reduce morbidity and mortality. The introduction of reduced exposure, reduced risk methods should never compromise these known methods and should optimally facilitate the achievement of abstinence. However, because more than 44.5 million people in the United States smoke (Centers for Disease Control and Prevention, 2005), as do about 1.2 billion people worldwide (www.treatobacco.net), it is important to consider alternative ways to reduce morbidity and mortality.
Conceptual model
A potential conceptual model to examine reduced exposure approaches is one that integrates the harm reduction framework described by MacCoun (1998) and the IOM report (Stratton et al., 2001), and the model for transdisciplinary treatment for tobacco addiction described by TTURC investigators (Baker et al., 2003). In this model as it applies to harm reduction (Figure 1), the smoker population is the general population from which the population participating in harm reduction approaches is drawn. The prevalence of the smoker population can be influenced by a number of factors, including tobacco control policies (increased cigarette costs, smoking bans), prevention campaigns and programs, and the availability of effective cessation treatments. The prevalence of smoking also is determined by the addictiveness of tobacco products, and the marketing and promotion of these products. It can be influenced by the availability and marketing of seemingly “safer” or “safe” tobacco products. The population participating in using reduced exposure, reduced risk products is determined by a number of factors, including the individual’s inability or unwillingness to quit smoking because of a number of personal and smoking history factors. In addition, the marketing of reduced exposure, reduced risk products, consumer perception of safety, governmental regulation of these products, and consumer education may play important roles in determining the participating population.
Figure 1.
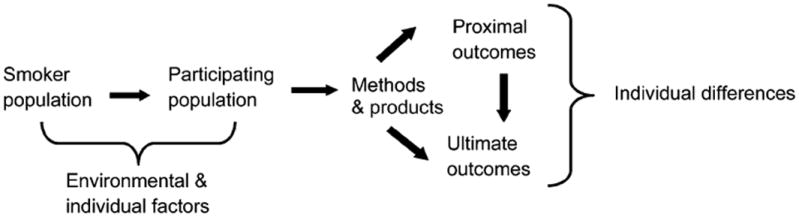
Conceptual model for analyses of tobacco harm reduction. Smoker population comprises the individuals from which the population participating in reduced exposure/risk methods and products is drawn. Environmental and individuals factors influence the individuals who constitute this population. Use of products and methods, with different mechanisms of action and effect, influence proximal (e.g., exposure biomarkers) and ultimate (e.g., disease risk, cessation) outcomes. Proximal outcomes can influence ultimate outcomes. These outcomes are influenced by individual differences.
The mechanisms by which different PREPs can reduce tobacco toxicant exposure (proximal outcome) and ultimately lead to abstinence or reduction in disease (ultimate outcome) include facilitating the reduction in tobacco use by replacing nicotine levels or the effects from nicotine (e.g., nicotine agonist treatment such as medicinal nicotine, bupropion, nortriptyline), reducing the reinforcing effects of tobacco by reducing the levels of nicotine delivery (e.g., nicotine-free cigarettes, nicotine vaccine), increasing or sustaining nicotine levels but reducing exposure to all other toxicants (e.g., high-nicotine, low-toxicant cigarettes), reducing all toxicant levels in the tobacco product including nicotine (e.g., low-toxicant cigarettes, no-nicotine-yield cigarette), or reducing or eliminating combustion products. Currently available reduced exposure, reduced risk methods or products include medications (e.g., medicinal nicotine, bupropion) or behavioral treatments to reduce cigarette consumption, modified tobacco products that have lower levels of toxicants and nicotine, cigarette-like delivery devices that heat rather than burn tobacco to reduce exposure to toxic combustion products, noncombusted lower nitrosamine oral tobacco products, and medicinal nicotine products. The use of policies represents another intervention that can reduce harm. These policies include increasing tobacco product taxes and smoking bans, both of which have been demonstrated to reduce cigarette consumption (Stratton et al., 2001).
The proximal outcome is measured both behaviorally and biologically. It can represent reductions in number of cigarettes or smoking topography parameters or in biomarkers for toxicant exposure or toxicity, that are related to disease risk. The ultimate outcome is abstinence or reduction in morbidity and mortality. The extent to which proximal and ultimate outcomes are achieved also depends on individual differences in response to these treatments. Different phenotypes (e.g., degree of dependence, nicotine metabolism, baseline nicotine intake, motives for smoking, negative affect, activation and detoxification of carcinogens) and genotypes (e.g., CYP2A6 or CYP2B6 polymorphisms) will determine the extent of exposure and response to toxicants and nicotine.
Current state of knowledge
This paper focuses on the following aspects of the model: (a) describing population characteristics, (b) developing and testing proximal outcomes (biomarkers), (c) exploring mechanisms and methods of current types of PREPs that might lead to harm reduction, (d) understanding individual differences or moderating factors that influence the effects from these approaches, and (e) determining the impact of the PREPs and methods on ultimate outcomes. Primarily we present data from our TTURC to illustrate some key points: (a) the importance of examining the characteristics of the population interested in harm reduction approaches, (b) the nascent stage of biomarker development and the systematic studies required to find valid biomarkers, (c) the challenges in determining the harm reduction potential of a PREP and the relatively few products likely to demonstrate a significant reduction in exposure and harm, and (d) the complexity of examining the harm potential of a product or method because of individual differences in response to nicotine or toxicants.
Population recruitment and characteristics
Studies show that smokers who express interest in trying PREPs tend to be concerned about the effects of smoking on their health (Hund et al., 2006), and among the low-tar, low-nicotine cigarette smokers who purchase them, the majority think PREPs are safer for their health than are their own cigarettes (Hughes, Keely, & Callas, 2005). Further examination of this population’s characteristics is critical to determine the impact of these products on tobacco use, health, and motivations for eventual cessation. We have examined the characteristics of smokers seeking cigarette reduction versus cessation interventions and of smokers who did and did not show a history of cigarette reduction. Smokers seeking to reduce smoking or who have done so have a higher prevalence of medical illness (Joseph, Bliss, Zhao, & Lando, 2005; Lemmonds, Mooney, Reich, & Hatsukami, 2004). Lemmonds et al. (2004) also found a higher prevalence of psychiatric disorders and more severe dependence in this population.
Limitations of these studies include the recruitment bias toward treatment-seekers and the fact that the results may not reflect the general population of smokers interested in harm reduction approaches. Nonetheless, these studies emphasize the importance of knowing the population that is interested in reduced exposure, reduced risk methods and products to determine the impact of these interventions on health outcomes. If the population that is seeking reduced exposure interventions is the most physically compromised, then the damage from tobacco use may have already been set in motion, thus minimizing any beneficial effects from PREPs.
Proximal outcomes: Biomarkers for tobacco toxicant exposure and toxicity
Biomarkers are critical in testing potential reduced exposure products and methods (Hatsukami, Benowitz, Rennard, Oncken, & Hecht, 2006; Hatsukami, Giovino et al., 2005; Stratton et al., 2001). Biomarkers can be categorized into two main types: (a) exposure biomarkers, which include specific chemical constituents of tobacco or products of tobacco combustion or their metabolites, for example, carbon monoxide (CO), nicotine, cotinine, 4-(methylnitrosamino)-1-(3-pyridyl)-butanol [NNAL] and its glucuronides [NNAL-Glucs], 1-hydroxypyrene [1-HOP]); and (b) toxicity biomarkers, which include biologically effective dose (e.g., 4-aminobiphenyl hemoglobin adducts), injury such as early biochemical or histological effects (e.g., lipoproteins, white cells, C-reactive protein, fibrinogen, F2 isoprostanes, platelet aggregation), or early health effects (e.g., hypertension, deterioration of lung function; Hatsukami, Benowitz et al., 2006).
To be valid, biomarkers for exposure and toxicity should (a) be specific to tobacco and tobacco smoke exposure, (b) be consistent in levels over time when tobacco use is sustained at the same rate, (c) differentiate tobacco users versus non–tobacco users, (d) demonstrate significant change after cessation of a tobacco product, and (e) demonstrate a dose–response relationship between intake and the biomarker or change with reduced use. Biomarkers also should be predictive of or related to disease risk. To date, the research on biomarkers is limited by the current knowledge of critical targets and restricted knowledge of the mechanisms associated with toxicity or disease, such as mechanisms of cell transformation. Therefore, the science and database on biomarkers remains incomplete. Furthermore, we have no knowledge of the extent of reduction in biomarkers that is required to reduce disease risk or the threshold of change that is required for reduced risk.
A review of potential biomarkers is provided elsewhere (Hatsukami, Benowitz et al., 2006; Stratton et al., 2001). Table 1 summarizes the biomarkers that show differences between smokers and nonsmokers, those that change with cessation, those that demonstrate a dose–response relationship, or those that change with cigarette reduction. Studies are ongoing by tobacco company and tobacco-funded researchers (Scherer et al., 2007; Zedler et al., 2006) and independent researchers (e.g., Jacob, Wilson, & Benowitz, 2007) to further identify, develop assays for, and validate biomarkers that can be used to assess exposure and toxicity. For example, our TTURC continues to explore biomarkers that measure exposure to different classes of carcinogens and potent carcinogens within a class of carcinogens. These biomarkers include urinary and blood total NNAL (NNAL plus NNAL-Glucs), which are metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; Carmella, Le Ka, Upadhyaya, & Hecht, 2002). In addition to serving as a biomarker of carcinogen exposure, the ratio of NNAL-Glucs to NNAL could potentially serve as an indicator of metabolic activation (NNAL) and detoxification (NNAL-Glucs) of NNK: the lower the ratio, the higher the exposure to free NNAL, and potentially the greater the risk for lung cancer. Investigations are ongoing to determine if phenotyping based on this ratio as well as level of exposure are predictive of lung cancer.
Table 1.
Panel of biomarkers.
| Measurement of | |
|---|---|
| Cancer | |
| NNAL and NNAL-Gluc in urine | Carcinogen (NNK) uptakeb |
| 3-Aminobiphenyl, 4 aminobiphenyl, and other aromatic amine-Hb adducts | Carcinogen (aromatic amines) uptake plus metabolic activationc |
| Urine mutagenicity | Mutagen uptaked |
| Sister chromatid exchange in peripheral lymphocytes | DNA damagec |
| Nonmalignant lung disease | |
| Macrophages | Inflammationd |
| Cardiovascular disease | |
| Carbon monoxidea | Chemical uptakeb |
| Nicotine/cotininea | Chemical uptake and metabolismb |
| Flow-mediated dilation | Endothelial functiond |
| Circulating endothelial precursor cells | Endothelial functiond |
| Fibrinogen | Hypercoagulable stated |
| Homocysteine | Hypercoagulable stated |
| White blood cell count | Inflammationd |
| C-reactive protein | Inflammationd |
| slCAM1 | Inflammationd |
| Glucose clamping studies | Insulin resistanced |
| Fetal toxicity | |
| Birth weight | Outcomee |
| Neurocognitive impairments in offspring | Outcomee |
| Maternal exhaled CO | Chemical uptakeb |
| Maternal cotinine | Chemical uptake and metabolismb |
| Maternal thiocyanate | Chemical uptake and metabolismb |
Note. From Hatsukami, Benowitz et al. (2006).
Should be included in all studies as general measures of tobacco constituent uptake.
Biomarker for exposure.
Biomarker for toxicity including biologically effective dose.
Biomarker for injury or potential harm.
Health outcome.
Our TTURC also has studied (a) urinary 1-HOP, a metabolite of the noncarcinogenic polycyclic aromatic hydrocarbon (PAH) pyrene (Hatsukami et al., 2004; Hecht, 2002; Hecht et al., 2004); (b) phenanthrene tetraol, a related PAH biomarker (Hecht, Chen, Yagi, Jerina, & Carmella, 2003); and (c) acetaldehyde DNA adducts in human leucocytes (Chen et al., 2007). Whereas 1-HOP gives information on PAH uptake, phenanthrene tetraol provides information on PAH uptake plus metabolic activation (Hecht et al., 2003). Other biomarkers under investigation include N′-nitrosonornicotine (NNN) and its glucuronides in urine; mercapturic acids of acrolein, butadiene, and benzene in urine; anti-7, 8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]-pyrene (BPDE)-hemoglobin adducts; ethylene oxide hemoglobin adducts; and hemoglobin adducts of ethylating agents. Systematic examinations of biomarkers across disease states (e.g., cardiovascular and pulmonary diseases) also are critical for the evaluation of harm reduction methods.
Methods and mechanisms
The mechanisms that can potentially lead to significant and meaningful exposure reduction via different products and methods are wide ranging and varied. These mechanisms can involve altering the amount of nicotine that is absorbed in the brain, the rapidity of this absorption, or both; providing relief of tobacco withdrawal symptoms or replacing the reinforcing effects of nicotine; altering the level of toxicants in the products or that is emitted from product use; or maintaining the palatability and sensory aspects of the product but reducing nicotine. To date, the reduced exposure intervention methods (excluding medicinal nicotine) include tobacco use reduction typically facilitated by pharmacotherapies, modified cigarettes or cigarette-like delivery devices, and noncombusted lower nitrosamine oral tobacco. Assessing the impact of these products requires integrating the results from studies that have used biomarkers for exposure and risk and epidemiological studies.
Cigarette reduction
Limiting the number of cigarettes smoked may be considered as a potential step toward ultimate cessation, particularly among those who do not have immediate plans to quit. Cigarette reduction also may serve as a method to reduce toxicant exposure in an effort to decrease mortality and morbidity. However, recent studies and analyses indicate that smoking reduction may not be a viable method to reduce harm, unless it leads to cessation. For example, Hecht and colleagues (2004, 2005) analyzed how reduced cigarette consumption facilitated by the use of nicotine replacement affected levels of NNAL and NNAL-Glucs and 1-HOP in smokers. They found that decreases were not only modest but were proportionally less than the reductions in cigarettes smoked per day. For example, a 74% reduction in self-reported cigarettes (from 24 to 6 cigarettes/day) led to a 29% reduction in total NNAL; and a 90% reduction in cigarettes was associated with a 46% reduction in total NNAL. Hatsukami et al. (2005) found that among smokers who reduced their smoking by a mean of about 70% (from 24 to 7 cigarettes/day), significant improvements in biomarkers for cardiovascular disease, such as white blood cell count, cholesterol concentrations, blood pressure, and heart rate were present. But, again, the changes were modest, and whether such reductions are associated with reduced disease risk is unknown. Other studies also have shown significant but modest reductions in hematological, fibrinogen, and some indices of lipoprotein biomarkers (Bolliger et al., 2002; Eliasson, Hjalmarson, Kruse, Landfeldt, & Westin, 2001; Haustein, Krause, Haustein, Rasmussen, & Cort, 2004).
These modest reductions in biomarkers agree with the findings from epidemiological studies examining the effects of cigarette reduction on disease risk. In a large cohort study conducted in Copenhagen, compared with regular smokers, heavy smokers who reduced their smoking by 50% or greater (643–858 reducers) and followed for 14–16 years did not experience fewer myocardial infarctions (Godtfredsen, Osler, Vestbo, Andersen, & Prescott, 2003), less hospitalizations for chronic obstructive pulmonary disease (Godtfredsen, Vestbo, Osler, & Prescott, 2002), or less overall mortality (Godtfredsen, Holst, Prescott, Vestbo, & Osler, 2002). Smokers did experience a 27% reduction in lung cancer (Godtfredsen, Prescott, & Osler, 2005), although other case–control studies showed no significant reduction in lung cancer among reducers (Benhamou, Benhamou, Auquier, & Flamant, 1989; Lubin, 1984). A large Norwegian cohort study followed, until December 2003, smokers who reduced their smoking by 50% or greater (348 males, 127 females; mean number of cigarettes was about 9–10 per day after reduction) between the first screening in the mid-1970s and the second screening 3–13 years later. Reducers experienced a similar relative risk of dying from any cause, cardiovascular disease, ischemic heart disease, and smoking-related cancers and a nonsignificant decrease in lung cancer compared with heavy smokers (17–19 cigarettes/day; Tverdal & Bjartveit, 2006). In a comprehensive literature review on this topic, Hughes & Carpenter (2006) concluded that decreases in risks for smoking-related disease and mortality with smoking reduction have not been tested adequately because of measurement limitations in the present studies (e.g., reliance on self-report, no information on duration of reduction). Future research, which can use existing cohort or case–control studies that have collected biosamples, also should examine the threshold of number of cigarettes reduced (absolute and percent) and biomarker levels when one would begin to see reductions in disease risk.
Compensatory smoking (e.g., smoking more on a cigarette) appears to account for the only modest reductions in biomarker measures despite dramatic reductions in cigarettes smoked per day. By measuring the levels of total NNAL, as a marker of extent of exposure, in light (low rate) smokers versus smokers who were reducing their cigarettes per day, Hatsukami, Le, and associates (2006) found that the mean level of this carcinogen biomarker was two- to threefold higher in reducers than in light smokers, even when the two groups smoked the same number of cigarettes per day. The limitation associated with this study is the short length of time in observing the effects of reduction (e.g., up to 6 months). Perhaps a longer duration is important for establishing a stabilized smoking pattern. However, these findings correspond with several epidemiological studies that showed that reducers (reducing cigarette intake by at least 50%) had less of a decrease in cancer risk than did persistent light smokers (Godtfredsen et al., 2005) and that, although persistent light smokers experienced fewer myocardial infarction and hospitalization for COPD, as previously noted reducers did not (Godtfredsen et al., 2003; Godtfredsen, Vestbo et al., 2002).
Other smoking reduction studies have shown greater reductions in smoking with the use of medicinal nicotine compared with placebo or no-treatment groups, but again no dramatic reductions in exposure biomarkers were observed as a result of using these treatments (see Hughes & Carpenter, 2005, for review). If smokers in these studies were compensating for the reduced nicotine concentrations that accompanied cigarette reductions, the observed compensatory smoking behavior may have resulted from insufficient nicotine replacement. In studies using rats, LeSage and associates (LeSage, Keyler, Collins, & Pentel, 2003; LeSage et al., 2002) found that continuous nicotine infusion, as a model of nicotine replacement therapy (NRT), suppressed nicotine self-administration in a rate-related fashion. At an infusion rate that provided venous serum nicotine concentrations equaling the peak arterial concentrations associated with self-administration (8.0 mg/kg/day), self-administration was reduced by 73%, similar to the level of suppression achieved by saline extinction of self-administration. NRT in smokers typically provides serum nicotine levels less than the mean levels they experience with smoking and substantially below peak arterial levels. Thus reduction of self-administration was greatest when the infusion provided nicotine doses and venous serum concentrations substantially higher than those typically associated with NRT in humans.
Similar observations were made in an inpatient study of high-dose NRT in humans (Benowitz, Zevin, & Jacob, 1998). In a recent outpatient pilot study, increasing doses of the nicotine patch led to dose-related decreases in number of cigarettes smoked; however, the presence of compensatory smoking behavior continued to be significant even at the highest dose (3- to 15-mg nicotine patches) as evidenced by a 166% increase in CO and a 245% increase in total NNAL per cigarette at the 45-mg dose, compared with baseline (Hatsukami, Mooney et al., 2007). Further research is needed to examine whether this persistence of compensation despite increasing NRT dose is a result of still insufficient nicotine replacement or non-nicotine factors (e.g., lack of sensory stimulation, smoking for constituents other than nicotine).
In summary, results to date suggest that reduction in smoking is unlikely to lead to significant reductions in toxicant exposure and that the extent of reduction in cigarettes per day necessary to lead to a 50% reduction in toxicants may be difficult to achieve or sustain. These results also might reflect the limitations of our current intervention methods. Cigarette reduction may be possible if the method of intervention (e.g., more potent agonist, more rapid delivery of agonists, antagonist, or immunotherapy) significantly minimizes compensatory smoking and level of exposure. For example, the use of nicotine immunotherapy may convert a regular smoker to an occasional, weekend smoker without the occurrence of relapse. Although this outcome is not ideal, this conversion might significantly reduce the tobacco-induced risk for disease. However, even with dramatic and sustained reduction in cigarette intake, it remains unclear whether this reduction would lead to clinically significant improvements in health risks or whether duration rather than amount of smoking—which has been found to be a significant predictor of life expectancy (Doll et al., 2004; Flanders et al., 2003)—is of greater significance for disease reduction.
In an another analysis conducted with the Norwegian prospective study, investigators found that even smoking 1–4 cigarettes/day is associated with increased risk of dying from ischemic heart disease, from all causes, and lung cancer in women compared with never-smokers (Bjartveit & Tverdal, 2005). Other studies have observed increased relative risk for fatal and nonfatal myocardial infarction (Kawachi et al., 1994; Rosengren, Wilhelmsen, & Wedel, 1992) and all-cause mortality (Rosengren et al., 1992) in smokers smoking 1–4 cigarettes/day. Therefore, to date, if the goal is to decrease tobacco-related morbidity and mortality significantly, cigarette reduction should not be considered as an end goal but rather as an intermediary step or part of a continuum of treatment, with cessation as the final treatment outcome.
Modified cigarettes
As with reductions in cigarette intake, use of modified tobacco cigarettes cannot be linked easily to a reduction in disease risk or even to significant reductions in carcinogen exposure biomarkers. Cigarettes are modified by various means: with genetic modification of tobacco, changes in tobacco curing and processing, addition of chemicals to the tobacco product, or the use of special filters. Reduced exposure cigarettes are not a novel concept. Low-yield cigarettes, with filters to reduce nicotine and tar, have been on the market for decades. However, as noted earlier, the promise of reducing health risks was unmet when these cigarettes, with varying tar yields as determined by machine smoking and primarily achieved through ventilation holes in filters, did not result in significant reduction in lung cancer rates. Hecht et al. (2005) found no significant differences in total NNAL, 1-HOP, or cotinine concentrations in the urine of smokers who smoked regular, light, or ultra-light types of cigarettes. Similar results were observed in another study: no differences in serum cotinine, urinary free NNAL and NNAL-Glucs, and hemoglobin adducts of 4-aminobiphenyl in smokers of regular or light cigarettes were found (Bernert et al., 2005). These results were consistent with epidemiological data showing no significant reduction in mortality between smokers of regular or light cigarettes (Harris, Thun, Mondul, & Calle, 2004) and are disconcerting given that smokers reported that smoking light and ultra-light cigarettes conferred a 25% and 33% reduction in risks, respectively, compared with regular brand (Shiffman, Pillitteri, Burton, Rohay, & Gitchell, 2001).
Assessments of the recent modified cigarettes have involved either short-term clinical trials that control the parameters of tobacco use or longer clinical trials. These studies are limited by small sample sizes; concern over whether the sample reflects smokers who are likely to use the PREP under study; short trial duration (no greater than 1.5 months), which may not allow for stabilization of product use and does not account for the long half-life of some biomarkers; limited biomarker assessments; and concern regarding compliance with product use, particularly in the nonlaboratory studies. Descriptions of the results from these trials can be found elsewhere (e.g., Hatsukami & Hecht, 2005). Here we provide some data on the longer clinical trials, which allows better assessment of exposure reduction.
Omni cigarettes (manufactured by Vector but no longer on the market) were advertised as a cigarette with reduced carcinogens, primarily nitrosamines, catechols, and PAHs. According to the manufacturer’s web site, the Omni cigarette was associated with a 53%–66% decrease in NNK and a 20%–29% decrease in pyrene, according to machine-determined yields using methods developed by the U.S. FTC and Massachusetts Department of Public Health. Hatsukami et al. (2004) measured carcinogen uptake in smokers who were randomized to use either the Omni cigarette (N=22) or medicinal nicotine (N=16) and assessed exposure biomarkers for a period of 4 weeks. Reductions in total NNAL were modest at best with the Omni cigarette (21%) compared with the usual brand. No significant reductions in 1-HOP were observed for Omni cigarettes (5%) or alveolar CO (<1%).
These results are in contrast to the significant reduction in these biomarkers (65%, 52%, and 92%, respectively) observed when smokers were switched from their usual brand of cigarettes to the nicotine patch, and significant differences in levels of these biomarkers were observed between Omni and nicotine patch. The results from the Omni cigarettes were similar to those observed in a study that assessed exposure during 6 weeks of Omni and of usual-brand cigarettes (N=34; Hughes, Hecht, Carmella, Murphy, & Callas, 2004). A nonsignificant reduction in total NNAL (17%) and 1-HOP (10%) and a significantly lower cotinine level (18%) were observed with Omni compared with ad libitum use of usualbrand cigarettes. However, CO increased significantly, by 21%. These results not only show limited reduction in exposure to carcinogens but also reiterate that machine-determined yields of cigarettes can be misleading to the consumer. The studies also point to the need for a broad range of biomarker assessments, because reductions can be observed for some biomarkers but not others.
Advance (manufactured by Star Scientific Tobacco and by Brown and Williamson) is a modified cigarette product that uses a curing process that reduces the levels of tobacco-specific nitrosamines. At one time, it was advertised to have “great taste, less toxins.” Only two trials spanning several days have been conducted with Advance. In one study, when use of Advance over 5 days was compared with usual-brand cigarettes (N=12), a significant reduction was observed for urinary total NNAL (51%) with no significant reductions in cotinine and a slight but significant reduction in CO levels (Breland, Acosta, & Eissenberg, 2003). In a more recent within-subject, 5-day study with Advance (N=35), a significant reduction was observed in the Advance condition for urinary total NNAL (37%) compared with own-brand cigarettes, but no differences were observed in 1-HOP, cotinine, or alveolar CO levels at day five (Breland, Kleykamp, & Eissenberg, 2006).
In summary, the results from these studies show modest reductions in exposure to one of the tobacco-specific nitrosamines. Modification of tobacco in cigarettes as a means to reduce toxicant exposure is unlikely to result in significant reductions in disease risk in general because of the number of toxicants associated with the burning of tobacco and the limited reduction in exposure to these toxicants.
Cigarette-like delivery devices
Cigarette-like delivery devices that heat rather than burn cigarettes are being sold in the United States and other countries. Two such devices that have been tested in humans with the results published are Eclipse (manufactured by R. J. Reynolds) and Accord (manufactured by Philip Morris). Although conceptually a significant reduction in combustion products can occur when a product is not burned, the consumer acceptability of these products has been problematic (e.g., Hughes et al., 2005). Furthermore, to date, few long-term studies with large sample sizes representative of the population likely to use these products have been conducted. For Eclipse, the existing studies have shown reductions, no change, or increases in toxicant exposure (see Hatsukami & Hecht, 2005). For example, compared with usual brands of cigarettes, Eclipse has shown a significant reduction in respiratory tract inflammation over 2 months of use in heavy smokers (35% improvement in alveolar inflammatory cells and 46% improvement in bronchitis index; Rennard et al., 2002), a significant reduction (70%–79%) in urine mutagenicity after 1 week of use (Bowman et al., 2002; Smith et al., 1996), improvement in high-density lipoprotein cholesterol and reduction in circulating lymphocyte activation after 2 or 4 weeks of use (Frampton et al., 2000); and modest increase in lung permeability half-life (indicative of improvement in pulmonary epithelial injury) after 2 and 4 weeks of use in some smokers (Stewart et al., 2006). Studies also have shown no changes in other biomarkers such as peripheral blood measures (e.g., fibrinogen, hemoglobin, platelets), lung function, pulmonary epithelial permeability (unlike the Stewart et al. [2006] findings), vital signs (Frampton et al., 2000; Rennard et al., 2002) or total NNAL and 1-HOP concentrations (Breland et al., 2006). Increases have been observed for CO exposure when using Eclipse (Breland et al., 2006; Frampton et al., 2000; Rennard et al., 2002; Stewart et al., 2006) and for some of the biomarkers for inflammation and oxidative stress (Stewart et al., 2006). Eclipse also has been examined when used in conjunction with cigarettes (Fagerström, Hughes, & Callas, 2002; Fagerström, Hughes, Rasmussen, & Callas, 2000), which may be more reflective of the naturalistic use of this product. The results from these two studies, which involved use of Eclipse for 2 or 8 weeks, showed a significant reduction in cigarette smoking but an increase in alveolar CO level.
Although the Accord cigarette has been examined in smoking sessions in the laboratory (Breland, Buchhalter, Evans, & Eissenberg, 2002; Buchhalter & Eissenberg, 2000; Buchhalter, Schrinel, & Eissenberg, 2001), only two studies have examined the toxicity profiles of first (Roethig, Kinser, Lau, Walk, & Wang, 2005) and second (Roethig et al., 2007) generations of an electrically heated cigarette smoking system (EHCSS, or Accord). These studies were conducted over the course of several days in a controlled and confined clinical setting. In Roethig et al. (2007), 100 smokers of light cigarettes (7- to 12-mg tar) were assigned to the EHCSS under a controlled (restricted number of Accords used per day) or an uncontrolled smoking condition, to an 11-mg tar cigarette, to a 1-mg tar ultra-light cigarette, or to no smoking for a period of 8 days. Results showed significant reductions in biomarkers for both controlled and uncontrolled EHCSS conditions compared with usual-brand cigarettes; values ranged from 48% to 60% for decreases in nicotine, plasma cotinine, total NNAL, and 3-hydroxypropylmercapturic acid; 66%–72% for decreases in 1-HOP and urine mutagenicity; and 85%–87% for decreases in carboxyhemoglobin and S-phenylmercapturic acid. The values of these biomarkers were two- to seven-fold less than the levels observed with the ultra-light cigarette. Even greater reductions were observed when these values were adjusted for residual effects from smoking the usual brand to account for the long elimination half-lives of some of these biomarkers.
In another study, 11 smokers were allowed to use Accord alone, as a substitute for cigarettes, or in addition to their own brand of cigarettes for 2 weeks. They also were assigned to a nicotine gum condition with the same instructions. Effects of increasing doses of Accord (5, 10, and 15 per day) on number of usual-brand cigarettes smoked, CO, and cotinine were measured and compared with usual-brand cigarettes and nicotine gum. Dose-related decreases in cigarette smoking and CO were observed, with mean reductions of 32% and 27%, respectively, in the 15 Accords/day condition. No changes were observed for cotinine levels. Although number of cigarettes smoked was lower in the 15 Accords/day condition than in the nicotine gum condition, no differences were observed for CO or cotinine concentrations.
In summary, the cigarette-like delivery devices appear to have more promise than the combustible products in significantly reducing some toxicants. However, the Eclipse cigarette led to significant increases or no change in some of the measured biomarkers. Accord appeared to significantly reduce biomarkers for nicotine, CO, and some carcinogens, but other biomarkers associated with inflammation, oxidative stress, and pulmonary function were not measured, and the studies were conducted in a restricted environment. Studies with more comprehensive panels of biomarkers, larger sample sizes, and long-term use in the natural environment need to be conducted, using designs that allow or disallow smoking of subjects’ usual brand of cigarettes.
Noncombusted oral products
Smokeless tobacco companies and cigarette manufacturing companies in the United States have been marketing reduced toxicant (lower nitrosamines), spitless oral tobacco products as a substitute for cigarettes. These newer products typically contain lower levels of tobacco-specific nitrosamines (TSNAs) than some of the conventional and most popular brands of smokeless tobacco sold in the United States or other parts of the world, such as India, and levels comparable with or lower than those from the smokeless tobacco products sold in Sweden. For example, Stepanov, Jensen, Hatsukami, and Hecht (2006) collected samples from 19 different brands of modified tobacco products, including five new oral or reduced exposure tobacco products: Ariva and Stonewall (compressed tobacco lozenge), Exalt and General (Swedish snus), and Revel (a spit-free American smokeless tobacco product). They also examined Smokey Mountain, a tobacco-free and nicotine-free herbal snuff, as well as medicinal nicotine products. With the exception of Smokey Mountain and medicinal nicotine, all of these newer products contained detectable levels of TSNAs. Two of these products—Ariva and Stonewall—had levels that were markedly less than those in conventional, popular smokeless tobacco products. Some of these reduced exposure oral tobacco products also had lower levels of TSNAs than were found in the tobacco of popular brands of cigarettes (Marlboro, Camel). Among the newer or lower nitrosamine products, the highest total TSNA levels were found in Exalt, and the lowest were identified in Ariva and Stonewall. In another study, Stepanov et al. (2006) found considerable levels of these carcinogens in some of the smokeless products from India.
These data suggest that if current users of conventional, popular high-nitrosamine smokeless tobacco products switched to the lower nitrosamine noncombusted oral tobacco products, health risks might be reduced. In a study conducted with smokeless tobacco users (Hatsukami et al., 2004), participants were asked to switch from their usual U.S. brand of smokeless tobacco to Swedish snus (General) or medicinal nicotine. Switching to Swedish snus resulted in a significant decrease in levels of urinary total NNAL (about 50%), although participants using medicinal nicotine had the greatest reductions (90%). Smokeless tobacco products with lower nitrosamine levels have been sold in the United States as Skoal Bandits for many years; however, this product is not as popular as brands that have higher nicotine content and has been considered as a starter product (Tomar, Giovino, & Eriksen, 1995). In another study conducted by Hatsukami, Ebbert et al. (2007), when smokeless tobacco users who used Copenhagen or Kodiak Wintergreen, which have NNK values of 0.76 and 0.41 μg/g product wet weight, respectively, were switched to Skoal Bandits (with an NNK value of 0.17 μg/g product wet weight), urinary total NNAL decreased by about 50%, a reduction similar to that achieved with the switch to Swedish snus.
Whether reductions in cancer risk would occur with a 50% reduction in NNK uptake is unclear. Swedish studies suggest that the risk of head and neck cancer is not significantly elevated in snus users, compared with those who do not use smokeless tobacco (Lewin, Norell, Johansson, Gustavsson, & Wennerberg, 1998; Schildt, Eriksson, Hardell, & Magnusson, 1998), but other risk factors such as metabolic syndrome (Norberg, Stenlund, Lindahl, Boman, & Weinehall, 2006) or pancreatic cancer (Boffetta, Aagnes, Weiderpass, & Andersen, 2005) remain a concern. One advantage of Skoal Bandits over the Swedish products is the lower nicotine levels. Notably, about 28% of the smokeless tobacco users assigned to ad lib use of Skoal Bandits were able to achieve 7-day point-prevalence abstinence.
Low-nitrosamine smokeless tobacco products are considered potential harm reduction products for smokers because they are believed to have less than 10% the health risk of cigarettes (Levy et al., 2004). Theoretically, if smokers switched completely to smokeless tobacco products, they should experience a reduction in tobacco-caused mortality and morbidity. The Swedish experience has been used to illustrate the harm reduction potential when smokers switch to Swedish snus. These studies have found that rates of lung cancer in men were significantly lower than the rates found in men in a neighboring country, Norway. This dramatic reduction in lung cancer was attributed to increased consumption of snus, which was associated with and may have led to reduced smoking rates (Foulds, Ramstrom, Burke, & Fagerström, 2003). Other researchers have pointed to tobacco control policies that may have led to reduction in lung cancer in Swedish men and have resulted in significantly lower smoking prevalence rates in other areas of the world, such as California (e.g., Tomar, 2006). Tomar (2006) also noted that some of the states in the United States have high rates of smokeless tobacco use without a corresponding low rate of cigarette smoking.
To date, few studies have examined the extent of reduction in exposure biomarkers when smokers were asked to switch from cigarettes to oral tobacco products, or if U.S. smokers would even find these products palatable. In a study conducted by Mendoza-Baumgart et al. (in press), cigarette smokers participated in a within-subjects, crossover design in which subjects were administered in random order 2 weeks of Exalt (pouched, spit-free, lower nitrosamine oral tobacco) or 2 weeks of medicinal nicotine. In another study, they were administered Ariva (low-nitrosamine tobacco lozenge) or medicinal nicotine using the same experimental design. In the first study, Exalt and medicinal nicotine led to significant reductions in urinary total NNAL compared with cigarettes; however, medicinal nicotine led to greater reductions of this biomarker. In the second study, Ariva and medicinal nicotine also led to greater reductions in urinary total NNAL compared with cigarettes, and no significant differences in reductions were seen between Ariva and medicinal nicotine. These studies show that tobacco carcinogen exposure can be reduced when smokers switch to smokeless tobacco products. However, this preliminary study is limited because smokers underwent only a short duration of product exposure and because biomarker assessment focused primarily on NNK and none of the other potential toxic constituents in smokeless tobacco.
To summarize, TSNA levels are lower in the newer noncombustible oral tobacco products; however, most of these products still have unacceptable levels of these toxicants. Only Ariva and Stonewall can be considered to have low TSNA levels. Nonetheless, data show that the use of some of the newer noncombustible oral products can lead to significant reductions in carcinogen exposure, compared with cigarettes and with some of the current conventional popular smokeless products sold in the United States. Whether this switch confers significant health benefits is unclear. One epidemiological study, using American Cancer Society Cancer Prevention Study II data, compared, after 20 years of follow-up, mortality among smokers who switched to smokeless tobacco with that of smokers who quit completely (Henley et al., 2007). Compared with complete quitters, switchers had significantly higher death rates from lung cancer (HR=1.46, 95% CI 1.24–1.73) and slightly higher death rates from coronary heart disease (HR=1.13, 95% CI 1.00–1.2), stroke (HR=1.24, 95% CI 1.01–1.53), and any cause (HR=1.08, 95% CI 1.01–1.15). One caveat in this study is that the smokeless tobacco products used by these former cigarette smokers are likely to be higher in nitrosamine levels than the current lower nitrosamine products. In addition, the authors point to a number of smoking history and lifestyle factors that may have contributed to the higher mortality risk for switchers, even though they statistically controlled for differences in these variables. Finally, because natural patterns of use were not assessed, it is unclear whether use of these products as substitutes for cigarettes or usual brand of smokeless tobacco would lead to increased risk for disease, particularly if dual use of products occurs.
Product summary
Current interventions that purportedly reduce exposure to toxicants appear to vary in their ability to do so (Figure2; Hatsukami & Hecht, 2005): Modified cigarettes demonstrate the least likely potential to reduce toxicants, and the noncombusted oral nicotine products have the most potential. The data generated by our TTURC and by others indicate that modified combustible products in any form are unlikely to reduce toxicant exposure to levels that might result in reduced health risk because of the numerous combustion products associated with smoking. One exception might be reducing the nicotine levels of products to a point that would render them nonaddicting (Benowitz & Henningfield, 1994; Gray et al., 2005); this would facilitate abstinence or minimize initiation of tobacco use. Another exception would be to develop a medication that would be able to significantly reduce compensatory smoking among those interested in reducing tobacco intake.
Figure 2.
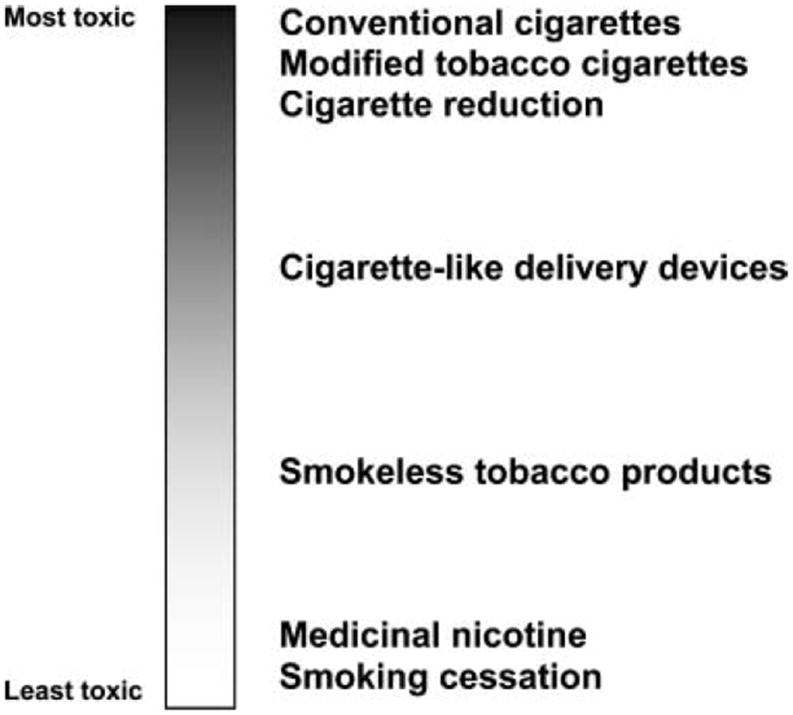
Spectrum of harm across tobacco products.
The level of toxicant exposure among the current cigarette-like devices and potential for reduction in harm are uncertain because of the limited number of studies conducted and the lack of appeal of these products; but these products are likely to lead to greater reduction in toxicant products than combustible products. The noncombusted oral, reduced exposure products have the greatest potential for reduced harm among all the tobacco products because they not only eliminate the combustion products but also can be manufactured to be low in toxicant levels. However, an urgent need exists for more extensive study of consumer use of these products, consumer perception of these products that would affect tobacco use behavior, and the population level impact of introducing these products as substitutes for smoking (both situational and permanent) or as a reduced exposure product for those smokeless tobacco users who are using products with higher toxicity. In addition, questions regarding whether these noncombusted oral products should be low in toxicants with moderate levels of nicotine, or low in toxicants with low levels of nicotine, have not been addressed. If the level of nicotine is too low, these products may not provide a suitable substitute for cigarettes, but if the level is too high, addiction would be easily acquired or sustained. The safest potential harm reduction method is providing only nicotine, but switching a tobacco user to these products might necessitate a high and rapid nicotine delivery and improved palatability.
Individual differences or moderating factors
Both individual and environmental factors can influence the population that is interested in using reduced exposure interventions and their responses to these interventions. On an individual level, careful consideration needs to be given to how gender, age, racial/ethnic groups, socioeconomic status, existing comorbidities that include physical and mental disease, degree of dependence, and motivation to quit might influence tobacco use behavior and proximal and ultimate outcomes (Hatsukami, Giovino et al., 2005). Genotypes similar to those identified by the University of Pennsylvania TTURC (Phillips et al., 2007) and endophenotypes (e.g., rate of nicotine metabolism, activation and detoxification of carcinogens) may also influence responses or toxicant exposure levels in tobacco users using the various exposure reduction methods.
Individual differences in response to both nicotine and carcinogens, which may influence the extent to which potential reduced exposure products may lead to reduced harm, have been the focus of a few studies. Given that compensation is a major barrier to achieving meaningful levels of smoking reduction, animal models of smoking reduction to examine factors that predict compensation are being developed. Such models may be useful to examine potential mechanisms underlying compensation in the context of smoking reduction. In these models, compensatory nicotine self-administration is induced by either (a) gradually decreasing the duration of access to nicotine from 23 hr/day to 2 hr/day or (b) decreasing the unit nicotine dose that is self-administered from 0.06 to 0.03 mg/kg/infusion.
To quantify the degree of compensation (i.e., the degree to which nicotine self-administration increases and intake is maintained), two forms of a compensation index (CI) are calculated: one as the hourly infusion rate during reduced access divided by the baseline infusion rate during the same 2-hr period of the day (hourly rate CI), and the other (daily intake CI) as 1 –(% change in intake divided by % change in access). The hourly rate CI controls for the variability in infusion rate that occurs during a 23-hr session, whereas the daily intake CI is a more conventional index that quantifies changes in overall nicotine exposure.
In a recent study using the access-reduction model (LeSage, Burroughs, & Pentel, 2006), correlations between each CI and baseline self-administration variables (infusion rate during the same 2 hr of the day during which reduced access occurred, proportion of infusions earned during the light phase) and nicotine pharmacokinetic variables (elimination half-life, clearance) were examined to determine if any would predict the degree of compensation during reduction. Results showed significant compensatory increases in nicotine self-administration when access was reduced (C=2.3, where values>1 indicate compensation), with considerable variability between rats (range=0.61–4.6), which is similar to humans undergoing reduction protocols (Hatsukami, Mooney et al., 2007). Although none of the baseline self-administration or pharmacokinetic variables predicted compensation in daily nicotine intake, compensation as indexed by the hourly rate CI was significantly negatively correlated with baseline infusion rate (r=.42, p<.05), indicating that rats were less likely to compensate if they already exhibited relatively high infusion rates during the same 2 hr of the day in which reduced access occurred. In addition, compensation for the hourly rate CI was to some extent correlated with nicotine clearance (r=.40, p=.05), suggesting that nicotine pharmacokinetics may play some role in individual differences in compensation during smoking reduction in humans. That is, smokers with slower nicotine clearance may exhibit less compensation.
An additional notable finding in this study was the observed transient increase in self-administration for 5 days after unlimited access was restored. The maximal level of this increase was correlated with the degree of compensation. This finding suggests that future studies in humans should consider the possibility that relapse from smoking reduction interventions could be associated with increases in smoking compared with prereduction levels, and that this effect could be related to the degree to which smokers compensate during smoking reduction.
Parallel human studies are being conducted to examine how nicotine clearance is related to nicotine regulation. P450 2A6 is the major catalyst of nicotine metabolism, and genetic variations in this enzyme clearly influence nicotine metabolism and hence its bioavailability (Hukkanen, Jacob, & Benowitz, 2005; Malaiyandi, Sellers, & Tyndale, 2005; Tyndale & Sellers, 2002). Yet CYP2A6 genotype alone is unlikely to explain the large variation in the extent and rate of nicotine metabolism among smokers. Therefore, more complete exploration of individual differences in nicotine metabolism requires a complete characterization of the important metabolic pathways and modulators that contribute to these differences. For example, Murphy and associates have been exploring the effect of nicotine on its own metabolism and the role of glucuronidation in nicotine metabolism. They have demonstrated that, in vitro, the metabolism of nicotine by CYP2A6 results in the time and concentration-dependent inactivation of these enzymes (von Weymarn, Brown, & Murphy, 2006). It is unclear how nicotine-mediated inactivation may contribute to nicotine bioavailability in smokers. However, it has been reported that the rate of nicotine clearance in smokers is longer than that in nonsmokers or in smokers who have abstained from smoking (Benowitz & Jacob, 1993, 2000).
In addition to CYP2A6-catalyzed 5′-oxidation, nicotine is metabolized by UGT-catalyzed N-glucuronidation. In smokers, nicotine glucuronidation typically accounts for less than 10% of total nicotine metabolism. However, the extent of nicotine glucuronidation varies significantly among individual smokers, and nicotine glucuronidation levels have been reported to be lower in Black smokers than in White smokers (Benowitz et al., 1999). We recently confirmed in a study of nicotine patch users that Blacks excreted less nicotine as its N-glucuronide conjugate than did Whites (unpublished manuscript). The lower level of nicotine glucuronidation in Blacks may contribute to the higher cotinine levels and lower cigarette consumption observed in this ethnic group.
Researchers are also exploring individual differences and associated genotypes in the activation and detoxification of carcinogens (Hecht et al., 2006). For example, to date we have observed that activation:detoxification ratios of the representative PAH phenanthrene correlate with polymorphisms in the CYP1A1 and CYP1B1 genes, although in different directions. We also have observed that high ratios—presumably indicating higher cancer risk—cannot be predicted by a combination of 11 different polymorphisms in PAH-metabolizing genes (Hecht et al., 2006), demonstrating some of the limitations of genotyping individuals to determine their response to carcinogens because of differences in metabolism.
Greater research attention toward individual differences in responses to products will provide greater insight into why individuals have a better response to some intervention methods (i.e., cigarette reduction) and are more or less vulnerable to the health effects of different types of PREPs.
Ultimate outcome
The ultimate outcome is reduction in morbidity and mortality. To date, the best method to achieve this outcome is abstinence from all tobacco products. Studies have shown that cigarette reduction attained through the use of nicotine replacement agents and bupropion SR results in 5%–36% (M=11%) point-prevalence abstinence rates among smokers who, at the time of enrollment, had no immediate quit plans (Hughes & Carpenter, 2005). A few studies have directly compared smoking reduction with advice to quit or no treatment. Joseph et al. (in press) randomized smokers who were diagnosed with cardiovascular disease and not ready to quit to usual-care cessation or to cigarette reduction arms. No differences were observed in cessation rates between the two conditions. These results were similar to those from a study conducted by Carpenter, Hughes, Solomon, and Callas (2004), in which the 7-day abstinence rate was 18% in smokers who received counseling for reduction with eventual cessation advice compared with 23% in those who received motivational counseling with cessation advice. The rates in both groups, however, were higher than the 4% in the no-treatment group. Similar observations were made in Tonnesen et al. (2005), in which abstinence rates were 15%, 15%, and 5%, respectively. These findings suggest that cigarette reduction in those initially unwilling to quit promotes cessation but produces no differences in rates compared with smokers counseled to quit. In a more extensive review of the literature, Hughes & Carpenter (2006) concluded that smoking reduction, in fact, increases the probability of smoking cessation.
Quest cigarettes, marketed by Vector, also have been tested as a method to quit smoking. These cigarettes are produced in three nicotine yields, “low,” “extra low,” and “nicotine free,” and are marketed as a step-down approach to becoming nicotine free. The Vector Tobacco web site (www.vectortobacco.com) recently indicated that “Nicotine-free cigarettes show promise in a new quit-smoking study; one out of three smokers quit by using Quest 3 (nicotine-free cigarettes).” This study is a small clinical trial, and the 33% abstinence rate was observed in the 15 smokers assigned the condition that used Quest 3 to gradually substitute for usual-brand cigarettes. To date, little is known about the toxicity of these products and how they may be used in a general population of smokers. Trials are continuing to determine the effects of these and other products on exposure biomarkers and cessation.
Few well-controlled studies have examined the extent to which the use of alternative tobacco products would lead to smoking cessation. Researchers have claimed that smokeless tobacco can be used clinically to facilitate abstinence in smoking (Rodu & Cole, 1999). Only one small, open-label clinical trial without a control group reported that smokers who were treated with smokeless tobacco experienced a 25% quit rate at 1 year. However, of the 16 abstainers, 13 were continuing to use smokeless tobacco at 1 year. Analysis of epidemiological studies, such as the ones conducted in Sweden, support the association of snus use with smoking cessation (Furberg et al., 2005; Ramstrom & Foulds, 2006) but also call for the need for a clinical trial (Furberg et al., 2005).
Conclusions
Addressing the controversial topic of tobacco harm reduction requires the efforts and coordinated interactions of multiple disciplines. Because of constantly evolving products developed by the tobacco companies and the consumer demand for ways that would allow them to sustain use of tobacco products with reduced risk, the scientific community needs to come together to rapidly generate the basic science needed to ensure the public health is protected and to avert any potential policy with negative consequences. Furthermore, strategies need to be developed so that the tobacco companies will be compelled to sell only products with the lowest risk to the millions of tobacco users who choose to continue to use tobacco products. The urgency of this research is evident because of the product evaluation articles under the Framework Convention for Tobacco Control and possible U.S. Food and Drug Administration regulation of tobacco products.
To achieve these goals, methods and measures for evaluating these products and interventions need to be developed and to cover preclinical testing of the toxicity of the products, in vitro tests and in vivo animal toxicology testing, human clinical trials, consumer perception, and postmarket surveillance (Hatsukami, Giovino et al., 2005; Stratton et al., 2001). To date, our research focus has been to (a) quantitate and test the reliability and validity, including predictive validity, of biomarkers, particularly for carcinogen exposure and toxicity; (b) develop animal and human behavioral models for examining exposure reduction methods and products; (c) examine various intervention mechanisms that would lead to the greatest reduction in exposure (high-nicotine, low-toxicants; or low-nicotine, low toxicants); and (d) examine individual differences that will determine how individuals might respond to the product, such as differences in nicotine metabolism, and activation and detoxification of carcinogens. This research covers only a few aspects of research on harm reduction. To address this topic more fully, other communities of scientists with a diverse range of expertise need to collaborate or coordinate their research efforts to provide a comprehensive science base for harm reduction.
Beyond individual responses to PREPs are environmental influences such as tobacco industry behavior and how the industry manufactures, promotes, advertises, and markets these products and even the reaction of the media and public figures to these products. The regulations that must be exerted on tobacco industry behavior to protect public health need to be studied and carefully considered. Measures must be developed to avoid (a) misleading the consumer (e.g., labeling cigarettes as “light” or “ultra-light,” which are terms that have been banned in the European Union, or “reduced carcinogens”), (b) facilitating the initiation of smoking among children and adolescents (e.g., the addition of flavorants to PREPs), (c) maintaining tobacco use in individuals who would otherwise quit (marketing smokeless tobacco as situational cigarette substitutes), and (d) continuing to manufacture extremely toxic products even though the technology to manufacture less toxic products is available.
Public policies also are important in determining the uptake of these products and the ultimate outcome. For example, policies such as differential taxation of products according to their toxicity have been discussed and need to be explored. Furthermore, the implementation of advertising and promotion restrictions or bans would have a significant impact on the uptake of PREPs and other tobacco products.
Finally, tobacco control researchers and advocates need to discuss and come to an agreement about the primary goals for harm reduction, the most effective methods to achieve these goals, and strategies to implement these effective methods (Martin, Warner, & Lantz, 2004). To understand the best mechanistic approach and eventually to promote it, the public health community needs to come to grips with whether or not it feels comfortable with a persistent addiction to nicotine, if it is provided in a safer form. To date, focus groups conducted with community tobacco control leaders indicated that they were skeptical of any harm reduction approach that involved modified tobacco because of the history and some of the current behaviors of the tobacco companies (Joseph, Hennrikus, Thoele, Krueger, & Hatsukami, 2004). They also indicated that long-term medicinal nicotine use may be helpful but only as a means to cessation. Yet they also admitted to a clear need to find ways to reduce harm among current smokers. The harm reduction dialogue is ongoing and a network system is being established that would accelerate the science and generate the data needed to make informed policy decisions. We live in an unprecedented time when a community of scientists is actively encouraged and engaged in interdisciplinary science and the technological tools are available to facilitate the collaborative process.
Acknowledgments
The TTURC studies in this paper were funded by NIH grant DA013333. The authors thank Irina Stepanov, Deborah Hennrikus, Harry Lando, Sharon Allen, Steven Fu, Marc Mooney, Jesse Mason, Ozlem Tulunay, Irene Mendoza Baumgart, Emily Stark, Kathy Longley, Steve Carmella, Yan Zhang, Robin Bliss, Ying Zhang, Tim Church, Kristin Anderson, and the many other faculty, students, and advisors who have contributed to our TTURC.
References
- Baker TB, Hatsukami DK, Lerman C, O’Malley SS, Shields AE, Fiore MC. Transdisciplinary science applied to the evaluation of treatments for tobacco use. Nicotine & Tobacco Research. 2003;5(Suppl. 1):S89–S99. doi: 10.1080/14622200310001625564. [DOI] [PubMed] [Google Scholar]
- Benhamou E, Benhamou S, Auquier A, Flamant R. Changes in patterns of cigarette smoking and lung cancer risk: Results of a case-control study. British Journal of Cancer. 1989;60(4):601–604. doi: 10.1038/bjc.1989.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N, Henningfield JE. Establishing a nicotine threshold for addiction. The New England Journal of Medicine. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clinical Pharmacology and Therapeutics. 1993;53(3):316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P., 3rd Effects of cigarette smoking and carbon monoxide on nicotine and cotinine metabolism. Clinical Pharmacology and Therapeutics. 2000;67(6):653–659. doi: 10.1067/mcp.2000.107086. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. The Journal of Pharmacology and Experimental Therapeutics. 1999;291(3):1196–1203. [PubMed] [Google Scholar]
- Benowitz N, Zevin S, Jacob P., III Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. The Journal of Pharmacology and Experimental Therapeutics. 1998;287(3):958–962. [PubMed] [Google Scholar]
- Bernert JT, Jain RB, Pirkle JL, Wang L, Miller BB, Sampson EJ. Urinary tobacco-specific nitrosamines and 4-aminobiphenyl hemoglobin adducts measured in smokers of either regular or light cigarettes. Nicotine & Tobacco Research. 2005;7(5):729–738. doi: 10.1080/14622200500259762. [DOI] [PubMed] [Google Scholar]
- Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tobacco Control. 2005;14(5):315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Aagnes B, Weiderpass E, Andersen A. Smokeless tobacco use and risk of cancer of the pancreas and other organs. International Journal of Cancer. 2005;114(6):992–995. doi: 10.1002/ijc.20811. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, Perruchoud AP, Sawe U. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research. 2002;4(4):433–439. doi: 10.1080/1462220021000018380. [DOI] [PubMed] [Google Scholar]
- Bowman DL, Smith CJ, Bombick BR, Avalos JT, Davis RA, Morgan WT, Doolittle DJ. Relationship between FTC ‘tar’ and urine mutagenicity in smokers of tobacco-burning or Eclipse cigarettes. Mutation Research. 2002;521(1–2):137–149. doi: 10.1016/s1383-5718(02)00219-x. [DOI] [PubMed] [Google Scholar]
- Breland AB, Acosta MC, Eissenberg T. Tobacco specific nitrosamines and potential reduced exposure products for smokers: A preliminary evaluation of Advance. Tobacco Control. 2003;12(3):317–321. doi: 10.1136/tc.12.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg Evaluating acute effects of potential reduced-exposure products for smokers: Clinical laboratory methodology. Nicotine & Tobacco Research. 2002;4(Suppl. 2):S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: Effects on subjective and physiological measures and on smoking behavior. Nicotine & Tobacco Research. 2000;2(1):39–43. doi: 10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: Comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine & Tobacco Research. 2001;3(2):111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Burns D. Estimating the benefits of a risk reduction strategy; Poster presented at the 3rd annual Society for Research on Nicotine and Tobacco conference; Nashville, TN. 1997. [Google Scholar]
- Burns D. Epidemiology of smoking-induced cardiovascular disease. Progress in Cardiovascular Disease. 2003;46(1):11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Le Ka KA, Upadhyaya P, Hecht SS. Analysis of N- and O-glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chemical Research in Toxicology. 2002;15(4):545–550. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72(3):371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2004. Morbidity and Mortality Weekly Report. 2005;54(44):1121–1124. [PubMed] [Google Scholar]
- Chen L, Wang M, Villalta PW, Luo X, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chemical Research in Toxicology. 2007;20(1):108–113. doi: 10.1021/tx060232x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R. Cigarette smoking and bronchial carcinoma: Dose and time relationships among regular smokers and lifelong non-smokers. Journal of Epidemiology and Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. British Medical Journal. 2004;328(7455):1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson B, Hjalmarson A, Kruse E, Landfeldt B, Westin A. Effect of smoking reduction and cessation on cardiovascular risk factors. Nicotine & Tobacco Research. 2001;3(3):249–255. doi: 10.1080/14622200110050510. [DOI] [PubMed] [Google Scholar]
- Etter J, Paernegger T, Ronchi A. Distributions of smokers by stage: International comparison and association with smoking prevalence. Preventive Medicine. 1997;26:580–585. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Hughes JR, Callas PW. Long-term effects of the Eclipse cigarette substitute and the nicotine inhaler in smokers not interested in quitting. Nicotine & Tobacco Research. 2002;4(Suppl. 2):S141–S145. doi: 10.1080/1462220021000032771. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Hughes JR, Rasmussen T, Callas PW. Randomised trial investigating effect of a novel nicotine delivery device (Eclipse) and a nicotine oral inhaler on smoking behaviour, nicotine and carbon monoxide exposure, and motivation to quit. Tobacco Control. 2000;9(3):327–333. doi: 10.1136/tc.9.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: Results from Cancer Prevention Study II. Cancer Research. 2003;63(19):6556–6562. [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerström K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12(4):349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Hyde RW, Torres A, et al. Lung injury and inflammation in smokers: Effects of switching to a “new” cigarette; Presented at World Congress on Lung Health and 10th ERS Annual Congress; Florence, Italy. 2000. [Google Scholar]
- Furberg H, Bulik CM, Lerman C, Lichtenstein P, Pedersen NL, Sullivan PF. Is Swedish snus associated with smoking initiation or smoking cessation? Tobacco Control. 2005;14(6):422–424. doi: 10.1136/tc.2005.012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godtfredsen NS, Holst C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: A 16-year follow-up of 19,732 men and women from the Copenhagen Centre for Prospective Population Studies. American Journal of Epidemiology. 2002;156(11):994–1001. doi: 10.1093/aje/kwf150. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Osler M, Vestbo J, Andersen I, Prescott E. Smoking reduction, smoking cessation, and incidence of fatal and non-fatal myocardial infarction in Denmark 1976-1998: A pooled cohort study. Journal of Epidemiology and Community Health. 2003;57(6):412–416. doi: 10.1136/jech.57.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. The Journal of the American Medical Association. 2005;294(12):1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
- Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: A Danish population study. Thorax. 2002;57(11):967–972. doi: 10.1136/thorax.57.11.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Henningfield JE, Benowitz NL, Connolly GN, Dresler C, Fagerström K, Jarvis MJ, Boyle P. Toward a comprehensive long term nicotine policy. Tobacco Control. 2005;14(3):161–165. doi: 10.1136/tc.2004.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Thun MJ, Mondul AM, Calle EE. Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II prospective cohort, 1982-8. British Medical Journal. 2004;328(7431):72. doi: 10.1136/bmj.37936.585382.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine & Tobacco Research. 2006;8(4):599–622. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Ebbert JO, Anderson A, Lin H, Le C, Hecht SS. Smokeless tobacco brand switching: A means to reduce toxicant exposure? Drug and Alcohol Dependence. 2007;87(2–3):217–224. doi: 10.1016/j.drugalcdep.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine & Tobacco Research. 2005;7(6):827–844. doi: 10.1080/14622200500266015. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Hecht SS. Hope or hazard: What research tells us about “potentially reduced-exposure” tobacco products. Minneapolis: University of Minnesota Transdisciplinary Tobacco Use Research Center; 2005. Available from www.tturc.umn.edu/documents/hope_or_hazard-3.pdf. [Google Scholar]
- Hatsukami D, Kotlyar M, Allen S, Jensen J, Li S, Le C, Murphy S. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128(4):2528–2537. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Le CT, Zhang Y, Joseph AM, Mooney ME, Carmella SG, Hecht SS. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiology, Biomarkers, and Prevention. 2006;15(12):2355–2358. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. Journal of the National Cancer Institute. 2004;96(11):844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacology, Biochemistry, and Behavior. 2007;86(1):132–139. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustein KO, Krause J, Haustein H, Rasmussen T, Cort N. Changes in hemorheological and biochemical parameters following short-term and long-term smoking cessation induced by nicotine replacement therapy (NRT) International Journal of Clinical Pharmacology and Therapeutics. 2004;42(2):83–92. doi: 10.5414/cpp42083. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Le KA, Murphy SE, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on levels of 1-hydroxypyrene in urine. Cancer Epidemiology, Biomarkers, and Prevention. 2004;13(5):834–842. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Yoder A, Chen M, Li ZZ, Le C, Dayton R, Jensen J, Hatsukami DK. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiology, Biomarkers, and Prevention. 2006;15(10):1805–1811. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: A potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiology, Biomarkers, and Prevention. 2003;12(12):1501–1508. [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, Joseph AM, Hatsukami DK. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiology, Biomarkers, and Prevention. 2005;14(3):693–698. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- Henley SJ, Connell CJ, Richter P, Husten C, Pechacek T, Calle EE, Thun MJ. Tobacco-related disease mortality among men who switched from cigarettes to spit tobacco. Tobacco Control. 2007;16(1):22–28. doi: 10.1136/tc.2006.018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield J. Introduction to tobacco harm reduction as a complementary strategy to smoking cessation. Tobacco Control. 1995;4(Suppl. 2):S25–S28. [Google Scholar]
- Hughes JR. Applying harm reduction to smoking. Tobacco Control. 1995;4(Suppl. 2):533–538. [Google Scholar]
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: An update. Addiction. 2005;100(8):1074–1089. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine & Tobacco Research. 2006;8(6):739–749. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hecht SS, Carmella SG, Murphy SE, Callas P. Smoking behaviour and toxin exposure during six weeks use of a potential reduced exposure product: Omni. Tobacco Control. 2004;13(2):175–179. doi: 10.1136/tc.2003.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Callas PW. Ever users versus never users of a “less risky” cigarette. Psychology of Addictive Behaviors. 2005;19(4):439–442. doi: 10.1037/0893-164X.19.4.439. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological Reviews. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hund LM, Farrelly MC, Allen JA, Chou RH, St Claire A W, Vallone DM, Healton CG. Findings and implications from a national study on potential reduced exposure products (PREPs) Nicotine & Tobacco Research. 2006;8(6):791–797. doi: 10.1080/14622200601004042. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2007;79(2):587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- Jimenez-Ruiz C, Kunze M, Fagerström K. Nicotine replacement: A new approach to reducing tobacco-related harm. European Respiratory Journal. 1998;11(2):263–264. doi: 10.1183/09031936.98.11020473. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Bliss RL, Zhao F, Lando H. Predictors of smoking reduction without formal intervention. Nicotine & Tobacco Research. 2005;7(2):277–282. doi: 10.1080/14622200500056176. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Lando H, Carmella SG, Han S, Gross M, Bliss R, Le CT, Hatsukami DK. Smoking reduction fails to improve clinical and biological biomarkers of cardiac disease: A randomized controlled trial. Nicotine & Tobacco Research. doi: 10.1080/14622200801901948. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Hennrikus D, Thoele MJ, Krueger R, Hatsukami D. Community tobacco control leaders’ perceptions of harm reduction. Tobacco Control. 2004;13(2):108–113. doi: 10.1136/tc.2003.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Archives of Internal Medicine. 1994;154(2):169–175. [PubMed] [Google Scholar]
- Lemmonds CA, Mooney M, Reich B, Hatsukami D. Characteristics of cigarette smokers seeking treatment for cessation versus reduction. Addictive Behaviors. 2004;29(2):357–364. doi: 10.1016/j.addbeh.2003.08.049. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Pentel PR. Compensatory nicotine self-administration during reduced access to nicotine: An animal model of nicotine exposure reduction; Presentation at the 11th annual meeting of the Society for Research on Nicotine and Tobacco; Orlando, FL. 2006. Feb 15–18, [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: Relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology. 2003;170(3):278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacology, Biochemistry, and Behavior. 2002;72(1–2):279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Levy DT, Mumford EA, Cummings KM, Gilpin EA, Giovino G, Hyland A, Sweanor D, Warner KE. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers, and Prevention. 2004;13(12):2035–2042. [PubMed] [Google Scholar]
- Lewin F, Norell S, Johansson H, Gustavsson P, Wennerberg J. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck. Cancer. 1998;82:1367–1374. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lubin JH. Modifying risk of developing lung cancer by changing habits of cigarette smoking. British Medical Journal. 1984;289(6449):921. doi: 10.1136/bmj.289.6449.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoun RJ. Toward a psychology of harm reduction. American Psychologist. 1998;53(11):1199–1208. doi: 10.1037//0003-066x.53.11.1199. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clinical Pharmacology and Therapeutics. 2005;77(3):145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Martin EG, Warner KE, Lantz PM. Tobacco harm reduction: What do the experts think? Tobacco Control. 2004;13(2):123–128. doi: 10.1136/tc.2003.006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Baumgart MI, Hecht SS, Zhang Y, Murphy SE, Le C, Jensen J, Hatsukami DK. Pilot study on lower nitrosamine smokeless tobacco products compared to medicinal nicotine. Nicotine & Tobacco Research. doi: 10.1080/14622200701704228. in press. [DOI] [PubMed] [Google Scholar]
- Menotti A, Blackburn H, Kromhout D, Nissinen A, Adachi H, Lanti M. Cardiovascular risk factors as determinants of 25-year all-cause mortality in the seven countries study. European Journal of Epidemiology. 2001;17:337–346. doi: 10.1023/a:1012757616119. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, Author; 2001. Smoking and Tobacco Control Monograph No.13; NIH Publication No.02-5074. [Google Scholar]
- Norberg M, Stenlund H, Lindahl B, Boman K, Weinehall L. Contribution of Swedish moist snuff to the metabolic syndrome: A wolf in sheep’s clothing? Scandinavian Journal of Public Health. 2006;34:576–583. doi: 10.1080/14034940600665143. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Siegel SJ, Shields AE, Patterson F, Gould TJ, Strasser AA, Ray R, Pinto A, Audrain-McGovern J, Rukstalis M, Perkins KA, Blendy JA, Lerman C. Translating basic science to improve pharmacotherapy for nicotine dependence. Nicotine & Tobacco Research. 2007 doi: 10.1080/14622200701691755. [DOI] [PubMed] [Google Scholar]
- Ramstrom LM, Foulds J. Role of snus in initiation and cessation of tobacco smoking in Sweden. Tobacco Control. 2006;15(3):210–214. doi: 10.1136/tc.2005.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard SI, Umino T, Millatmal T, Daughton DM, Manouilova LS, Ullrich FA, Patil KD, Romberger DJ, Floreani AA, Anderson JR. Evaluation of subclinical respiratory tract inflammation in heavy smokers who switch to a cigarette-like nicotine delivery device that primarily heats tobacco. Nicotine & Tobacco Research. 2002;4(4):467–476. doi: 10.1080/1462220021000018407. [DOI] [PubMed] [Google Scholar]
- Rodu B, Cole P. Nicotine maintenance for inveterate smokers. Technology. 1999;6:17–21. [Google Scholar]
- Roethig HJ, Kinser RD, Lau RW, Walk RA, Wang N. Long-term exposure evaluation of adult smokers switching from conventional to first-generation electrically heated cigarettes during controlled smoking. Journal of Clinical Pharmacology. 2005;45(2):133–145. doi: 10.1177/0091270004271253. [DOI] [PubMed] [Google Scholar]
- Roethig HJ, Zedler BK, Kinser RD, Feng S, Nelson BL, Liang Q. Short-term clinical exposure evaluation of a second-generation electrically heated cigarette smoking system. Journal of Clinical Pharmacology. 2007;47(4):518–530. doi: 10.1177/0091270006297686. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Wilhelmsen L, Wedel H. Coronary heart disease, cancer and mortality in male middle-aged light smokers. Journal of Internal Medicine. 1992;231(4):357–362. doi: 10.1111/j.1365-2796.1992.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regulatory Toxicology and Pharmacology. 2007;47(2):171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Schildt E, Eriksson M, Hardell L, Magnusson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. International Journal of Cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Mason KM, Henningfield JE. Tobacco dependence treatments: Review and prospectus. Annual Review of Public Health. 1998;19:335–358. doi: 10.1146/annurev.publhealth.19.1.335. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers’ beliefs about “light” and “ultra light” cigarettes. Tobacco Control. 2001;10(Suppl. 1):i17–i23. doi: 10.1136/tc.10.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, McKarns SC, Davis RA, Livingston SD, Bombick BR, Avalos JT, Morgan WT, Doolittle DJ. Human urine mutagenicity study comparing cigarettes which burn or primarily heat tobacco. Mutation Research. 1996;361(1):1–9. doi: 10.1016/s0165-1161(96)90222-8. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8(2):309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Hyde RW, Boscia J, Chow MY, O’Mara RE, Perillo I, Pietropaoli A, Smith CJ, Torres A, Utell MJ, Frampton MW. Changes in markers of epithelial permeability and inflammation in chronic smokers switching to a nonburning tobacco device (Eclipse) Nicotine & Tobacco Research. 2006;8(6):773–783. doi: 10.1080/14622200601004091. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- Thun M, Day-Lally C, Calle E, Flanders W, Heath C. Excess mortality among cigarette smokers: Changes in a 20-year interval. American Journal of Public Health. 1995;85(9):1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL. Epidemiological perspective for tobacco risk reduction; Presentation at the NIH State-of-the-Science Conference on Tobacco Use: Prevention, Cessation and Control; Bethesda, MD. 2006. Jun 12–14, [Google Scholar]
- Tomar SL, Giovino GA, Eriksen MP. Smokeless tobacco brand preference and brand switching among US adolescents and young adults. Tobacco Control. 1995;4:67–72. [Google Scholar]
- Tonnesen P, Pisinger C, Hvidberg S, Wennike P, Bremann L, Westin A, Thomsen C, Nilsson F. Effects of smoking cessation and reduction in asthmatics. Nicotine & Tobacco Research. 2005;7(1):139–148. doi: 10.1080/14622200412331328411. [DOI] [PubMed] [Google Scholar]
- Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tobacco Control. 2006;15(6):472–480. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Therapeutic Drug Monitoring. 2002;24(1):163–171. doi: 10.1097/00007691-200202000-00026. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services . Reducing tobacco use: A report of the surgeon general. Atlanta, GA: Author, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2000. [Google Scholar]
- U.K. Department of Health. Smoking kills. A white paper on tobacco. London: The Stationery Office; 1998. [Google Scholar]
- UN Focal Point on Tobacco or Health. Social and economic aspects of reduction of tobacco smoking by use of alternative nicotine delivery systems (ANDS) Geneva: United Nations; 1998. [Google Scholar]
- von Weymarn LB, Brown KM, Murphy SE. Inactivation of CYP2A6 and CYP2A13 during nicotine metabolism. The Journal of Pharmacology and Experimental Therapeutics. 2006;316(1):295–303. doi: 10.1124/jpet.105.091306. [DOI] [PubMed] [Google Scholar]
- Warner KE, Burns DM. Hardening and the hard-core smoker: Concepts, evidence, and implications. Nicotine & Tobacco Research. 2003;5:37–48. doi: 10.1080/1462220021000060428. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Preventive Medicine. 2003;36(6):710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- Wynder E, Stellman S. Impact of long-term filter cigarette usage on lung and larynx cancer risk: A case-control study. Journal of the National Cancer Institute. 1979;62:471–477. doi: 10.1093/jnci/62.3.471. [DOI] [PubMed] [Google Scholar]
- Zedler BK, Kinser R, Oey J, Nelson B, Roethig HJ, Walk RA, Kuhl P, Rustemeier K, Schepers G, Von Holt K, Tricker AR. Biomarkers of exposure and potential harm in adult smokers of 3-7 mg tar yield (Federal Trade Commission) cigarettes and in adult non-smokers. Biomarkers. 2006;11(3):201–220. doi: 10.1080/13547500600576260. [DOI] [PubMed] [Google Scholar]


