Abstract
Adult-born granule cells in the mammalian dentate gyrus have long been implicated in hippocampal dependent spatial learning and behavioral effects of chronic antidepressant treatment. Although recent anatomical and functional evidence indicates a dissociation of the dorsal and ventral regions of the hippocampus, it is not known if adult neurogenesis within each region specifically contributes to distinct functions or whether adult-born cells along the entire dorsoventral axis are required for these behaviors. We examined the role of distinct subpopulations of adult-born hippocampal granule cells in learning- and anxiety-related behaviors using low-dose focal x-irradiation directed specifically to the dorsal or ventral dentate gyrus. Our findings indicate a functional dissociation between adult-born neurons along the longitudinal axis of the dentate gyrus wherein new neurons in the dorsal dentate gyrus are required for timely acquisition of contextual discrimination while immature neurons in the ventral dentate gyrus are necessary for anxiolytic/antidepressant-related effects of fluoxetine. Interestingly, when contexts are presented with altered temporal cues, or fluoxetine is administered alongside chronic glucocorticoid treatment, this dissociation is abrogated such that adult-born neurons across the entire dorsoventral extent of the dentate gyrus appear to contribute to these behaviors. Our results suggest that individual subpopulations of adult-born hippocampal neurons may be sufficient to mediate distinct behaviors in certain conditions, but are required to act in concert in more challenging situations.
Keywords: hippocampus, longitudinal axis, pattern separation, antidepressant, stress
INTRODUCTION
In addition to the classic view of the hippocampus as a neural structure that is primarily responsible for spatial memory, recent studies indicate a role for the hippocampus in emotional behavior. Lesions along the longitudinal axis of the hippocampus reveal that the dorsal pole appears to regulate cognitive-related behaviors while the ventral pole mediates mood-related responses (Moser et al., 1995; Ferbinteanu and McDonald, 2001; Bannerman et al., 2002; Kjelstrup et al., 2002; Maren and Holt, 2004). This segregation of the dorsal and ventral hippocampus (Moser and Moser, 1998; Fanselow and Dong, 2010) is also supported by anatomical evidence as shown by both tract tracing (Swanson and Cowan, 1977; Witter et al., 1989; Burwell and Amaral, 1998; Dolorfo and Amaral, 1998; Cenquizca and Swanson, 2007) and gene expression studies (Hortnagl et al., 1991; Jinno et al., 1998; Leonardo et al., 2006; Thompson et al., 2008; Dong et al., 2009; Christensen et al., 2010; Jinno and Kosaka, 2010). Consistent with these findings, a recent study has demonstrated that acute activation of granule cells specifically in the dorsal or ventral hippocampal dentate gyrus differentially suppresses contextual learning or innate anxiety, respectively (Kheirbek et al., 2013).
The hippocampal dentate gyrus is one of the few structures in the healthy adult mammalian brain where neural progenitor cells continue to proliferate and differentiate into mature neurons (Ming and Song, 2011). Several studies have indicated a role for adult neurogenesis in hippocampal function. Ablation of adult-born neurons results in deficits ranging from spatial learning and memory (Raber et al., 2004; Clelland et al., 2009; Deng et al., 2010; Arruda-Carvalho et al., 2011; Sahay et al., 2011; Denny et al., 2012) to behavioral responses to antidepressants and regulation of the hypothalamic-pituitary-adrenal stress axis (Becker and Wojtowicz, 2007; Surget et al., 2008; David et al., 2009; Snyder et al., 2011). In the same manner, manipulations that increase neurogenesis, including exercise, environmental enrichment, antidepressant administration, and genetic approaches, both enhance spatial learning and contextual discrimination (van Praag et al., 1999; Sahay et al., 2011) and produce anxiolytic and antidepressant effects (Chapillon et al., 1999; Malberg et al., 2000; Surget et al., 2008; David et al., 2009). While these studies support a role for adult hippocampal neurogenesis in both the cognitive functions that are thought to be mediated by the dorsal hippocampus and emotional regulation that has been attributed to the ventral hippocampus, it is not known to what extent adult-born cells located within each region contribute to these specific functions.
To dissect the distinct functional contribution of adult-born neurons along the longitudinal axis of the hippocampus, we used focal x-irradiation to specifically ablate adult-born neurons in either the dorsal or ventral dentate gyrus. We find that in a contextual discrimination task in which similar contexts are presented in the same order each day, ablation of adult neurogenesis only in the dorsal, but not ventral, dentate gyrus results in delayed acquisition of discrimination. When discrimination is tested under conditions in which contexts are presented in a randomized order though, adult neurogenesis at both poles of the dentate gyrus becomes necessary. Interestingly, adult-born neurons in the ventral, but not dorsal, dentate gyrus appear to be required for anxiolytic/antidepressant effects of fluoxetine under situations of chronic stress. However, adult neurogenesis at both poles of the dentate gyrus is required for fluoxetine-mediated behavioral effects when stress is induced by chronic administration of glucocorticoids. Taken together, our study suggests a condition-dependent functional dissociation of adult-born neurons at the dorsal and ventral poles of the dentate gyrus.
MATERIALS AND METHODS
Animals
Seven-week-old C57Bl6/J male mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were group housed, maintained on a 12:12 light cycle, and given food and water ad libitum. All procedures were performed in the light cycle and complied with NIH AALAC and NYSPI IACUC guidelines.
X-Irradiation
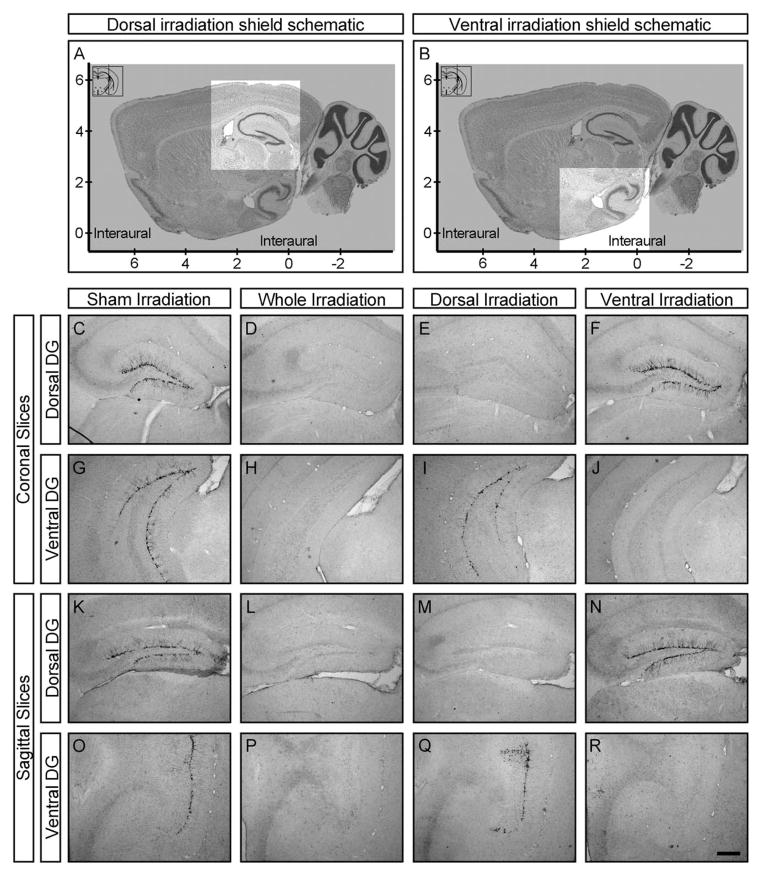
Irradiation was performed at 8 weeks of age in a manner similar to previous reports (Santarelli et al., 2003; David et al., 2009; Drew et al., 2010). Briefly, animals were anesthetized with 6 mg kg−1 sodium pentobarbital (Nembutal), placed in a stereotaxic frame, and exposed to cranial irradiation using an X-RAD 320 X-ray system (PXI; North Branford, CT) operated at 300 kV and 12 mA using a 2-mm Al filter. Three 2.5-Gy doses were delivered with a 2 day inter-dose interval for a total of 7.5 Gy over a 1-week period. Behavioral studies were carried out 6 weeks later. Whole hippocampus irradiated animals were protected with a lead shield that covered the entire body, but left unshielded a 3.22 × 11 mm2 treatment field above the hippocampus (interaural 3.00 to 0.00). Sham animals were anesthetized but not irradiated. Dorsal and ventral hippocampus irradiated animals were placed in the stereotaxic frame and then rotated 90° clockwise along the coronal plane. A lead shield containing a sliding 3.5 × 3.5mm2 window was then used to expose either the dorsal (2.5–6 mm above the interaural line) or ventral hippocampus (1 mm below to 2.5 mm above the interaural line) to x-irradiation (Figs. 1A, B).
FIGURE 1.
Focal ablation of distinct hippocampal adult-born neuronal subpopulations. (A, B) Schematic design of a sliding shield allowing for focal irradiation of the dorsal (A) or ventral (B) hippocampus. Shaded areas are protected by the lead shield; unshaded regions are targeted by x-irradiation. (C–R) Representative coronal (C–J) and sagittal (K–R) sections through the dorsal (C–F, K–N) or ventral (G–J, O–R) dentate gyrus (DG) of sham (C, G, K, O), whole (D, H, L, P), dorsal (E, I, M, Q), or ventral hippocampus (F, J, N, R) irradiated adult male mice stained for doublecortin. Scalebar equals 250 μm.
Drugs
35 μg mL−1 corticosterone (C2505, Sigma; St. Louis, MO) was dissolved in 0.45% β-cyclodextrin (C4767, Sigma; St. Louis, MO) in water. Fluoxetine (BG0197, Biotrend; Destin, FL) was dissolved at 160 μg mL−1. Corticosterone (5 mg kg−1 day−1) was delivered in the drinking water either alone or in the presence of fluoxetine (18 mg kg−1 day−1).
Contextual Discrimination
Testing was conducted in one side of a shuttle box (20.3 × 15.9 × 21.3 cm3, Med-Associates; St Albans, VT) with two clear plexiglass walls, two aluminum walls, and a stainless steel grid floor, which was encased in a sound-dampening cubicle. Animals were held outside the experimental room in their home cages prior to testing.
For training context A, experimental room lights were on, the chamber was lit from above with a house light, and ventilation was provided with a house fan. A mild lemon scent was used as an olfactory cue (by using a cotton-tipped applicator to place six drops of lemon extract (McCormick; Sparks, MD) on a paper towel located beneath the grid floor), a nonalcoholic antiseptic was used to clean the chamber and grid floor between runs, and animals were transported from their home cages to the chambers and back using cardboard buckets. Mice were placed in the context for 180 s, received a single two second foot shock of 0.75 mA delivered through the grid floor, and then removed from the context 15 s after the shock ended. For similar context B, experimental room lights were dimmed, the house light and fan were turned off, two plastic inserts were used to cover the walls in a circular fashion, and the encasing cubicle door was left ajar. A mild anise scent was used as an olfactory cue (by using a cotton-tipped applicator to place six drops of anise extract on a paper towel located beneath the grid floor), 70% ethanol was used to clean the inserts and grid floor between runs, and animals were transported using standard mouse cages. Mice were placed in the context for 180 seconds and removed, with no shock delivery.
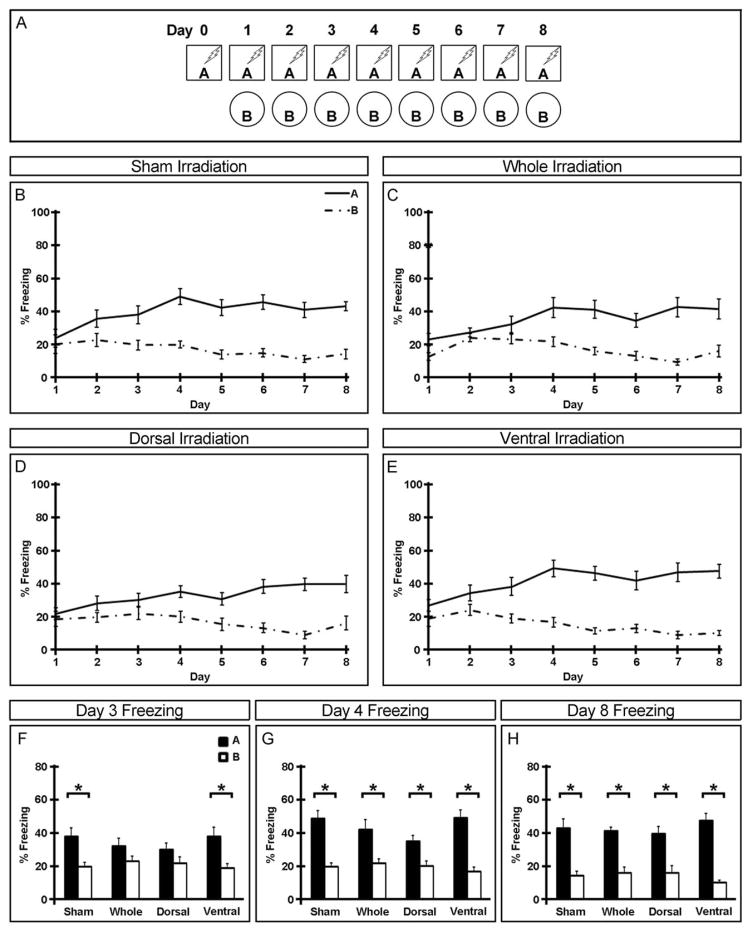
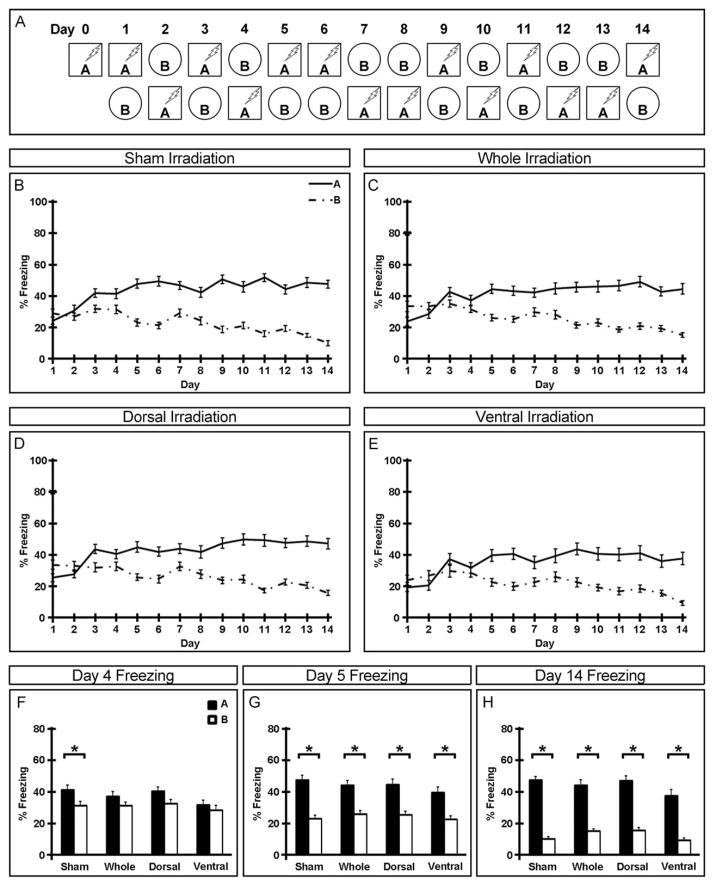
Behavior was recorded by a digital video camera mounted above the chamber and freezing behavior was analyzed with Freezeview software (Actimetrics; Evanston, IL). Percentage of time freezing in the first minute after placement in each context is presented. All animals were trained in context A on day 0 and tested in both contexts starting on day 1. In the nonrandomized condition, animals were always tested in context A in the morning, returned to the vivarium, and tested in context B in the afternoon ≥2 h later (Fig. 2A). In the randomized condition, order of testing was pseudo-randomized but animals were always returned to the vivarium for ≥2 h between contexts (Fig. 3A). Freezing data is presented within groups across all days of testing (Figs. 2B–E and 3B–E) as well as between groups on the last day of testing (Figs. 2H and 3H), the first day all groups distinguished between contexts (Figs. 2G and 3G), and the day immediately preceding (Figs. 2F and 3F).
FIGURE 2.
Requirement for adult-born neurons in the dorsal dentate gyrus in timely acquisition of non-randomized contextual discrimination. (A) Experimental paradigm for nonrandomized contextual discrimination. (B–E) Freezing behavior of sham (B), whole (C), dorsal (D), and ventral (E) hippocampus irradiated mice over the duration of the experiment. (F–H) Freezing behavior in two similar contexts on days 3 (F), 4 (G), and 8 (H) of the nonrandomized paradigm. Mean ± SEM. n ≥ 13. *P < 0.05.
FIGURE 3.
Adult-born neurons in both the dorsal and ventral dentate gyrus are required for normal acquisition of randomized contextual discrimination. (A) Experimental paradigm for randomized contextual discrimination. (B–E) Freezing behavior of sham (B), whole (C), dorsal (D), and ventral (E) hippocampus irradiated mice over the duration of the experiment. (F–H) Freezing behavior in two similar contexts on days 4 (F), 5 (G) and 14 (H) of the randomized paradigm. Mean ± SEM. n ≥ 28. *P < 0.05.
Stress Paradigms
Chronic stress
Five weeks after nonrandomized contextual discrimination, anxiety- and depression-related tests were performed immediately following 30 min of restraint (Snyder et al., 2011). Animals were then subjected to a chronic stress paradigm consisting of daily unpredictable foot shocks for 21 consecutive days. One week later, fluoxetine was provided in the drinking water for 4 weeks and animals were then retested in anxiety- and depression-related assays (Fig. 4A).
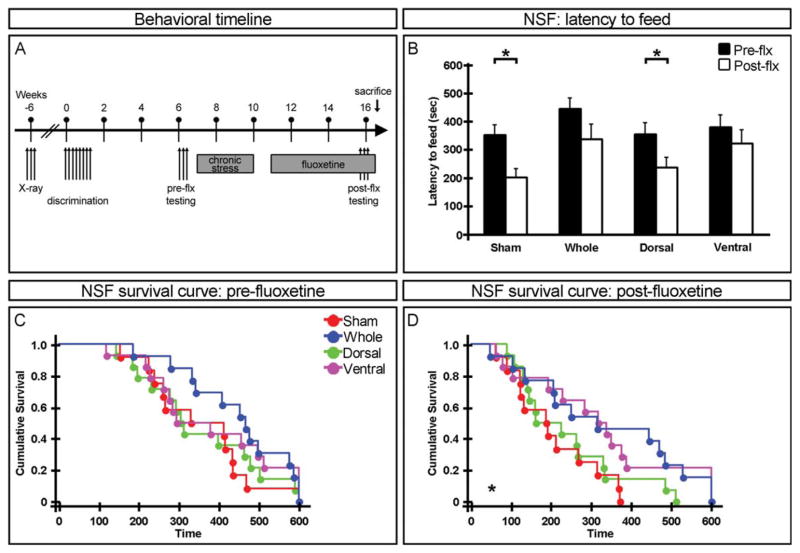
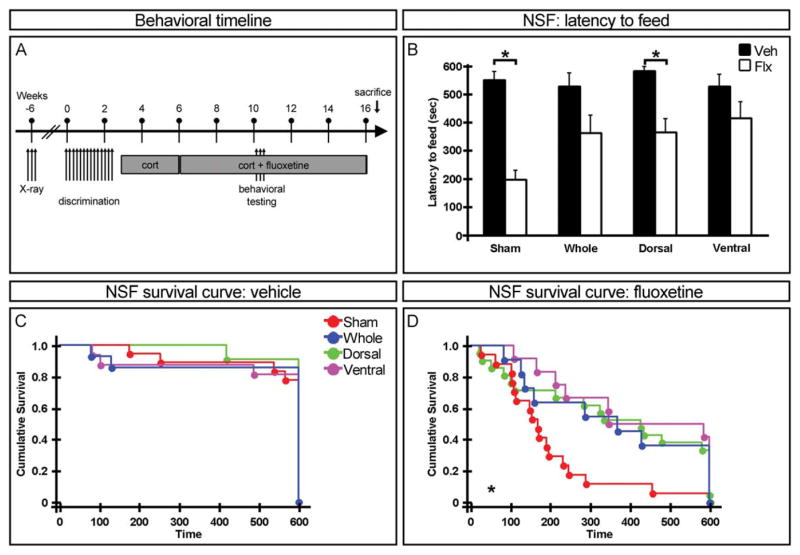
FIGURE 4.
Requirement for adult-born neurons in the ventral dentate gyrus for anxiolytic/antidepressant effects of fluoxetine (flx) in a chronic stress model. (A) Experimental timeline of the chronic stress model. (B) Latency to feed in the novelty suppressed feeding paradigm. Chronic fluoxetine decreases the latency to feed in sham and dorsal hippocampus irradiated mice but not in whole or ventral hippocampus irradiated animals. Mean ± SEM. (C, D) Cumulative survival curve of latency to feed before (pre) (C) and after (post) (D) chronic fluoxetine treatment. n ≥ 12. *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Chronic corticosterone
One week after randomized contextual discrimination, animals were placed on a corticosterone regimen as previously described (David et al., 2009). After 3 weeks, half the animals were given fluoxetine in addition to corticosterone while the remainder continued to receive corticosterone alone. Anxiety-and depression-related behaviors were tested following 4 weeks of fluoxetine or vehicle treatment (for a total of 7 weeks corticosterone administration) (Fig. 5A).
FIGURE 5.
Adult-born neurons in both the dorsal and ventral dentate gyrus are required for neurogenesis dependent effects of fluoxetine (flx) in a corticosterone stress model. (A) Experimental timeline of the corticosterone stress model. (B) Latency to feed in the novelty suppressed feeding paradigm. Mean ± SEM. (C, D) Cumulative survival curve of latency to feed in vehicle (C) and fluoxetine (D) treated animals. n ≥ 11. *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Anxiety- and Depression-Related Behavioral Testing
Animals were tested in brightly lit experimental rooms during the light cycle. Assays were carried out in the following order with at least 48 h between behavioral tests.
Open field
Animals were placed in the corner of a 43 × 43 cm2 plexiglass enclosed arena. Ambulatory activity was automatically detected by infrared photobeam crosses over a 30-min period (Med-Associates; St Albans, VT). Percent time spent in the center of the arena is reported.
Novelty suppressed feeding
Prior to the test, animals were food deprived for 24 h. The testing arena consisted of a plastic box (45 × 30 × 15 cm3) covered with ~2 cm of sawdust bedding. A single pellet of standard chow was affixed to a raised platform covered with white filter paper. A mouse was placed in a corner of the arena and the latency to feed (defined as the mouse sitting on its haunches and biting the pellet with the use of its forepaws) was recorded (with a ceiling of ten min).
Forced swim test
The testing arena consisted of a transparent beaker (19 cm diameter × 23 cm deep) filled with room temperature water. Animals were placed in the water for six minutes and their behavior was recorded using VideoTrack, an automated video-tracking system (Viewpoint Life Sciences; Montreal, Canada). Testing was carried out over two consecutive days. Time spent immobile in the last 4 min on the second day is reported.
Histology
To confirm neurogenesis ablation, immunolabeling against doublecortin (DCX), a marker for immature neurons, was performed at the conclusion of all behavioral procedures as previously described (Wang et al., 2008; Denny et al., 2012). Briefly, animals were given an overdose of ketamine/xylazine, then transcardially perfused with 0.1M phosphate buffered saline (PBS) followed by ice cold 4% paraformaldehyde (PFA) in PBS. Brains were dissected and postfixed overnight in 4% PFA followed by cryoprotection in 30% sucrose in PBS at 4°C. Serial 35-μm sections were then collected and stored in 0.02% NaN3 in PBS at 4°C. Every sixth section was washed, incubated in H2O2 to quench endogenous peroxidases, blocked with normal donkey serum, and incubated overnight in primary antibody at 4°C (goat anti-DCX, 1:500, SCBT sc-8066; Dallas, TX). Following washes, sections were incubated in secondary antibody (biotinylated donkey anti-goat, 1:250, Jackson Immunoresearch; West Grove, PA) for 2 h at room temperature, treated with avidin-biotin-peroxidase complex, and exposed to 3,3′ diaminobenzidine substrate (Vector Labs; Burlingame, CA). Sections were then slide mounted, dehydrated through an ethanol series, and coverslipped with Permount before being imaged on an AxioObserver A.1 (Zeiss; Oberkochen, Germany).
Statistical Analysis
All behavioral testing was performed with investigators blinded to x-irradiation and drug treatment. Data was analyzed by ANOVAs followed by post-hoc tests as appropriate. Latency to feed in the NSF was also analyzed using Kaplan–Meier survival curves followed by Mantel-Cox log-rank tests. P values <0.05 were deemed statistically significant (Supporting Information Table S1).
RESULTS
Targeted Ablation of Adult-Born Neurons
To specifically ablate adult neurogenesis in the dorsal or ventral dentate gyrus, we utilized low-dose x-irradiation which has previously been shown to result in permanent disruption of progenitor cell proliferation (Parent et al., 1999; Santarelli et al., 2003). A modified lead shield was designed to protect nontargeted regions of the hippocampus as well as the rest of the brain and body from irradiation (Figs. 1A, B). Six weeks after sham, whole, dorsal, or ventral hippocampus irradiation, immature neurons, as evinced by doublecortin (DCX) expression, are absent from targeted subregions of the dentate gyrus, but spared elsewhere (Figs. 1C–R, Supporting Information S1). Our ablation strategy was indeed permanent even in the presence of fluoxetine administration, as DCX expression is still absent 22 weeks after irradiation (Supporting Information Fig. S2).
Adult Neurogenesis in the Dorsal Dentate Gyrus is Required for Contextual Discrimination
To examine the contribution of distinct subpopulations of adult-born neurons to cognitive-related tasks, we subjected irradiated animals to a contextual discrimination learning paradigm. Performance in this task has previously been shown to be impaired in animals lacking, and enhanced in animals with increased, adult neurogenesis (Sahay et al., 2011). Six weeks after irradiation, animals were first trained to associate a neutral context (A) with an aversive footshock. When tested 24 h after training, freezing in context A was indistinguishable between groups (F3,175 = 0.27, P > 0.84), indicating normal acquisition of contextual fear conditioning. Two hours later, mice were then tested in a similar context (B) with no shock delivery, and all groups indistinguishably displayed comparable levels of freezing in context B and A (X-ray: F3,350 = 1.36, P > 0.25; context: F1,350 = 2.57, P > 0.11), suggesting that all groups similarly generalized contextual fear responses.
Non-Randomized Contextual Discrimination
Half of the mice continued to be tested daily in both contexts in a nonrandomized fashion, such that exposure to context A always preceded exposure to context B, for seven additional days (Fig. 2). All animals reliably distinguished between contexts by the eighth day of testing, as demonstrated by significantly higher levels of freezing in context A compared to B (Fig. 2H). However, while both sham and ventral hippocampus irradiated animals distinguished between contexts A and B by day 3 (Fig. 2F), neither whole nor dorsal hippocampus irradiated animals were able to contextually discriminate until the fourth day (Fig. 2G) of testing. Together, this suggests that while adult-born neurons in the dorsal dentate gyrus are required for rapid acquisition of this non-randomized contextual fear discrimination task, adult neurogenesis in the ventral dentate gyrus is not.
Randomized Contextual Discrimination
To test animals in a more demanding version of contextual discrimination where temporal cues such as time-of-day or order-of-context-presentation cannot be used to reliably predict shock, half of the mice were tested daily in both contexts in a randomized fashion for 13 additional days (Fig. 3). Similar to those tested in non-randomized conditions, all animals were able to reliably distinguish between contexts by the last day of testing (Fig. 3H). In this more demanding cognitive task, sham irradiated animals were not able to distinguish between contexts until day 4 (Fig. 3F). Interestingly, all three irradiated groups (whole, dorsal, and ventral hippocampus) required 5 days of testing before reliably discriminating between contexts (Fig. 3G) though in a different strain, acquisition of the randomized contextual discrimination task was seen to be sensitive to whole and dorsal, but not ventral, hippocampus irradiation (Supporting Information Fig. S3). Taken together, this suggests that the pool of adult-born neurons in the dorsal dentate gyrus by itself may no longer be sufficient to support normal acquisition of cognitive ability in this task.
Adult Neurogenesis in the Ventral Dentate Gyrus is Required for Some Behavioral Effects of Fluoxetine
We next assessed the effects of fluoxetine on anxiety/depression- related behaviors in irradiated animals under stressed conditions as mice of the C57Bl6 strain do not exhibit behavioral effects of fluoxetine at baseline (David et al., 2009). As expected, fluoxetine treatment was sufficient to rescue behavior in two tasks that are not dependent on neurogenesis equivalently across all groups ([open field (Supporting Information Fig. S4)—X-ray: F3,49 = 0.54, P > 0.66; drug: F1,49 = 5.64, P < 0.02 (chronic stress). X-ray: F3,112 = 0.83, P > 0.48; drug: F1,112 = 20.26, P < 0.0001 (chronic corticosterone)] [forced swim test (data not shown)—x-ray: F3,49 = 3.43, P < 0.03; drug: F1,49 = 142.14, P < 0.0001 (chronic stress). X-ray: F3,112 = 1.28, P > 0.29; drug: F1,112 = 5.92, P < 0.02 (chronic corticosterone).]). We also tested the effects of fluoxetine on irradiated animals in the novelty suppressed feeding assay, which has previously been shown to be dependent on adult neurogenesis (Santarelli et al., 2003; Surget et al., 2008; David et al., 2009; Rainer et al., 2011). In this assay, conflict between hunger and the anxiety of entering the center of the testing arena is measured by the latency to begin feeding.
Chronic Stress
Five weeks after nonrandomized contextual discrimination, the first cohort of animals was tested in mood-related behavioral assays. They were then subjected to 3 weeks of chronic stress followed by fluoxetine administered through the drinking water for 4 weeks before retesting (Fig. 4A). Prior to fluoxetine treatment, no significant difference was found in the latency to feed in the novelty suppressed feeding task across irradiation groups (Fig. 4C) though there is a trend difference between sham and whole hippocampus irradiated animals. Following chronic stress and fluoxetine administration, latency to feed was significantly decreased in sham and dorsal hippocampus irradiated animals but not whole or ventral hippocampus irradiated mice (Fig. 4B). Additionally, there was a significant difference between sham and both whole and ventral, but not dorsal, hippocampus irradiated animals in latency to feed (Fig. 4D), thus suggesting that adult-born neurons in the ventral, but not dorsal, dentate gyrus are required for this anxiolytic/antidepressant effect of fluoxetine.
Chronic Corticosterone
To examine the requirement for specific subpopulations of adult-born neurons in an alternate model of stress-related behavior, we administered corticosterone to the second cohort of irradiated animals for 3 weeks (David et al., 2009). Half the animals were then treated with both corticosterone and fluoxetine for 4 weeks while the other half remained on corticosterone alone (Fig. 5A). As previously shown, all animals administered corticosterone alone indistinguishably exhibited a high latency to feed in the novelty suppressed feeding task (Fig. 5C), indicating that anxiety overrode hunger drive in these mice irrespective of the presence of adult hippocampal neurogenesis. Fluoxetine administration resulted in a decrease in latency to feed in sham, and to a lesser extent in dorsal hippocampus, irradiated animals, but not in whole or ventral hippocampus irradiated mice (Fig. 5B). Survival analysis of latency to feed in fluoxetine treated mice reveals that all three (whole, dorsal, and ventral hippocampus) irradiated groups are significantly different from sham animals (and indistinguishable from each other) (Fig. 5D). Together, this suggests that adult-born neurons in both the dorsal and ventral subregions of the dentate gyrus are required for behavioral effects of fluoxetine on novelty suppressed feeding under conditions of chronic corticosterone administration.
DISCUSSION
Recent work has strengthened the theory that the hippocampus can be subdivided not only into the classic DG-CA3-CA1 trisynaptic loop, but also into subregions along its longitudinal axis [for review see (Moser and Moser, 1998; Fanselow and Dong, 2010)]. Given that adult neurogenesis has been implicated in a dichotomous set of behaviors that have been associated with either the dorsal or ventral hippocampus and that adult-born neurons functionally integrate into, and are indistinguishable from, the surrounding network as they mature (Laplagne et al., 2006; Toni et al., 2008), it has been hypothesized that dorsal and ventral subpopulations of adult-born neurons respectively regulate separable cognitive or mood-related behaviors [for review regarding the latter see (Tanti and Belzung, 2013)]. However, this is the first, to our knowledge, demonstration of a double dissociation in the functional requirement for adult neurogenesis along the dorsoventral axis of the dentate gyrus.
We do recognize that several factors should be addressed in follow-up studies. First, in order to increase internal validity and statistical power, animals subjected to the chronic stress paradigm were tested in a within-subject manner, raising the possibility that initial exposure to the novel environment may have influenced behavior in the re-exposure test. However, previous studies that have involved repeated testing in the novelty suppressed feeding test indicate no evidence of habituation to the novel environment (Mendez-David et al., 2013). Additionally, we see a specific requirement for adult-born neurons in the ventral aspect of the dentate gyrus when animals were subjected to an alternate stress paradigm and tested in a between-subject manner (Supporting Information Fig. S5). Similarly, anxiety and depression related tests were always performed following contextual discrimination. Though our results are comparable to those observed in other studies that have examined novelty suppressed feeding without subjecting animals to prior discrimination tasks (David et al., 2009; Mendez-David et al., 2013), suggesting that exposure to the discrimination task did not interfere with performance on the novelty suppressed feeding task, it will be important to examine these effects in behaviorally naïve mice in future studies. Finally, we note that we cannot provide an explanation for why the difference in freezing between contexts A and B on day one is not identical between animals in the randomized (Figs. 2B–E) and nonrandomized (Figs. 3B–E) contextual discrimination tasks (though there is no statistical difference between groups) and therefore cannot rule out the possibility that this discrepancy contributes to differences in rates of acquisition between the two tasks. However, this difference in freezing behavior on day one does not appear to correlate with time to acquire contextual discrimination as whole and ventral hippocampus irradiated animals show the greatest difference in freezing on day one in the nonrandomized task while it is the sham and ventral irradiated groups that are quickest to acquire discrimination learning. Similarly, whole and dorsal hippocampus irradiated animals show the greatest difference in freezing on day one in the randomized task but all three irradiated groups are slower to discriminate compared to sham irradiated animals.
Interestingly, we find that distinct subpopulations of adult-born neurons appear to be able to uniquely contribute to specific hippocampal functions only in certain situations but not others. Under normal conditions, a small pool of adult-born neurons, either in the dorsal or ventral dentate gyrus, appears to be sufficient to support either cognitive- or anxiolytic-related behavior, respectively, by itself. However, when faced with a more difficult contextual discrimination task or corticosterone administration induced stress, these subpopulations are unable to support the demands of the tasks on their own and a requirement for immature neurons throughout the dentate gyrus is revealed such that there appear to be no behaviors primarily dependent on neurogenesis specifically in the dorsal or ventral subregion of the dentate gyrus. This milieu-dependent necessity is reminiscent of previous work demonstrating that adult neurogenesis is not required for initial learning of, but instead for cognitive flexibility in, an active place avoidance task (Burghardt et al., 2012). In this paradigm, animals with ablated adult neurogenesis show no deficit in learning to avoid a shock zone on a rotating platform, but are impaired when suppression of the initial learned response is required in order to successfully avoid a new, conflicting, shock zone location. Similarly, it has recently been hypothesized that rather than mediating the etiology of anxiety- or depression- related disorders, adult-born neurons are required for mounting appropriate behavioral responses in the face of additional stressors in already anxious or depressed individuals (Petrik et al., 2012). Taken together, these results support a model where adult-born hippocampal neurons function not so much as behavioral master regulators, but instead as modulators of the local underlying network under conditions of high demand (Piatti et al., 2013). Indeed, ablation of adult hippocampal neurogenesis has been shown to alter the overall network circuitry of mature granule cells in the dentate gyrus (Lacefield et al., 2012; Burghardt et al., 2012). It will therefore be interesting to examine the effects of our targeted ablation strategy on local hippocampal network activity.
It is also possible that the loss of functional dissociation we see in the randomized contextual discrimination and chronic corticosterone paradigms is not simply due to change in load demand. Freezing behavior in the nonrandomized contextual learning task may be informed by circadian cues (Moron et al., 2002) that are not available in the randomized condition. Given that our randomized task does not allow us to evaluate the relative importance of time-of-day or order-of-context-presentation, it will be important to independently examine the contribution of each of these factors in future studies. Similarly, resultant atrophy of the adrenal glands from corticosterone overexpression (Ingle and Kendall, 1937) may alter endogenous hypothalamic-pituitary-adrenal (HPA) axis responses required in the novelty suppressed feeding task. Interestingly, adult hippocampal neurogenesis has been postulated to be dependent on both circadian cues (Holmes et al., 2004; Tamai et al., 2008) and diurnal fluctuations in corticosterone levels (Huang and Herbert, 2006). This would suggest that regardless of the underlying mechanism, possibly through longitudinal communication across the septotemporal axis via mossy cells (Amaral and Witter, 1989; Vivar et al., 2012), there is a point at which adult neurogenesis across the entire hippocampus is necessary to support certain behavioral functions. We note that both our dorsal and ventral irradiation strategies lead to partial ablation of adult neurogenesis in the intermediate dentate gyrus (Supporting Information Fig. S1) and thus cannot exclude the possibility that it is these intermediate adult-born neurons that contribute to the conditional dependency of our observed behavioral effects. Further work will be necessary to more finely dissect the conditions in which functional specialization of adult-born neurons occurs.
Our results also provide potential target specificity for therapeutic strategies aimed at cognitive or mood related disorders (Sahay et al., 2007; Kheirbek et al., 2012). Some studies have suggested that select antidepressants may specifically increase adult neurogenesis in the ventral dentate gyrus (Banasr et al., 2006; Jayatissa et al., 2006), but these changes appear to be dependent on the strain and emotional status of the animal (Jayatissa et al., 2006; Rainer et al., 2011). Recent work has also shown that environmental enrichment increases neurogenesis in the dorsal, but not ventral dentate gyrus, while unpredictable chronic mild stress reduces, and fluoxetine induces, neurogenesis preferentially in the ventral dentate gyrus (Tanti et al., 2012). In humans, chronic antidepressant administration has also been shown to result in increased numbers of neural progenitors and mature granule cells specifically in the rostral (homologous to the ventral in rodents) dentate gyrus granule cell layer of depressed patients compared to untreated controls (Boldrini et al., 2012, 2013). Given the results of our study, it will be interesting to examine the behavioral effects, and potential translational implications, of using a genetic strategy (Sahay et al., 2011) to selectively increase adult neurogenesis in the dorsal or ventral dentate gyrus.
Supplementary Material
Acknowledgments
Grant sponsor: NIMH; Grant numbers: T32-MH015174, R37-MH068542; Grant sponsor: NYSTEM; Grant number: C024330; Grant sponsor: Hope for Depression Research Foundation.
The authors thank DJ David, CA Denny, and AS Hill for consultation on irradiation and behavioral testing; A Jonathan for animal care; A Jacobs, M Lauring, and KB Shakman for technical assistance; and NS Burghardt and AS Hill for critical reading of the manuscript. Behavioral and cognitive testing was performed with support from the Rodent Neurobehavioral Analysis Core at The New York State Psychiatric Institute. Irradiation was performed in the Radiation Research Core Facility of the Herbert Irving Comprehensive Cancer Center at Columbia University. RH receives compensation as a consultant for Lundbeck, Roche, and Servier.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: Interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapillon P, Manneche C, Belzung C, Caston J. Rearing environmental enrichment in two inbred strains of mice: 1. Effects on emotional reactivity. Behav Genet. 1999;29:41–46. doi: 10.1023/a:1021437905913. [DOI] [PubMed] [Google Scholar]
- Christensen T, Bisgaard CF, Nielsen HB, Wiborg O. Transcriptome differentiation along the dorso-ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience. 2010;170:731–741. doi: 10.1016/j.neuroscience.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. The 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: Topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA. 2009;106:11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Hortnagl H, Berger ML, Sperk G, Pifl C. Regional heterogeneity in the distribution of neurotransmitter markers in the rat hippocampus. Neuroscience. 1991;45:261–272. doi: 10.1016/0306-4522(91)90224-c. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ingle DJ, Kendall EC. Atrophy of the adrenal cortex of the rat produced by the administration of large amounts of cortin. Science. 1937;86:245. doi: 10.1126/science.86.2228.245. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus. 2010;20:829–840. doi: 10.1002/hipo.20685. [DOI] [PubMed] [Google Scholar]
- Jinno S, Aika Y, Fukuda T, Kosaka T. Quantitative analysis of GABAergic neurons in the mouse hippocampus, with optical disector using confocal laser scanning microscope. Brain Res. 1998;814:55–70. doi: 10.1016/s0006-8993(98)01075-0. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo ED, Richardson-Jones JW, Sibille E, Kottman A, Hen R. Molecular heterogeneity along the dorsal-ventral axis of the murine hippocampal CA1 field: A microarray analysis of gene expression. Neuroscience. 2006;137:177–186. doi: 10.1016/j.neuroscience.2005.08.082. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Romer S, Gardier AM, Hen R. Rapid anxiolytic effects of a 5-HT receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron I, Manrique T, Molero A, Ballesteros MA, Gallo M, Fenton A. The contextual modulation of conditioned taste aversions by the physical environment and time of day is similar. Learn Mem. 2002;9:218–223. doi: 10.1101/lm.52202. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: Are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Ewell LA, Leutgeb JK. Neurogenesis in the dentate gyrus: Carrying the message or dictating the tone. Front Neurosci. 2013;7:50. doi: 10.3389/fnins.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaer E, Hen R, Enhamre E, Gardier AM, David DJ. Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol. 2011;15:321–335. doi: 10.1017/S1461145711000356. [DOI] [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tamai S, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS One. 2008;3:e3835. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, Belzung C. Neurogenesis along the septo-temporal axis of the hippocampus: Are depression and the action of antidepressants region-specific? Neuroscience. 2013;252:234–252. doi: 10.1016/j.neuroscience.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Tanti A, Rainer Q, Minier F, Surget A, Belzung C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63:374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ, Lein ES. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.