Abstract
Plant biochemical processes result in the release of an array of volatile chemical substances into the environment, some of which are known to play important plant fitness enhancing functions, such as attracting pollinators, thermal tolerance of photosynthesis, and defense against herbivores. Cunningly, phytophagous insects have evolved mechanisms to utilize these volatiles to their own advantage, either to colonize a suitable host for feeding, reproduction and oviposition or avoid an unsuitable one. The volatile compounds involved in plant–insect chemical interactions have been widely exploited in the management of agricultural pests. On the other hand, use of plant volatiles in the management of medically important insects is limited, mainly due to paucity of information on their role in disease vector–plant interactions. To date, a total of 29 plant volatile compounds from various chemical classes, including phenols, aldehydes, alcohols, ketones and terpenes, have been identified as mosquito semiochemicals. In this review, we present highlights of mosquito–plant interactions, the available evidence of nectar feeding, with particular emphasis on sources of plant attractants, methods of plant volatile collection and the candidate plant volatile compounds that attract mosquitoes to nectar sources. We also highlight the potential application of these phytochemical attractants in integrated mosquito management.
Keywords: Plant–insect interactions, Phytochemicals, Semiochemicals, Attractants, Mosquitoes, Disease vectors
1. Introduction
Plants synthesize and emit a wide range of volatile organic compounds including terpenoids, fatty-acid derivatives, benzenoids, nitrogen containing compounds and other scented substances (Knudsen et al., 1993). These compounds serve a variety of functions for the plants such as pollinator attraction, direct and indirect self-defense against herbivores, and for their own metabolic processes (Paré and Tumlinson, 1999; Berkov et al., 2000; Schiestl et al., 2000; Loreto and Velikova, 2001; Schiestl and Ayasse, 2001; Sharkey et al., 2001; Van Poecke et al., 2001). In an evolutionary arms race, herbivorous insects have adapted mechanisms to identify suitable host plants and to evade non-host plants using their floral scents (Visser, 1986). These plant–insect interactions that involve volatile chemical communication have been widely exploited in the management of agricultural pests. However, their use in the management of medically important insects, such as mosquitoes, remains largely unexplored.
Several studies on plant–mosquito interactions have shown that floral nectar forms an important component of both male and female mosquito diet. Among these, are the mosquito species Anopheles, Culex and Aedes that transmit diseases such as malaria, yellow fever, dengue fever, West Nile virus, Rift Valley fever, chikungunya, St. Louis encephalitis, and lymphatic filariasis to man and his livestock. Reisen et al. (1986) showed that 75% females and 68% males of field collected Culex tarsalis tested positive for fructose, indicating that plant nectar feeding constituted a normal diet of this mosquito species. Similar results were obtained from field collected Aedes albopictus in coastal Israel (Müller et al., 2010c). In mosquitoes collected in western Kenya, Beier (1996) demonstrated that 6.3% of the indoor-resting and 14.4% of host-seeking An. gambiae s.l. and An. funestus tested positive for fructose. Several other studies have documented evidence of facultative or obligate nectar feeding of mosquitoes in nature (Foster, 1995; Stone and Foster, 2013).
Sugar feeding has been identified as essential in mosquito energetic budget. While only females mosquitoes feed on vertebrate blood for gonotrophic development, both sexes of all ages and gonotrophic stages require sugar meals, derived from plant sources for important processes such as flight, metabolism and fecundity (Nayar and Sauerman, 1971; Magnarelli, 1977, 1978; Van Handel and Day, 1988; Manda et al., 2007). In addition, the excessive growth of fat body and elevation of lipid reserves that are associated with adult diapauses have been linked to a boost in sugar feeding accompanied with up regulation of fatty acid synthase genes in some mosquito species such as Culex pipiens (Jaenson and Ameneshewa, 1991; Bowen, 1992a; Robich and Denlinger, 2005; Sim and Denlinger, 2009). Sugar feeding has been shown to continue throughout diapause in Culex tarsalis during mild winters (Reisen et al., 1986). Furthermore, newly emerged females of small size have been shown to require an initial sugar or blood meal to develop their follicles to stage II before undergoing vitellogenesis and egg maturation (Lounibos and Conn, 1991; Briegel and Horler, 1993). Sugar also plays an important role in the early stages of adult development and, in nature, the availability and abundance of sugar sources determine the frequency of sugar feeding (Van Handel et al., 1994; Martinez-Ibarra et al., 1997; Gu et al., 2011).
In this review, we highlight on some of the sources of attractive plant compounds that likely direct mosquitoes to a sugar meal, the various volatile collection techniques that have been employed in these studies, as well as their advantages and disadvantages, and identities of the plant compounds attractive to mosquitoes. We also give an insight into the prospects for deployment of plant volatile compounds in surveillance and control of disease transmitting mosquitoes.
2. Sources of attractive plant odours
The potential for plant volatiles to lure mosquitoes has been known since the 1960s with the observation by Sandholm and Price (1962) that various mosquitoes species in the field were attracted to light-coloured flowers with distinct fragrances. Almost two decades later, the individual contributing roles of visual and olfactory cues in mosquito attraction was established for Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Healy and Jepson, 1988; Jepson and Healy, 1988). In separate studies using a wind tunnel designed to evaluate long range attraction of mosquitoes, Jepson and Healy demonstrated an upwind flight and landing of these two mosquito species to the inflorescences of Achillea millefolium and Leucanthemum vulgare respectively both in the presence and absence of visual cues. Prior to these studies, Joseph (1970) trapped various species of Aedes, Culex, Anopheles, Psorophora, and Culiseta mosquitoes using damaged and over ripe apples, grapes, peaches and water-melons in the field. The attractiveness of flowers and fruits/pods to wild mosquitoes has also been demonstrated for An. gambiae (Müller et al., 2010b) and Aedes albopictus (Müller et al., 2011). Laboratory assays have further shown that floral and vegetative scents play a vital role in the attraction of Ae. aegypti to Asclepias syriaca (Vargo and Foster, 1982), Culex pipiens pipiens to L. vulgare, A. millefolium, Asclepias syriaca, and Solidago canadensis (Mauer and Rowley, 1999; Otienoburu et al., 2012), and An. gambiae to Parthenium hysterophorus, Ricinus communis and Bidens pilosa (Nyasembe et al., 2012).
Plant scents emanate from both the floral and vegetative parts (Knudsen et al., 1993; Pichersky and Gershenzon, 2002). These plant scents are synthesized either in situ and stored in glandular trichomes or de novo when plants are under attack by herbivores (Jakobsen and Olsen, 1994; Paré and Tumlinson, 1997). While plant scents has been well established for the floral parts, in particular for pollinator attraction, similar studies on the vegetative parts are continuously being explored because of their complexity and functions they play (Knudsen et al., 1993). The general plant volatiles are those formed via biosynthetic pathways common in most plants. These include fatty acid derivatives from unsaturated fatty acids formed through lipooxygenase pathway (Kunst and Samuels, 2003) and terpenoids through melanovate and methylerythritol phosphate pathways (Lichtenthaler et al., 1997; Eisenreich et al., 1998). Besides fatty acids, plant volatiles contain terpenes, which constitute the largest and most structurally diverse group of compounds (Degenhardt et al., 2009). Specific volatile components arise from further modification of these secondary metabolites through reduction or removal of carboxyl groups, addition of hydroxyl groups, or formation of esters and ethers catalyzed by various enzyme families (Dudareva et al., 2004). The fruit aroma consists of a complex mixture of compounds including terpenes, esters, aldehydes and alcohol, and sulphur-based compounds among others, the biosynthesis of which is regulated by ethylene (Sanz et al., 1996; Lalel et al., 2003).
3. Volatile collection techniques
With the realization of the significance of plant volatile organic compounds in plant–insect interactions, there has been a growing interest in the chemistry, biochemistry, physiology, and ecology of these compounds. This has led to the development of a variety of techniques for the collection and analysis of plant volatiles (Harborne, 1998; Millar and Sims, 1998). The choice of the most suitable system for collection of plant volatiles is dependent on the biological problem and the plant material being investigated (Tholl et al., 2006). Several volatile collection and analysis techniques have been used in identifying various mosquito attractive compounds (Table 1).
Table 1.
Chemical analysis of mosquito attractive plant compounds: source, plant part, methods of volatile collection and analysis and the identified compounds.
| Source | Targeted mosquito species | Plant part | Volatile collection method | Method of analysis | Compounds identified | References |
|---|---|---|---|---|---|---|
| Synthetic standards | Ae. aegypti | – | – | Single sensilla recording | Terpeniol, geraniol, eugenol, citral, citronellol and fatty acids | Lacher (1967) |
| Synthetic standards | Ae. aegypti | – | – | Single sensilla recording | Geraniol, eugenol, amyl acetate, toluene | Davis (1977) |
| A. millefolium | An. arabiensis | Flowers | Dynamic headspace air entrainment, activated charcoal, pentane | GC-MS | Cyclic and bicyclic monoterpene | Healy and Jepson (1988) |
| Synthetic standards | Cx. pipiens | – | – | Single sensilla recording | Thujone, verbenone, α-pinene, citral, nerol, limonene, and farnesol, hexanal, 1-hexenol, and (Z)-3-hexen-1-ol, ethyl propanoate, methyl propanoate, ethyl butyrate and ethyl acetate | Bowen (1992b) |
| S. otites | Cx. pipiens molestus and Ae. aegypti | Flowers | Dynamic headspace, Tenax-TA and Carbotrap tube, acetone | GC-EAD and GC-MS | Phenylethyl alcohol, phenylacetaldehyde, lilac aldehydes, (Z)-3-hexenyl acetate, linalool oxide, linalool, benzaldehyde, lilac alcohol, acetophenone, methyl salicylate and hexanal, 1-hexenol, Z-3-Hexen-1-ol | Jhumur et al. (2007, 2008) |
| A. syriaca | Cx. pipiens | Flowers | Solvent extraction in pentane and static headspace, DVB/CAR/PDMS SPME fibre | GC-MS | Phenylacetaldehyde, benzaldehyde and (E)-2-nonenal | Otienoburu et al. (2012) |
| P. hysterophorus, R. communis, B. pilosa | An. gambiae | Intact plant | Dynamic headspace, Super-Q, dichloromethane | GC-EAD and GC-MS | Hexanal, limonene, (Z)-and (E)-linalool oxide, β-pinene, (Z)-and (E)-β-ocimene, and (E)-β-farnesene | Nyasembe et al. (2012) |
GC-EAD = gas chromatography-electroantennography, DVB/CAR/PDMS = divinylbenzene/carboxen/polydimethylsiloxane, SPME = solid phase micro-extraction.
Solvent extraction has been widely used to collect plant volatiles for elucidation of their potential attractiveness to various mosquito species, with a single solvent (Otienoburu et al., 2012) or a series of solvents (Vargo and Foster, 1982; Jepson and Healy, 1988) used to extract floral compounds of different plant species. Solvent extraction is advantageous in that it is possible to get the full volatile organic profile of the plant by varying the type of solvent used. However, this method suffers a major limitation in that it does not give a realistic picture of the actual volatile compounds emitted by plants that are likely to play key ecological roles in plant–insect interactions. Furthermore, this method is liable to contamination by impurities present in the solvent.
Another method that has been employed collecting volatiles from mosquito host plants is the static headspace volatile collection techniques (Otienoburu et al., 2012). In their study, Otienoburu et al. (2012) placed Asclepias syriaca florets in a glass vial and collected the volatiles on solid phase micro-extraction (SPME) fibre with no circulation of air. Various SPME fibres that adsorb volatile organic compounds of varying polarity and molecular weights are available and have been reviewed in Tholl et al. (2006). The amount of compounds collected depends on the thickness of the fibre coating and the distribution constant of the analyte. This method is advantageous in sampling volatile organic compounds from low emitting plants and eliminates the need for solvents which might introduce impurities. The major disadvantage of this method is that it does not allow for repeated injection of the sample, quantification is not possible when dealing with a wide range of compounds with different distribution constants, and accumulation of humidity, heat and deleterious chemicals which might interfere with the physiology of the plant (Tholl et al., 2006).
Healy and Jepson (1988) used dynamic headspace volatile collection technique in which they placed freshly cut inflorescences of Achillea millefolium in a glass jar and passed charcoal filtered air over the inflorescences through adsorbent matrix (activated charcoal). Similar method was used by Jhumur et al. (2007, 2008) and Nyasembe et al. (2012) to collect volatiles from flowers on to adsorbent Tenax-TA and Carbotrap and from intact plants on to Super-Q traps respectively. However, both studies by Healy and Jepson (1988) and Jhumur et al. (2007, 2008) used a ‘closed-loop stripping’ system while Nyasembe et al. (2012) used a ‘push–pull’ system. In the former system, volatiles were collected through continuous circulation of headspace air inside closed chambers, while in the later air was pulled over the plant sample through an adsorbent trap connected to a vacuum pump.
Overall, the dynamic headspace volatile collection has several advantages including providing sufficient amount of volatiles for detection and structure elucidation, no increase in temperature and humidity and reduced accumulation of deleterious chemicals in the headspace (Tholl et al., 2006). However, this volatile collection method is limited by incomplete adsorption of volatile organic compounds due to different trapping matrices having specific affinities for volatile organic compounds. For instance, activated charcoal is less efficient in trapping aromatic aldehydes, while Tenax-TA and Super-Q have low affinity for low molecular weight or polar compounds. This often calls for combination of two or more of the adsorbent matrices to increase the range of volatile organic compounds trapped. For instance, Jhumur et al. (2007, 2008) used both Tenax-TA (high affinity for lipophilic to medium polar organic compounds) and Carbotrap (wide range of organic compounds but with easy decomposition of terpenes).
4. Plant volatiles detected by mosquitoes
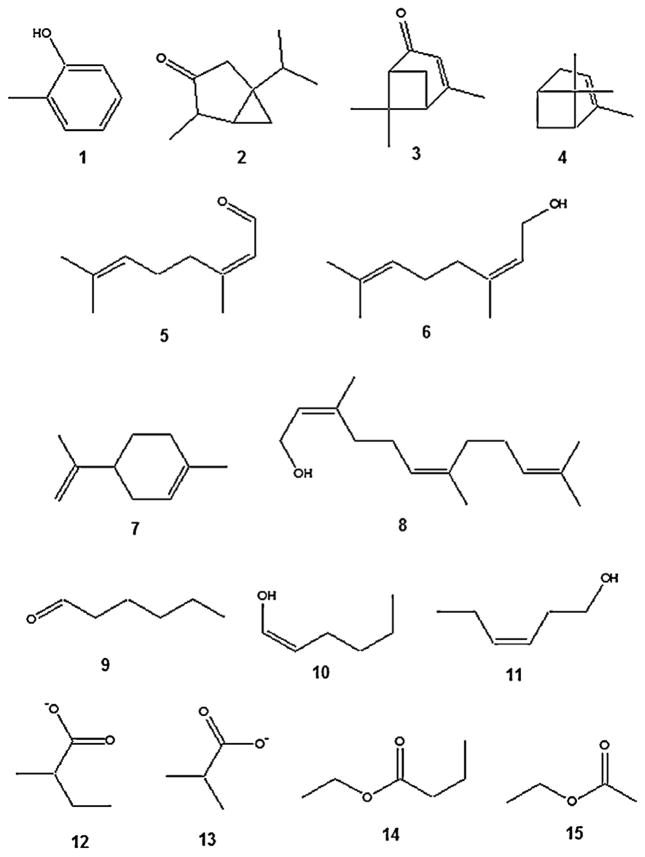
Volatile compounds emitted by plants vary greatly, and their activity on various insect species depends on their quality and quantity and the recipient insect species. Several plant compounds have been identified as responsible for host plant location by foraging mosquitoes (Table 1). Pioneer studies aimed at identifying antennally active plant compounds relied on antennal sensilla recordings to synthetic plant compounds. Notable was the study carried out by Lacher (1967) who demonstrated antennal sensilla response to terpenes in Ae. aegypti. Further evidence of mosquito antennal response to plant-related odours was demonstrated in Ae. aegypti and Ae. triseriatus which were shown to respond to o-cresol (1, Fig. 1) and related compounds (Davis, 1976; Bentley et al., 1982). Using Cx. pipiens, Bowen (1992b) discovered a high proportion of both broadly- and narrowly-tuned antennal receptor neurons sensitive to a group of terpenes (thujone 2, verbenone 3, α-pinene 4, citral 5, nerol 6, limonene 7, and farnesol 8, Fig. 1), green leaf volatiles (hexanal 9, 1-hexenol 10, and (Z)-3-hexen-1-ol 11, Fig. 1) and fatty acid esters (ethyl propanoate 12, methyl propanoate 13, ethyl butyrate 14 and ethyl acetate 15) (Fig. 1). However, behavioural assays with thujone, the major constituent, only elicited a close-range dose-dependent probing response in Cx. pipiens but did not stimulate upwind flight in a wind-tunnel olfactometer. Although these studies did not conclusively demonstrate behavioural activity of the identified compounds, they served as a foundation for more elaborate studies into the involvement of chemical cues in plant –mosquito interactions.
Fig. 1.
Structures of electrophysiologically-active phytochemicals for mosquitoes. They include o-cresol 1, thujone 2, verbenone 3, α-pinene 4, citral 5, nerol 6, limonene 7, and farnesol 8, hexanal 9, 1-hexenol 10, and (Z)-3-hexen-1-ol 11, ethyl propanoate 12, methyl propanoate 13, ethyl butyrate 14, and ethyl acetate 15.
Several years later, the advancement of more refined volatile entrainment and analytical techniques that allow characterization of the full profile of plant volatiles have seen more mosquito attractive plant compounds identified. Jhumur et al. (2007) demonstrated antennal responses of Cx. pipiens and Ae. aegypti to twelve compounds of Silene otite inflorescences including phenylethyl alcohol 16, phenylacetaldehyde 17, lilac aldehydes 18, (Z)-3-hexenyl acetate 19, linalool oxide 20, linalool 21, benzaldehyde 22, lilac alcohol 23, acetophenone 24, methyl salicylate 25 and hexanal 9 (Fig. 2). Of these, linalool oxide, linalool and hexenyl acetate elicited the strongest antennal response (Jhumur et al., 2008). Only four compounds viz. acetophenone, linalool oxide, phenylacetaldehyde, and phenylethyl alcohol elicited significant behavioural response in Cx. pipiens in olfactometer assays. The attraction of Cx. pipiens to Asclepias syriaca (Asclepiadaceae) has also been attributed to three major constituents of the floral scent; phenylacetaldehyde 17, benzaldehyde 22 and (E)-2-nonenal 26 (Fig. 2) (Otienoburu et al., 2012). In our previous study (Nyasembe et al., 2012), we demonstrated electrophysiological and behavioural activity of the malaria vector An. gambiae, to six plant compounds identified from P. hysterophorus, R. communis and B. pilosa. These included hexanal, limonene, (Z)- and (E)-linalool oxide, β-pinene 27, (Z)- and (E)-β-ocimene 28, and (E)-β-farnesene 29 (Fig. 2).
Fig. 2.
Structures of electrophysiologically- and behaviourally-active phytochemicals for mosquitoes. They include phenylethyl alcohol 16, phenylacetaldehyde 17, lilac aldehydes 18, (Z)-3-hexenyl acetate 19, linalool oxide 20, linalool 21, benzaldehyde 22, lilac alcohol 23, acetophenone 24, methyl salicyilate 25, (E)-2-nonenal 26, β-pinene 27, (Z)- and (E)-β-ocimene 28, and (E)-β-farnesene 29.
Some of these plant compounds have previously been shown to play a role in insect–plant interactions. For instance, (E)-β-farnesene and (E)-linalool oxide have been shown to elicit electrophysiological and behavioural responses in the western flower thrips, Frankliniella occidentalis, while phenylacetaldehyde has been shown to be attractive to the cabbage butterfly, Pieris rapae, and the cotton bollworm, Helicoverpa armigera (Honda et al., 1998; Agelopoulos et al., 1999; Bruce and Cork, 2001). Linalool oxide, lilac aldehydes and lilac alcohol are isomers derived from oxidation of linalool. These isomers have been associated with the fragrance of various plants and have been shown to elicit electrophysiological activity in the noctuid moth, Hadena bicruris, which is known to rely on lilac aldehydes to locate its host plants (Dötterl et al., 2006). Their detection by various mosquito species highlights the significance of this group of plant compounds in insect–plant interactions. Overall, the coincidence in detection of identical volatile compounds from different plant species by different insect species supports the argument that it is the qualitative and quantitative composition of plant compounds rather than the presence of a certain individual compound that imparts specific sensory impression on insects (Najar-Rodriguez et al., 2010). Most plants have a common biosynthetic pathway resulting in release of similar groups of compounds but of varying proportions (Schwab et al., 2008), hence it is not surprising that insects have evolved a way of utilizing different combinations of plant volatiles to locate a suitable host. Careful cellular screening of these compounds can reveal their bioactivity in mosquito vectors and the knowledge generated combined with molecular understanding employed in developing better management tools for these disease vectors.
Plant-derived volatiles are important for many nectar feeding and herbivorous insects which associate them with particular plant species, with many relying on specific blends of these compounds for identifying the particular plant species exploited as feeding or oviposition sites (Pichersky and Gershenzon, 2002; Bruce et al., 2005; Bruce and Pickett, 2011). Varied semiochemicals play important roles in host-plant location by insects which can vary in structural and chemical complexity in different habitats (Bruce et al., 2005; Najar-Rodriguez et al., 2010; Gols et al., 2012). The list of attractive volatile chemicals identified from different mosquito host plants vary considerably, and although some of these compounds may be common across the plant species, the ratios in which they are released is important (Visser, 1986; Bruce et al., 2005). The success of any insect–resource interaction is dependent on the ability of the insect to successfully locate the resource. Therefore, the observed variations could possibly indicate an adaptive or innate evolution on the part of various mosquito species to identify their potential host plants using specific or general cues and their proportions to locate potential nectar sources.
Variation in plant volatile emission among different plant species and even within the same species but in different geographical locations has been reported (Knudsen, 2002; Jhumur et al., 2008). Although the evolutionary significance of this variability in mosquito–plant interactions is not known, genetic drift or natural selection may account for such differences (Tollsten and Bergström, 2008).
5. Potential application of phytochemicals in mosquito vector management
Effective control of disease vectors often depends on several complementary approaches that combine to form an integrated vector management strategy. Despite many years of concerted efforts to control vector-borne diseases, the burden associated with these diseases still remains unacceptably high (The malERA Consultative Group on Monitoring, 2011). Of particular concern includes malaria and arboviral diseases transmitted by various mosquito species. This has prompted the need to develop new control strategies with the exploitation of vector ecology seen as a viable target for new and more environmentally friendly control tools (Ferguson et al., 2010; Govella and Ferguson, 2012). In addition to application as general toxicants against immature mosquitoes, phytochemicals may also have potential uses as repellents, larvicidal, ovicidal and oviposition deterrents, growth and reproduction inhibitors as well as attractants (Rajkumar and Jebanesan, 2005; Pushpanathan et al., 2006; Foster, 2008; Stone and Foster, 2013).
Recent advances in the knowledge of chemical mediation in mosquito–plant interactions presents a unique opportunity for development of new vector control tools. Unlike vertebrate host odour baited traps which target mainly blood seeking female mosquitoes, traps baited with plant volatile compounds have the potential of attracting both male and female mosquitoes of diverse age groups and varying gonotrophic stages (Foster, 2008). Besides their potential for deployment as attractive lures for surveillance of mosquito population, phytochemical attractants can also be used in mass trapping in control operations. This would particularly add more arsenal to vector control strategies with the recent discovery of outdoor biting fractions of An. gambiae which has sustained the transmission of malaria even in communities where use of insecticide treated bed nets is high (Riehle et al., 2011; Zhou et al., 2011).
Studies have shown that spraying of vegetations around water bodies with attractive toxic sugar baits can reduce mosquito populations by up to 98% (Schlein and Müller, 2008; Müller et al., 2010a; Beier et al., 2012). Furthermore, knowledge of the semiochemicals mediating mosquito–plant interaction can be utilized in luring mosquitoes into traps baited with highly selective insecticides or entomopathogenic agents such as fungi and viruses. The potential of this approach in integrated vector management is exemplified in Culex quinquefasciatus oviposition pheromone ((5R,6S)-acetoxy-5-hexadecanolide) which when combined with the insect growth regulator pyriproxifen resulted in increased oviposition accompanied by killing of the emerging larvae (Agelopoulos et al., 1999; Mboera et al., 2000).
Furthermore, advances in the biochemical pathways of semiochemical production, perception and processing as well as molecular characterization of odour receptors in mosquitoes should open avenues for molecular approaches that promise to spearhead a new wave of research to establish the role of semiochemicals in the future of integrated vector management (Renwick, 2009). Efforts to identify mosquito odour binding proteins for both vertebrate and plant hosts would provide a starting point for the potential use of odour binding proteins as targets to interfere with mosquito host location (Zhou et al., 2010). Such non-insecticidal approaches could play an important role as part of integrated vector management strategies and broaden the arsenal of available tools for disease vector control.
It is however worth noting that semiochemicals alone might not be sufficient as a control tool against mosquitoes, but their use can be maximized through integration with other existing mosquito vector control strategies which can provide a powerful tool that can help reduce and even eliminate vector populations.
Acknowledgments
We are very grateful to International Centre of Insect Physiology and Ecology for the core support provided in writing this review and to the U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) grant R01A1077722 for partial financial support for this work.
References
- Agelopoulos N, Birkett MA, Hick AJ, Hooper AM, Pickett JA, Pow EM, Smart LE, Smiley DW, Wadhams LJ, Woodcock CM. Exploiting semiochemicals in insect control. Pestic Sci. 1999;55:225–235. [Google Scholar]
- Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae sl and Anopheles funestus (Diptera: Culicidae) in western Kenya. J Med Entomol. 1996;33:613–618. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malaria J. 2012;11:1–7. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley MD, McDaniel IN, Davis EE. Studies of 4-methylcyclohexano: an Aedes triseriatus (Diptera: Culicidae) oviposition attractant. J Med Entomol. 1982;19:589–592. doi: 10.1093/jmedent/19.5.589. [DOI] [PubMed] [Google Scholar]
- Berkov A, Meurer-Grimes B, Purzycki KL. Do lecythidaceae specialists (Coleoptera, Cerambycidae) shun fetid tree species? Biotropica. 2000;32:440–451. [Google Scholar]
- Bowen M. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J Med Entomol. 1992a;29:843–849. doi: 10.1093/jmedent/29.5.843. [DOI] [PubMed] [Google Scholar]
- Bowen M. Terpene-sensitive receptors in female Culex pipiens mosquitoes: electrophysiology and behaviour. J Insect Physiol. 1992b;38:759–764. [Google Scholar]
- Briegel H, Horler E. Multiple blood meals as a reproductive strategy in Anopheles (Diptera: Culicidae) J Med Entomol. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Cork A. Electrophysiological and behavioral responses of female Helicoverpa armigera to compounds identified in flowers of African marigold, Tagetes erecta. J Chem Ecol. 2001;27:1119–1131. doi: 10.1023/a:1010359811418. [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Pickett JA. Perception of plant volatile blends by herbivorous insects – finding the right mix. Phytochemistry. 2011;72:1605–1611. doi: 10.1016/j.phytochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Wadhams LJ, Woodcock CM. Insect host location: a volatile situation. Trends Plant Sci. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Davis EE. A receptor sensitive to oviposition site attractants on the antennae of the mosquito, Aedes aegypti. J Insect Physiol. 1976;22:1371–1376. doi: 10.1016/0022-1910(76)90160-8. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Burkhardt D, Weißbecker B, Jürgens A, Schütz S, Mosandl A. Linalool and lilac aldehyde/alcohol in flower scents: electrophysiological detection of lilac aldehyde stereoisomers by a moth. J Chromatogr A. 2006;1113:231–238. doi: 10.1016/j.chroma.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Ann Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Foster WA. Phytochemicals as population sampling lures. J Am Mosq Control Assoc. 2008;24:138–146. doi: 10.2987/8756-971X(2008)24[138:PAPSL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gols R, Veenemans C, Potting RPJ, Smid HM, Dicke M, Harvey JA, Bukovinsky T. Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim Behav. 2012;83:1231–1242. [Google Scholar]
- Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3:199. doi: 10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Müller G, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS ONE. 2011;6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB. A Guide to Modern Techniques of Plant Analysis. 3. Chapman and Hall; London, UK: 1998. Phytochemical Methods. [Google Scholar]
- Healy T, Jepson P. The location of floral nectar sources by mosquitoes: the long-range responses of Anopheles arabiensis Patton (Diptera: Culicidae) to Achillea millefolium flowers and isolated floral odour. Bull Entomol Res. 1988;78:651–657. [Google Scholar]
- Honda K, Ômura H, Hayashi N. Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly, Pieris rapae. J Chem Ecol. 1998;24:2167–2180. [Google Scholar]
- Jaenson TG, Ameneshewa B. Prehibernation diet and reproductive condition of female Anopheles messeae in Sweden. Med Vet Entomol. 1991;5:243. doi: 10.1111/j.1365-2915.1991.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen HB, Olsen CE. Influence of climatic factors on emission of flower volatiles in situ. Planta. 1994;192:365–371. [Google Scholar]
- Jepson P, Healy T. The location of floral nectar sources by mosquitoes: an advanced bioassay for volatile plant odours and initial studies with Aedes aegypti (L.) (Diptera: Culicidae) Bull Entomol Res. 1988;78:641–650. [Google Scholar]
- Jhumur US, Dötterl S, Jürgens A. Electrophysiological and behavioural responses of mosquitoes to volatiles of Silene otites (Caryophyllaceae) Arthropod–Plant Interact. 2007;1:245–254. [Google Scholar]
- Jhumur US, Dötterl S, Jürgens A. Floral odors of Silene otites: their variability and attractiveness to mosquitoes. J Chem Ecol. 2008;34:14–25. doi: 10.1007/s10886-007-9392-0. [DOI] [PubMed] [Google Scholar]
- Joseph SR. Fruit feeding of mosquitoes in nature. Proc NJ Mosq Exterm Assoc. 1970;57:125–131. [Google Scholar]
- Knudsen JT. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon. Am J Bot. 2002;89:1772–1778. doi: 10.3732/ajb.89.11.1772. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Tollsten L, Bergström LG. Floral scents – a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Kunst L, Samuels A. Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res. 2003;42:51–80. doi: 10.1016/s0163-7827(02)00045-0. [DOI] [PubMed] [Google Scholar]
- Lacher V. Elektrophysiologische Untersuchungen an einzelnen Geruchsre-zeptoren auf den antennen weiblicher Moskitos (Aëdes aegypti L.) J Insect Physiol. 1967;13:1461–1470. doi: 10.1016/0022-1910(67)90171-0. [DOI] [PubMed] [Google Scholar]
- Lalel H, Singh Z, Tan S. The role of ethylene in mango fruit aroma volatiles biosynthesis. J Hortic Sci Biotechnol. 2003;78:485–496. [Google Scholar]
- Lichtenthaler HK, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Conn J. Fecundity, parity, and adult feeding relationships among Nyssorhynchus malaria vectors from venezuela. Mem Inst Oswaldo Cruz. 1991;86:57–66. doi: 10.1590/s0074-02761991000100010. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. Nectar feeding by Aedes sollicitans and its relation to gonotrophic activity. Environ Entomol. 1977;6:237–242. [Google Scholar]
- Magnarelli LA. Nectar-feeding by female mosquitoes and its relation to follicular development and parity. J Med Entomol. 1978;14:527–530. doi: 10.1093/jmedent/14.5.527. [DOI] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malaria J. 2007;6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ibarra JA, Rodriguez MH, Arredondo-Jimenez JI, Yuval B. Influence of plant abundance on nectar feeding by Aedes aegypti (Diptera: Culicidae) in southern Mexico. J Med Entomol. 1997;34:589–593. doi: 10.1093/jmedent/34.6.589. [DOI] [PubMed] [Google Scholar]
- Mauer DJ, Rowley WA. Attraction of Culex pipiens pipiens (Diptera: Culicidae) to flower volatiles. J Med Entomol. 1999;36:503–507. doi: 10.1093/jmedent/36.4.503. [DOI] [PubMed] [Google Scholar]
- Mboera LEG, Takken W, Mdira K, Pickett J. Sampling gravid Culex quinquefasciatus (Diptera: Culicidae) in Tanzania with traps baited with synthetic oviposition pheromone and grass infusions. J Med Entomol. 2000;37:172–176. doi: 10.1603/0022-2585-37.1.172. [DOI] [PubMed] [Google Scholar]
- Millar JG, Sims JJ. Preparation, cleanup, and preliminary fractionation of extracts. In: Miller JG, Haynes KF, editors. Methods in Chemical Ecololgy. Kluwer Academic Publishers; Boston: 1998. pp. 1–37. [Google Scholar]
- Müller G, Junnila A, Qualls W, Revay E, Kline D, Allan S, Schlein Y, Xue RD. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. J Med Vet Entomol. 2010a;24:346–351. doi: 10.1111/j.1365-2915.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malaria J. 2010b;9:262. doi: 10.1186/1475-2875-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Xue RD, Schlein Y. Seed pods of the carob tree Ceratonia siliqua are a favored sugar source for the mosquito Aedes albopictus in coastal Israel. Acta Trop. 2010c;116:235–239. doi: 10.1016/j.actatropica.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Müller GC, Xue RD, Schlein Y. Differential attraction of Aedes albopictus in the field to flowers, fruits and honeydew. Acta Trop. 2011;118:45–49. doi: 10.1016/j.actatropica.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Najar-Rodriguez A, Galizia C, Stierle J, Dorn S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J Exp Biol. 2010;213:3388–3397. doi: 10.1242/jeb.046284. [DOI] [PubMed] [Google Scholar]
- Nayar J, Sauerman D. The effects of diet on life-span, fecundity and flight potential of Aedes taeniorhynchus adults. J Med Entomol. 1971;8:506–513. doi: 10.1093/jmedent/8.5.506. [DOI] [PubMed] [Google Scholar]
- Nyasembe VO, Teal PEA, Mukabana WR, Tumlinson JH, Torto B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit Vectors. 2012;5:234. doi: 10.1186/1756-3305-5-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA. Analysisand optimization of a synthetic milkweed floral attractant for mosquitoes. J Chem Ecol. 2012:1–9. doi: 10.1007/s10886-012-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121:325–332. [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Pushpanathan T, Jebanesan A, Govindarajan M. Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae) Trop Biomed. 2006;23:208–212. [PubMed] [Google Scholar]
- Rajkumar S, Jebanesan A. Oviposition deterrent and skin repellent activities of Solanum trilobatum leaf extract against the malarial vector Anopheles stephensi. J Insect Sci. 2005;5 doi: 10.1093/jis/5.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Meyer RP, Milby MM. Patterns of fructose feeding by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 1986;23:366–373. doi: 10.1093/jmedent/23.4.366. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Guelbeogo WM, Gneme A, Eiglmeier K, Holm I, Bischoff E, Garnier T, Snyder GM, Li X, Markianos K. A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science. 2011;331:596–598. doi: 10.1126/science.1196759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm H, Price R. Field observations on the nectar feeding habits of some Minnesota mosquitoes. Mosq News. 1962;22:346–349. [Google Scholar]
- Sanz C, Olias JM, Perez A. Proc-Phytochem Soc Europe. Oxford University Press Inc; 1996. Aroma biochemistry of fruits and vegetables; pp. 125–156. [Google Scholar]
- Schiestl F, Ayasse M, Paulus H, Löfstedt C, Hansson B, Ibarra F, Francke W. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): patterns of hydrocarbons as the key mechanism for pollination by sexual deception. J Comp Physiol A: Neur Sens Neural Behav Physiol. 2000;186:567–574. doi: 10.1007/s003590000112. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M. Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximising reproductive success? Oecologia. 2001;126:531–534. doi: 10.1007/s004420000552. [DOI] [PubMed] [Google Scholar]
- Schlein Y, Müller GC. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordanis trees against Culex pipiens. J Med Entomol. 2008;45:384–390. doi: 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schwab W, Davidovich-Rikanati R, Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001;125 doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol Genom. 2009;39:202–209. doi: 10.1152/physiolgenomics.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Foster WA. Plant-sugar feeding and vectorial capacity. In: Takken W, Koenraadt CJM, editors. Ecology of Parasite–Vector Interactions. Wageningen Academic Publishers; Wageningen: 2013. pp. 35–79. [Google Scholar]
- The malERA Consultative Group on Monitoring, and Surveillance. A research agenda for malaria eradication: monitoring, evaluation, and surveillance. PLoS Med. 2011;8:e1000400. doi: 10.1371/journal.pmed.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Röse US, Schnitzler JP. Practical approaches to plant volatile analysis. Plant J. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- Tollsten L, Bergström LG. Fragrance chemotypes of Platanthera (Orchidaceae) – the result of adaptation to pollinating moths? Nordic J Bot. 2008;13:607–613. [Google Scholar]
- Van Handel E, Day J. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J Am Mosq Control Assoc. 1988;4:549–550. [PubMed] [Google Scholar]
- Van Handel E, Edman JD, Day JF, Scott TW, Clark CG, Reiter P, Lynn HC. Plant-sugar, glycogen, and lipid assay of Aedes aegypti collected in urban Puerto Rico and Rural Florida. J Am Mosq Control Assoc. 1994;10:149–153. [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol. 2001;27:1911–1928. doi: 10.1023/a:1012213116515. [DOI] [PubMed] [Google Scholar]
- Vargo AM, Foster WA. Responsiveness of female Aedes aegypti (Diptera: Culicidae) to flower extracts. J Med Entomol. 1982;19:710–718. [Google Scholar]
- Visser J. Host odor perception in phytophagous insects. Annu Rev Entomol. 1986;31:121–144. [Google Scholar]
- Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, Himeidan YE, Minakawa N, Githeko AK, Yan G. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS ONE. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]