Abstract
Mitotically active progenitor cells from the anterior portion of the forebrain subventricular zone (SVZa), which give rise throughout life to olfactory bulb interneurons, bear processes and express neuronal markers. To understand how rodent SVZa neuronal progenitors coordinate division and process formation, we used time-lapse videomicroscopy to analyse the proliferative behavior of SVZa progenitors in dissociated cell culture continuously for up to five generations. The cell cycle time of these cultured SVZa cells assessed videomicroscopically (cytokinesis to cytokinesis) was similar to the cell cycle time along the rostral migratory stream in vivo (14–17 h). The relationship between process extension, process retraction and cytokinesis was assessed quantitatively for 120 cells undergoing cytokinesis. Although all of these cells had elaborated processes, virtually all of them completely withdrew their processes prior to cytokinesis. Process withdrawal was rapid and tightly coupled to cytokinesis; 50% of the cells studied initiated process retraction within 30 min of cytokinesis and 96% had begun to withdraw their processes within 60 min of cytokinesis. In SVZa progenitor cell lineages, the sequence of process extension, process retraction and division is repeated over multiple generations. This complete withdrawal of processes prior to division differentiates SVZa progenitor cells from the characteristics reported for several other process-bearing types of neural progenitor cells, including sympathetic neuroblasts, cerebral cortical radial glia, and cerebellar and retinal progenitors. Collectively, our findings indicate that SVZa progenitors employ different cellular mechanisms than other neural progenitors to regulate proliferation and differentiation.
Keywords: cell cycle, neurogenesis, proliferation, Rattus norvegicus, rostral migratory stream, videomicroscopy
Introduction
The GABAergic granule and periglomerular cell interneurons of the olfactory bulb are produced and functionally integrated into the existing olfactory bulb circuitry throughout life (Luskin, 1993; Lois & Alvarez-Buylla, 1994; Doetsch & Hen, 2005). In the neonate, these neurons derive from neuronally restricted progenitor cells concentrated in the anterior portion of the forebrain subventricular zone (SVZa) (see Falls & Luskin, 2005). Mitosis within the SVZa continuously produces new progenitor cells, some of which leave the SVZa and migrate to the olfactory bulb along a well-defined pathway known as the rostral migratory stream (RMS) (Fig. 1A). While en route to the olfactory bulb, SVZa-derived cells divide, display leading and trailing processes, and express proteins characteristic of postmitotic neurons such as neuron-specific tubulin (recognized by the antibody TuJ1; Menezes et al., 1995; Zigova et al., 1996), microtubule-associated protein 2 (Pencea & Luskin, 2003) and doublecortin (Gleeson et al., 1999). Cells in the SVZa and along the RMS have been demonstrated to exhibit these neuronal characteristics virtually immediately after incorporating the thymidine analog bromodeoxyuridine (BrdU) during the S-phase of the mitotic cycle, suggesting that the progenitor cells of olfactory bulb interneurons retain the capacity for cell division after they initiate neuronal differentiation and process formation (Luskin, 1993; Menezes et al., 1995; Luskin et al., 1997; Coskun & Luskin, 2001; Kornack & Rakic, 2001a; Pencea et al., 2001).
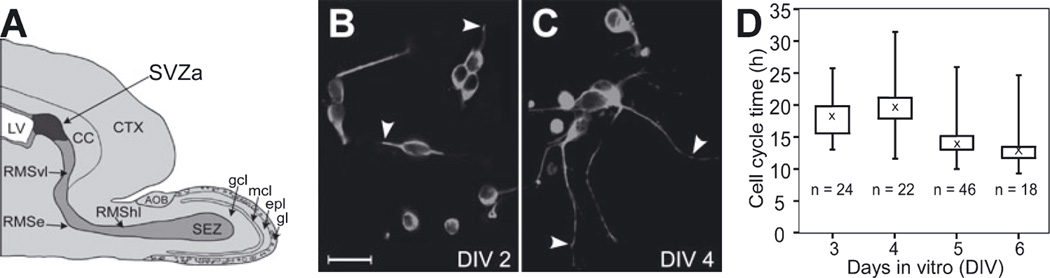
Fig. 1.
Expression of neuron-specific markers and division rate of neonatal anterior subventricular zone (SVZa) progenitor cells in culture. (A) Diagram of a parasagittal section of the P1 rat forebrain depicting the SVZa (dark gray), the beginning of the rostral migratory stream (RMS; light gray) that was microdissected, dissociated and cultured for immunohistochemical and videomicroscopic analysis. Other subdivisions of the RMS [vertical limb of the RMS (RMSvl), elbow of the RMS (RMSe), horizontal limb of the RMS (RMShl) and subependymal zone (SEZ)] as well as the layers of the olfactory bulb are also indicated. SVZa-derived progenitor cells are fated to become the granule and periglomerular cell interneurons of the olfactory bulb. To reach their destination, SVZa-derived progenitor cells migrate along the RMS to the SEZ. At the SEZ they exit from the RMS and migrate radially into the overlying granule cell and glomerular layers of the olfactory bulb. Anterior is to the right and dorsal is up. AOB, accessory olfactory bulb; CC, corpus callosum; CTX, cerebral cortex; epl, external plexiform layer; gcl, granule cell layer; gl, glomerular layer; LV, lateral ventricle; mcl, mitral cell layer. (B and C) Representative fluorescent photomicrographs of SVZa progenitor cells cultured in serum-free medium for 2 (B) or 4 (C) DIV and then double-labeled with an antibody to neuron-specific tubulin (TuJ1) and an antibody to glial fibrillary acidic protein (GFAP); DAPI was used to label nuclei (not shown). In the fields shown all cells were TuJ1-positive and GFAP-negative. The images shown were captured using fluorescein optics to visualize TuJ1. The results are consistent with our previous analysis of SVZa progenitor cells cultured in serum-containing medium demonstrating that nearly all cultured neonatal SVZa progenitor cells were TuJ1-positive and GFAP-negative (see Luskin et al., 1997). As shown here, at 2 DIV, the majority of the TuJ1-positive cells have extended one or two processes (e.g. arrowheads in B) and, at 4 DIV, TuJ1-positive cells are observed with processes that are longer and more branched (e.g. arrowheads in C). Collectively, these findings demonstrate that the SVZa progenitor cells retain a neuronal phenotype and undergo differentiation in culture. (D) The distribution of cell cycle times for cultured SVZa neuronal progenitor cells (n = 110 cell cycles) as a function of the DIV. The data are presented as box plots. The median value (50th percentile) is marked by an ‘X’. The top and bottom of the box indicate the 75th and 25th percentiles, respectively. The horizontal bars above and below the box represent the maximum and minimum values observed. The DIV (X-axis) to which a cell cycle time is assigned is the day on which the cell under consideration divided. At 1 DIV, the cells were allowed to equilibrate in the incubator. 2 DIV was the first day on which videomicroscopic images were recorded and 3 DIV was the first day for which sufficient numbers of cell cycles ended to allow meaningful display of cell cycle time distribution. The median cell cycle times observed in this culture are similar to the cell cycle times measured in vivo for the neonatal RMS (Smith & Luskin, 1998). Scale bar, 10 µm in B (also applies to C).
The question of whether process-bearing SVZa-derived cells go on to divide can best be answered by directly analysing the proliferative and morphological behavior of living, identified SVZa progenitors and their progeny over time using time-lapse videomicroscopy. This procedure overcomes the limitation of BrdU incorporation studies, both in vivo (Luskin, 1993; Menezes et al., 1995; Luskin et al., 1997; Smith & Luskin, 1998; Coskun & Luskin, 2001; Kornack & Rakic, 2001a; Pencea et al., 2001) and in vitro (Luskin et al., 1997), because such studies cannot directly demonstrate that SVZa-derived cells had processes prior to incorporating BrdU. For these experiments we adapted a culture system that we had previously established and characterized (Luskin et al., 1997; Stewart et al., 1999, 2002). We chose to analyse dissociated cultured cells rather than cells in a slice culture or in living animals because in slices or living animals the movement of cells throughout the width of the RMS, and thus out of the plane of focus, would preclude successfully following a large number of identified cells and their progeny over several days. Tracking living cells in vitro by videomicroscopy, in many cases over multiple generations of a lineage, allowed us to investigate whether processes remain extended at the time of division or are withdrawn prior to division.
Materials and methods
SVZa progenitor cell cultures
All animal procedures were approved by the Emory University Institutional Animal Care and Use Committee. Sprague-Dawley postnatal day 1 P1 rat pups were anesthetized by hypothermia, decapitated and their brains removed. Each hemisphere was cut in the sagittal plane approximately 1.0 mm lateral to the midline; this cut extended through the middle of the olfactory bulb. The tissue lateral to the cut was discarded. From the medial forebrain slices (i.e. left and right hemisphere slices) the region of the SVZa (Fig. 1A) was isolated and processed as previously described (Luskin et al., 1997; Zigova & Newman, 2002). The SVZa can be identified by the transparency of the SVZa tissue relative to surrounding structures. Care was taken to avoid the overlying corpus callosum or underlying striatal and septal tissue during the dissections. SVZa progenitor cells were dissociated using trypsin (Gibco, Invitrogen, Carlsbad, CA, USA; cat. no. 15090; 0.1% w/v) and DNase I (Roche Scientific, Indianapolis, IN, USA; cat. no. 10104159001; 0.1 and 0.4 mg/mL). Dissociated SVZa progenitor cells were plated at a density of 104–106 cells/mL in ‘flaskettes’ (Nunc, Rochester, NY, USA; cat. no. 177453) in a serum-free medium containing neurobasal medium (Gibco; cat. no. 10888), B-27 supplement (Gibco; cat. no. 17504; 2% v/v), l-glutamine (Gibco; cat. no. 25030; 2 mm), penicillin (100 U/mL), streptomycin (Gibco; cat. no. 15140; 100 ng/mL), basic fibroblast growth factor (bFGF; Upstate Biotechnology, Waltham, MA, USA; cat. no. 01–106; 20 ng/mL) and epidermal growth factor (EGF; Upstate Biotechnology; cat. no. 01–102; 20 ng/mL). The growing surface of the flaskette is a glass slide, which was coated with poly-d-lysine (Sigma, St Louis, MO, USA; cat. no. P7280; 10 µg/mL in water) prior to plating the cells.
After plating, the flaskettes were placed overnight in the incubator (37 °C, 5% CO2) with their caps loosely applied to permit air exchange between the flask environment and that of the incubator. The next afternoon, designated as the beginning of the second day in vitro (DIV), the caps were closed tightly and the flaskettes were placed on the stage of a Zeiss Axiovert microscope equipped for videomicros-copy. The stage, condenser and lenses of this microscope were enclosed in a temperature-controlled chamber, which allowed the temperature of the flaskette to be maintained at 37 °C throughout the period of videomicroscopic imaging (1–6 days).
Immunocytochemistry
In order to determine the phenotype of cultured SVZa progenitor cells, cultures were labeled after 2 or 4 days in the incubator with cell-type-specific antibodies. The cultures were fixed with 4% paraformaldehyde in 0.1 m phosphate-buffered saline (100 mm sodium phosphate, 150 mm NaCl, pH 7.4) for 10 min, rinsed in phosphate-buffered saline and then incubated with blocking solution (10% v/v normal goat serum and 0.01% v/v Triton X-100 in phosphate-buffered saline) for 1 h. Subsequently, the sections were double-labeled using a mouse antibody to neuron-specific class III β-tubulin (also known as TuJ1 antibody; Promega, Madison, WI, USA, clone 5G8) to identify neurons and a rabbit antibody to glial fibrillary acidic protein (Dako, Denmark; cat. no. Z0334) to identify astrocytes at a dilution of 1 : 4000 and 1 : 5000, respectively, in blocking solution for 1 h at 4 °C. The cultures were then incubated in a cocktail of fluorescein-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; cat. no. 115-095-003) and rhodamine-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories; cat. no. 111-026-045) secondary antibodies, each at a dilution of 1 : 500 in blocking solution, incubated in DNA dye DAPI (4′6-diamidino-2-phenylindole 2 HCL) for 10 min and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA, USA; cat. no. H-1000). The slides were examined on a Zeiss Axiophot microscope and epifluores-cence images were captured using an MTI Dage DC330 CCD camera and iplab software (Scanalytics, Fairfax, VA, USA).
Time-lapse videomicroscopy
Cultures were imaged with a 20× long-working-distance phase objective. Four to 10 fields were selected from each flask for videomicroscopic analysis. Using a computer-controlled motorized stage, images were captured from each field every 20 or 60 s for up to 6 days with an MTI Dage CCD camera (100 ms exposure time) and saved as sequential jpeg files. The camera and stage were controlled by our custom-written software, which time-stamped each image with the acquisition date and time. Sequential images were then imported into iplab (Scanalytics; Release 4.6.1) or premier (Adobe, San Jose, CA, USA; Version 6.01) as a time sequence, compiled and exported as avi or mpeg format movies. To prepare Supplementary material, Videos S1 and S2, the original full-field images were cropped around the cells of interest. The interactive controls of supplementary Video S2 were created using Macromedia Flash MX 2004 (www.macromedia.com). The images shown in Figs 2–4 and 6 were digitally masked to remove floating debris and thus improve image clarity. The cell shown in Fig. 4 is also shown in supplementary Videos S1 and S2 (Cell A); the movie images were not processed to remove debris.
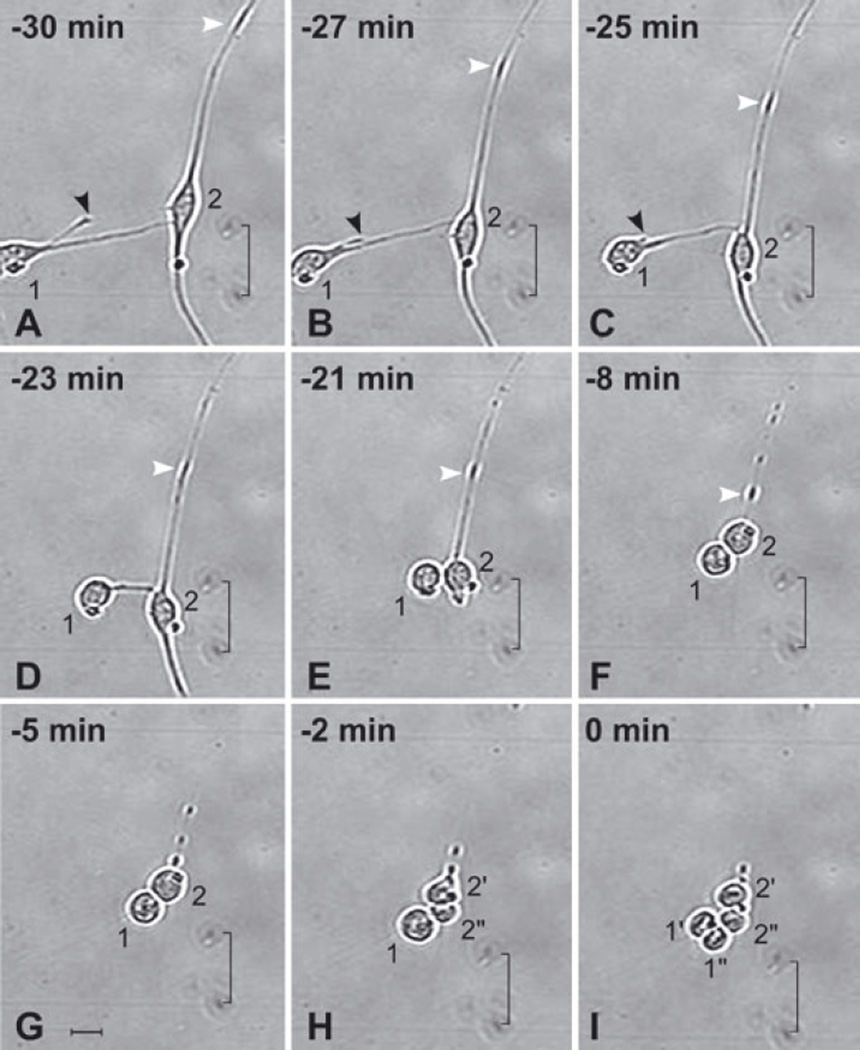
Fig. 2.
Anterior subventricular zone (SVZa) neuronal progenitor cells retract their processes prior to cytokinesis. A 30 min series of time-lapse videomicroscopic photomicrographs of two SVZa neuronal progenitor cells beginning 5 DIV. The time that Cell no. 1 undergoes cytokinesis is considered t = 0. The position of each cell is visualized relative to marks on the underside of the growing surface (indicated by a bracket). The behavior of Cell no. 1 is described first and then Cell no. 2. (i) Cell no. 1. (A–C) At t = −30 min (A), Cell no. 1 has relatively long processes. Between 30 and 25 min prior to cytokinesis, Cell no. 1 retracts its shorter process (black arrowhead). (C–E) Between 25 and 21 min prior to cytokinesis, the longer process of Cell no. 1 (the process contacting Cell no. 2) retracts. Concurrently, the soma of Cell no. 1 moves towards Cell no. 2. (C–F) Cell no. 1 rounds up. (G and H) Cell no. 1 remains in contact with Cell no. 2 and, after Cell no. 2 divides, with its progeny. (I) At t = 0 min, Cell no. 1 divides, generating two daughter cells (labeled 1′ and 1″). (ii) Cell no. 2. (A–G) From t = −30 to t = −5 min, Cell no. 2 retracts both of its processes. Note that the shorter process (below the soma) retracts more quickly (see E) than the longer process (above the soma). The longer process is in contact with the process of another cell (the soma of which is located beyond the edge of the image at the upper right) giving the appearance of a single long process. The change in the position of an enlarged dense segment of the longer process of Cell no. 2 (white arrowhead in A–F) is indicative of the retraction of the process towards its soma. While Cell no. 2 is withdrawing its processes, its somatic morphology is transforming from elongated (A–C) to round (D–G). (H and I) At t = −2 min, Cell no. 2 undergoes cytokinesis, generating two daughter cells (labeled 2′ and 2″). Note that, although Cell no. 1 completes the retraction of its processes before Cell no. 2 (D–F), Cell no. 2 divides first (compare H with I). The alignment of the chromosomes is evident in the daughter cells of both Cell no. 1 (1′ and 1‴) and Cell no. 2 (2′ and 2″). See also supplementary Video S1, which shows the dynamics of process retraction of other cells. Scale bar, 20 µm in G (applies to all panels).
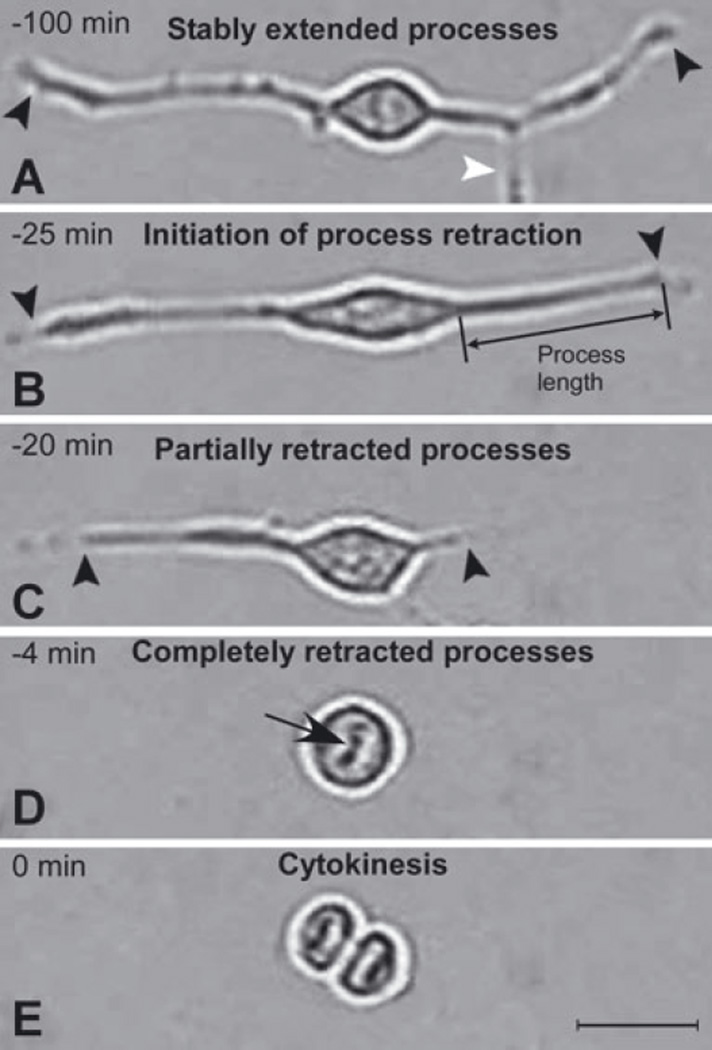
Fig. 4.
Stages of process retraction and cell cycle progression. Images of a typical dividing SVZa progenitor cell are shown in A–E to illustrate the operational definitions used to determine the progression of process retraction relative to cytokinesis. Black arrowheads in A–C indicate the ends of the cell’s processes and the white arrowhead in A marks a process of another cell, the soma of which is outside the field. The images shown are of a cell in a 5 DIV culture but are representative of cells at earlier and later times in culture. The original videomicroscopic data from which these static images are taken are presented in supplementary Videos S1 and S2 (Cell A). (A) Stably extended processes. For at least 75 min prior to cytokinesis, the length of the processes was relatively stable (< 11% variation in length; compare A with B). (B) Initiation of process retraction. Processes were measured along a straight line from the base of the growth cone to the junction of the process with the soma. At 25 min prior to cytokinesis the processes began to rapidly withdraw, a distinct change in behavior that is defined as ‘initiation of process retraction’. (C) Partially retracted processes. By 20 min prior to cytokinesis the processes are considerably shortened. (D) Completely retracted processes. The time of ‘complete retraction’ is defined as the first time at which an uninterrupted phase-bright ‘halo’ surrounds the cell soma. In contrast, in cells with processes extended there is a break in the halo at the base of processes (e.g. see B and C). Withdrawal of processes continued until they had completely retracted, in this example by 4 min prior to cytokinesis. Note also the chromosomes lined up at the metaphase plate (black arrow; see also supplementary Videos S1 and S2). (E) Cytokinesis. Cytokinesis is defined as the first time at which there is a distinct phase-bright partition separating the two daughter cells. The condensed, aligned chromosomes in the daughter cells can be seen in this image (see also Fig. 2I). Scale bar, 20 µm in E (applies to all panels).
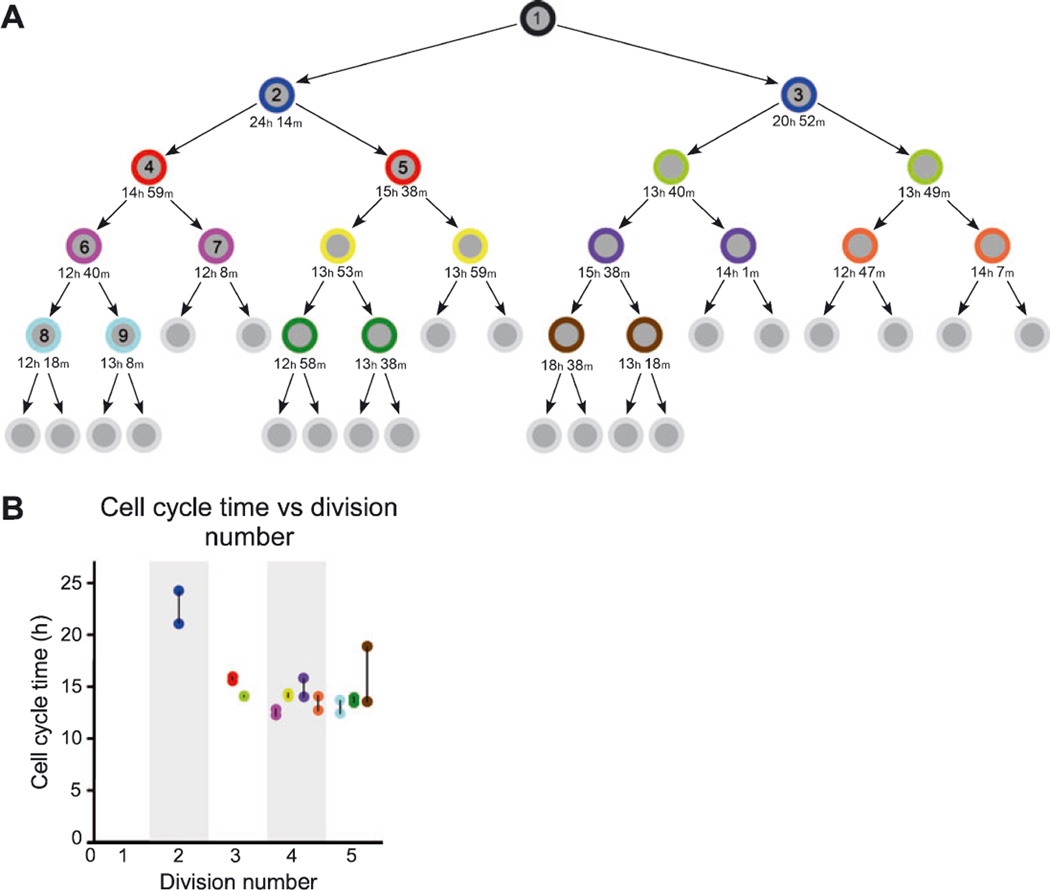
Fig. 6.
Lineage diagram depicting the clonal progeny of an SVZa progenitor cell and the cell cycle time over five successive generations. (A) The first cytokinesis observed in this lineage, the division of Cell no. 1 generating Cells no. 2 and no. 3, occurred on 3 DIV. The last cytokinesis of this lineage that could be tracked occurred on 6 DIV. Sibling cells are represented by an identically colored outer ring. The cell cycle length for individual cells is stated below the cell in hours (h) and minutes (m). The cell cycle time is the interval from the cell’s birth to its subsequent division (cytokinesis to cytokinesis). The cells not assigned a cell cycle time may have continued to divide but could not be followed for technical reasons. The lineage diagram with individual cell cycle times extends over five successive divisions. Note that many of the sibling cells have nearly identical cell cycle times (e.g. see yellow sibling pair in fourth row). The cells that are numbered on the left half of the lineage tree (i.e. Cell nos 1–9) are further analysed in Fig. 7. (B) Graph showing the cell cycle time as a function of the division number for the clone depicted in A. Cells are represented with the same color as used in A and the siblings pairs are linked together with a black line. The near identity of cell cycle times for several sibling pairs (causing some dots to overlap) suggests that the control of cell cycle time may be regulated by an autonomous timing mechanism.
Quantitative analysis of time-lapse videomicroscopic data: process retraction and extension dynamics
xtrack (Mehes et al., 2002) and iplab were used in the analysis of videomicroscopic image sequences. The xtrack and iplab software allow an investigator to cycle back-and-forth through the movie files frame-by-frame (minute-by-minute), facilitating the accurate determination of event time. All times were normalized to cytokinesis (t = 0). Process lengths were measured directly in iplab using the ‘measure length’ function, which was calibrated to the images (image size, 640 × 480 pixels, corresponding to an 800 × 600 µm field).
To quantitatively analyse the dynamics of process retraction and extension that accompany cell division, 120 cytokinesis events were randomly selected from movies at 4, 5 and 6 DIV. 1–3 DIVand 7 DIV were excluded from the measurements to allow cells to acclimate to the culture conditions and to avoid high cell density, respectively. For each of these 120 cells undergoing cytokinesis, five parameters were measured.
Initiation of process retraction time. Over a period of 2 or more hours prior to cytokinesis, a cell typically exhibits relatively stable process pattern and length; the cell then begins to rapidly and progressively withdraw all of its processes. This change in behavior can be timed to an accuracy of a few minutes.
Number and length of processes at the initiation of process retraction. At the time-point defined in (i), all of the processes of a cell were counted and their length determined. Process length was measured along a straight line from the base of the growth cone (at the tip of the process) to the junction of the process with the cell soma (see Fig. 4B). There was no lower limit beyond which processes were excluded from the count and length determination; the shortest process that we observed in these experiments was 4.5 µm in length.
Time of complete retraction. The time of complete retraction was defined as the first time at which the cell soma was surrounded by an unbroken phase-bright halo. This can be measured to an accuracy of ±1 min.
Time of cytokinesis. The time at which a cell underwent cytokinesis was defined as the first time a phase-bright line separating the two daughters was first observed. This can be measured to an accuracy of ±1 min.
Time of process extension. The time at which a cell initiated process extension was defined as the first time following cytokinesis at which the phase-bright halo surrounding the cell soma was interrupted by newly formed processes. This can be measured to an accuracy of ± 1 min.
Analysis of time-lapse videomicroscopic data: clonal analysis
To assess process extension and retraction during sequential cell cycles of lineally related cells, the clonal expansion of SVZa progenitor cells was analysed by tracking their progeny for 1–6 DIV. Tracking of cells was performed by visual observation of image sequences as described above. In some cases the cells were semiautomatically followed in xtrack by attaching a number to the cell, which was propagated from frame to frame. Lineage trees (e.g. Fig. 6 and supplementary Fig. S1) were constructed for each clone. Tracking was performed forwards and backwards in time from each identified cytokinesis to maximize the number of lineally related cytokineses identified. The lineage trees were used to calculate the cell cycle times (times between birth cytokinesis and division cytokinesis) presented in Figs 1D and 6 and supplementary Fig. S1.
Results
In vivo, migrating SVZa-derived progenitor cells in the RMS divide, have a leading and trailing process (Luskin, 1993; Luskin & Boone, 1994; Zigova et al., 1996), and express marker proteins typical of immature postmitotic neurons (Bonfanti & Theodosis, 1994; Menezes et al., 1995; Rousselot et al., 1995; Zigova et al., 1996; Gleeson et al., 1999; Pencea & Luskin, 2003). Cells along the pathway express these neuronal characteristics shortly after incorporating BrdU, and perhaps continuously, suggesting that these cells may already have elaborated processes prior to exiting the cell cycle (Menezes et al., 1995; Luskin et al., 1997). To determine how SVZa-derived progenitor cells coordinate proliferation and process formation, we cultured SVZa progenitor cells from P1 rat pups (Fig. 1A) and analysed the proliferative behavior of individual cells and their descendants using time-lapse videomicroscopy. Cultured cells were tracked continuously for up to 6 days.
Cultured neonatal SVZa progenitor cells maintain their neuronal phenotype
In vivo the vast majority of cells in the neonatal SVZa and other regions of the RMS have a neuronal phenotype (Menezes et al., 1995; Law et al., 1999; Coskun & Luskin, 2001). We had previously demonstrated that, when neonatal SVZa progenitor cells were put in culture, virtually all of them maintained their expression of neuron-specific markers (Luskin et al., 1997; Stewart et al., 2002). For those studies SVZa progenitor cells were cultured in a medium containing 10% fetal calf serum. For the present study we used a defined serum-free medium containing FGF and EGF that supports the survival of cultured neonatal SVZa progenitor cells and that is known to promote the survival of other neural progenitor cells (Reynolds & Weiss, 1992; Craig et al., 1996; Kuhn et al., 1997; Gritti et al., 1999; Gage, 2000). To validate the neuronal phenotype of cells grown in this medium, P1 SVZa progenitor cells were analysed after 2 and 4 DIV using double-label immunocytochemistry. An antibody to neuron-specific tubulin (TuJ1) was used to identify neurons (Lee et al., 1990a,b; Luskin et al., 1997) and an antibody to GFAP was used to identify astrocytes (Bignami et al., 1972; Luskin et al., 1997). Nearly all cultured neonatal SVZa progenitor cells were TuJ1-positive (Fig. 1B and C) and GFAP-negative (not shown). This confirms and extends our previous findings that SVZa progenitor cells retain a neuronal phenotype in vitro.
Cell cycle time of neonatal SVZa progenitor cells is similar in vivo and in vitro
To characterize the mitotic behavior of the cells in the culture system we compared the cell cycle time of SVZa progenitor cells in vitro, determined by time-lapse videomicroscopy, with the reported in vivo cell cycle time of these cells, determined by using cumulative BrdU labeling (Smith & Luskin, 1998). By sealing the culture vessel and maintaining a temperature of 37 °C we were able to culture SVZa progenitors for up to 6 DIV. Cell cycle time was defined as the interval between the cytokinesis at which a cell was generated and the subsequent cytokinesis when the cell divided, giving birth to two daughter cells. The time at which cytokinesis occurred was considered to be the first appearance of a distinct border between two daughter cells in videomicroscopic image sequences (discussed more fully in Materials and methods and below). At 3,4, 5 and 6 DIV, the median length of the cell cycle was 18.2, 19.6, 13.9 and 12.8 h, respectively (Fig. 1D). For each of these days the variation was small; the difference between the cell cycle time at the 25th percentile and that at the 75th percentile (Fig. 1D) was < 15% of the median. Previous in vivo studies using cumulative BrdU labeling to determine cell cycle time (Nowakowski et al., 1989) demonstrated that neonatal SVZa-derived progenitor cells divide with an average cell cycle time of 14–17 h along the RMS (Smith & Luskin, 1998). Thus, the cell cycle times in our cultures, directly measured using videomicroscopy, are similar to the cell cycle times in vivo, assessed by cumulative BrdU labeling. Moreover, the fact that cell cycle times are fairly constant over up to 6 days in culture attests to the health of these cultures throughout the period examined.
SVZa progenitor cells retract their processes prior to division and extend processes following division
Before presenting a quantitative analysis of the dynamics of process formation in our culture system, a qualitative description of proliferating neonatal SVZa neuronal progenitor cells in vitro will be given. A representative time-lapse sequence from a 5 DIV culture is shown in Figs 2 and 3. The culture shown in these figures is typical of the cultures from our videomicroscopic experiments. The SVZa progenitor cells, which had been sealed in a flaskette and on the stage of the videomicroscope for 5 days at the time the images shown in Figs 2 and 3 were recorded, have morphologies similar to those in SVZa progenitor cell cultures maintained in the incubator (compare with Fig. 1C; see Luskin et al., 1997), suggesting that the conditions used for the videomicroscopy experiments support normal development of SVZa progenitor cells. The typical neuronal appearance of these cells, distinct from that of astrocytes (Bignami et al., 1972), is displayed by the two SVZa progenitor cells shown in Fig. 2A. However, over the course of the 30 min sequence shown in Fig. 2, both of these SVZa progenitor cells withdraw their processes and, shortly after their processes are withdrawn, they divide (see also supplementary Video S1). Thus, SVZa progenitor cells may divide after extending processes and this division can be preceded by retraction of the processes.
Fig. 3.
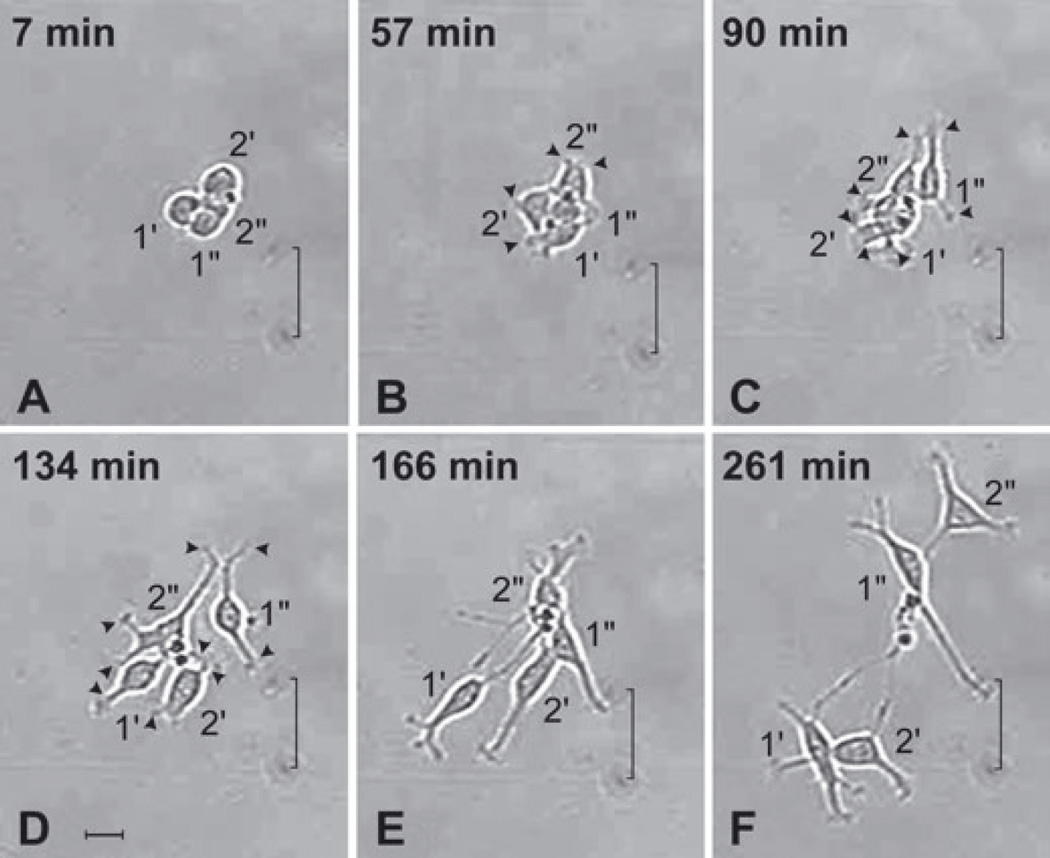
Anterior subventricular zone (SVZa) progenitor cells extend processes following cytokinesis. A series of time-lapse videomicroscopic photomicrographs of the four daughters of Cell nos 1 and 2 (continued from Fig. 2I) as they elaborate processes during the first 4.5 h following their generation. Time is measured relative to t = 0 in Fig. 2I. The position of each cell is visualized relative to marks on the underside of the growing surface (indicated by a bracket). (A) Following cytokinesis, the four newly generated cells are clustered and lack processes. (B and C) The newly generated cells initiate process extension at nearly the same time. All four cells have short processes ending in an active, broad growth cone-like structure (e.g. arrowheads). Most cells have two or more processes. (D) The cells begin to disperse by t = 134 min. At this time most of the cells have long processes (e.g. arrowheads). (E and F) The cells continue to separate from each other, form new processes and lengthen their existing processes. Together the panels of this figure demonstrate that newly generated SVZa progenitor cells begin to extend elaborate processes within a short time after division. See also supplementary Video S1, which shows the dynamics of process extension of other cells. Scale bar, 20 µm in D (applies to all panels).
The outgrowth of processes from newly generated progenitor cells is quite dynamic. Although the newly generated SVZa progenitor cells depicted in Figs 2I and 3A lacked processes, within 60 min after cytokinesis each daughter cell exhibited short processes (Fig. 3B; see also supplementary Video S1) that extended and retracted quickly from the edge of its soma at multiple locations; and within 90 min after their generation these cells had one or more processes extending up to 20 µm (Fig. 3C). Within 4.5 h, these newly generated cells each extended two or more processes, some of which were 60 µm or longer (e.g. see Cell no. 1′ in Fig. 3C – F). Immediately following cytokinesis, the newly generated siblings were round (Fig. 3A) but subsequently the cells acquired an oval or elongated soma in association with process formation (Fig. 3C – F), reminiscent of the shape of the soma of migrating cells in the RMS. Whereas, for dividing sympathetic neurons, the number and pattern of the processes on the daughter cells recapitulate the pattern of processes of the parent cell (Wolf et al., 1996), this was not the case for these dividing SVZa progenitor cells (compare Fig. 2A with 3F). In summary, SVZa-derived neurons with extensive process formation can fully withdraw their processes, divide and extend new processes following division.
In vivo, SVZa-derived progenitor cells migrate several centimeters along the RMS from the SVZa to the subependymal zone (SEZ) in the middle of the olfactory bulb. Although there is a gradient in the RMS such that proliferation is high in the SVZa and low in the SEZ (Coskun & Luskin, 2001), in vivo observations have indicated that these progenitor cells are mitotically active during their entire migration (Menezes et al., 1995). Consistent with this, the proliferative SVZa progenitor cells in culture were mobile. Sibling cells showed variable displacement from each other following cytokinesis (Fig. 3 and supplementary Video S1). Some sibling cells dispersed and some stayed together, although we could not predict which would move apart. Moreover, some sibling cells moved apart and then back together before dividing again. In a number of cases, a soma was observed to translocate along a process (supplementary Video S1). Thus, SVZa-derived progenitor cells do not necessarily remain clustered as clonally related units but instead siblings may disperse from each other. Similarly, siblings disperse in vivo as shown by the fact that clonally related SVZa-derived interneurons are found widely scattered in the olfactory bulb (Reid et al., 1999).
Dynamics of process retraction prior to cytokinesis
Our qualitative assessment of SVZa progenitor cells suggested several hypotheses: (i) all dividing SVZa progenitor cells elaborate substantial processes prior to cytokinesis; (ii) all process-bearing SVZa-derived progenitor cells fully withdraw their processes prior to dividing; and (iii) the time-course of process retraction is short relative to the cell cycle time. We tested these hypotheses by quantitative analysis of cultured SVZa progenitor cells.
Operational definitions of the events measured to address these hypotheses are schematized in Fig. 4 and videomicroscopic sequences annotated to illustrate the application of these definitions to our data are presented in supplementary Video S2. Process length and number were quantified at the initiation of process retraction prior to cytokinesis. Process length was measured along a straight line extending from the outer edge of the phase-bright halo surrounding the soma at the base of the process to the tip of the process. As processes may be curved, this straight-line measure may underestimate total process length. Although the length and morphology of processes were dynamic throughout the interphase of the mitotic cycle, prior to cytokinesis there was an evident change in behavior such that the cell rapidly and progressively withdrew all of its processes. This change in behavior could be timed to an accuracy of a few minutes. For the cell illustrated in Fig. 4, the initiation of process retraction began 25 min prior to cytokinesis (see also supplementary Video S2). Shortly following the complete retraction of processes (Fig. 4D), defined as the first time at which the soma was surrounded by an unbroken phase-bright halo, the cell underwent cytokinesis. For quantification purposes the time at which a cell underwent cytokinesis was defined as the time a phase-bright line separating the two daughters was first observed (Fig. 4E). In summary, based on morphological criteria, we could determine from the videomicroscopic image sequences the time at which process retraction began, process retraction was complete and cytokinesis occurred, and we could also determine process length at the time process retraction began.
It is worth noting that the entire sequence of morphological changes involved in cytokinesis (cell division) and in mitosis (nuclear division) occurred rapidly, as shown in Fig. 4. For the cell illustrated, the cell was spherical at t = −4 min. A distortion in the spherical shape of the parent cell was first detected 2 min later, at t = − 2 min (not shown), and cytokinesis occurred only 2 min after this (Fig. 4D). Preceding many cell divisions, the metaphase plate (alignment of condensed chromosomes) could be observed (e.g. Fig. 4D), and in cases in which the metaphase plate was observed, it was first seen after process retraction was complete and within ~10 min of cytokinesis (not shown). Even when the metaphase plate was not evident in the parent, the aligned chromosomes could often be seen in the daughters for a short period following cytokinesis (e.g. Fig. 4E). Thus, the events of SVZa-derived progenitor cell mitosis and cytokinesis discernible in the videomicroscopic sequences typically occurred within ~10 min, or ~1% of the 13–18 h cell cycle.
We identified 120 cells undergoing cytokinesis in 4, 5 and 6 DIV cultures and quantitatively analysed the dynamics of process retraction for these cells (Fig. 5A). More than 80% of these cells had at least two or three processes at the initiation of process retraction prior to cytokinesis and 98% had at least one process of a length exceeding 10 µm. Virtually all 120 SVZa progenitor cells fully retracted their processes prior to cytokinesis. For 50% of the 120 cells, the interval from initiation of process retraction to cytokinesis was 30 min or less and > 95% began process retraction within 60 min of cytokinesis (Fig. 5B). Once process retraction was complete, 50% of the cells studied divided within 12 min and 95% underwent cytokinesis within 20 min of fully retracting their processes (Fig. 5B). These data indicate that almost all 4–6 DIV SVZa progenitor cells extend processes of substantial length prior to dividing and completely and rapidly retract these processes immediately before division. We have found that this is also the case at earlier and later times in culture (Falls, Lane, Coskun & Luskin, unpublished findings).
Fig. 5.
SVZa progenitor cells retract their processes prior to cytokinesis: quantification. (A) Schematic illustrating the two time intervals assessed in the analysis of the dynamics of process retraction prior to cytokinesis. (B) Cumulative distribution of time intervals from initiation of process retraction to cytokinesis (closed squares) and from complete process retraction to cytokinesis (open circles) for 120 cells (40 cells each from 4, 5 and 6 DIV). Virtually every cell analysed retracted its processes prior to cytokinesis.
Cycle of process retraction, cytokinesis and process extension is sequentially repeated in a lineage
Although the data presented above demonstrate that SVZa neuronal progenitor cells can divide at least once after elaborating processes (Figs 2–5), these results do not address the question of whether the daughters produced remain viable or whether they can also divide. To address this issue, we attempted to track the lineage relationships of every cell undergoing division within a selected culture. In this culture, activity in 10 microscopic fields was followed; each of these fields was imaged every minute for the 6 days of the experiment. Due to aggregation of cells and to cells moving out of an imaged field, not all cells in these fields were successfully tracked for the duration of the experiment. From our observations, we were able to construct lineage trees for eight clones in which cell cycle times could be determined for cells in three or more sequential generations. For these clones, a total of 62 cells undergoing division were analysed. The results from the largest of the eight clones identified, which we tracked for five generations, are presented in Fig. 6. However, these results are representative of findings from analysis of each of the other clones (e.g. supplementary Fig. S1). The cell cycle time is shown below each of the 20 cells that were tracked in this lineage in Fig. 6A and is plotted as a function of generation (division number) in Fig. 6B. The cell cycle times observed in this individual clone are similar to the cell cycle times observed for the entire sample of cultured SVZa progenitor cells assessed (Fig. 1D).
We examined process formation and retraction for lineally related cells over five generations of the clone illustrated in Fig. 6. Following each division, each cell elaborated extensive processes and subsequently completely retracted these processes prior to its own division (Fig. 7). The time-course of process retraction and extension was consistent with that observed in the analysis of randomly selected cells undergoing division (Fig. 5). Thus, SVZa-derived progenitor cells are neuronal progenitors capable of repeatedly developing a neuron-like morphology, morphologically de-differentiating, dividing and then again developing a neuron-like morphology.
Fig. 7.
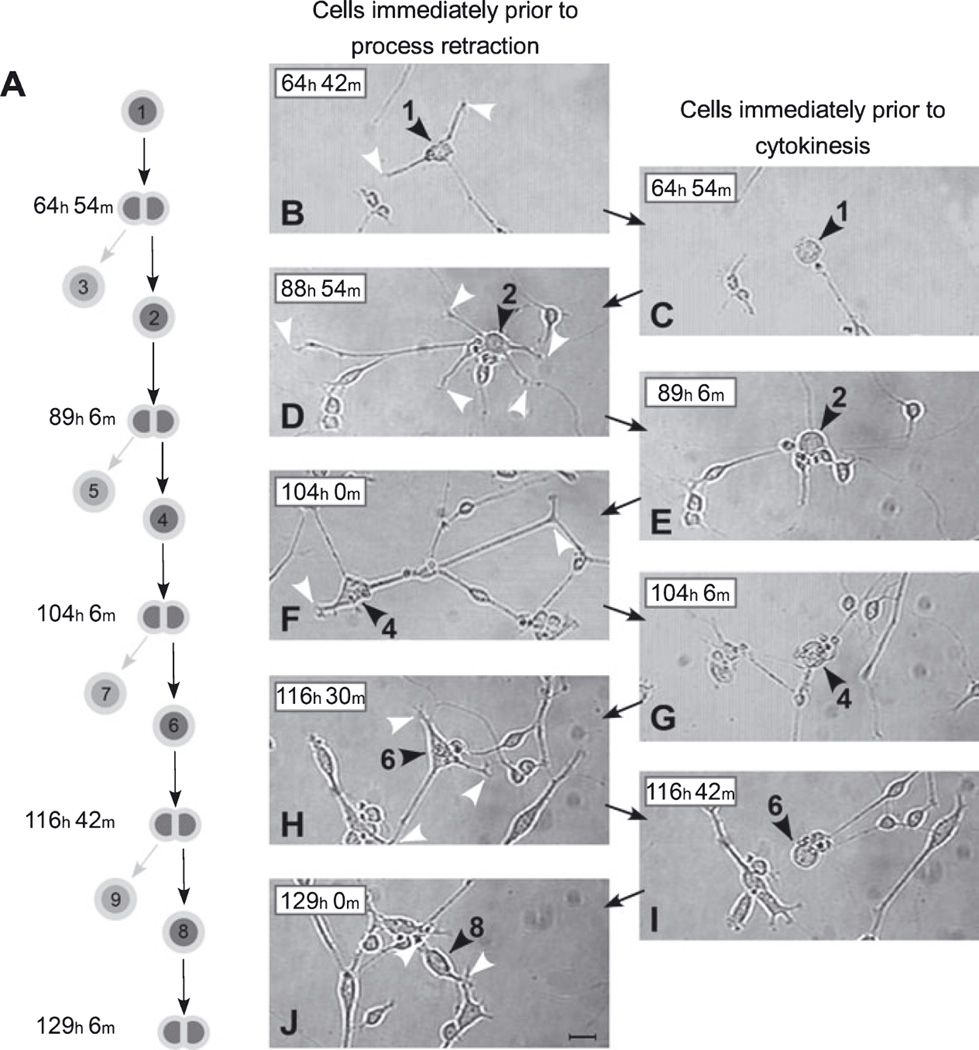
Process retraction precedes each division of lineally related SVZa progenitors in a clone. (A) Lineage relationships for cells illustrated in photomicrographs B–J. These cells are representative members of the lineage depicted in Fig. 6. Only one daughter produced by each cytokinesis, those numbered 2, 4, 6 and 8, is illustrated in the photomicrographs. The daughter cells pointed to by the light arrows in A, those numbered 3, 5, 7 and 9, are not tracked in these images. (B–J) The left column of photomicrographs (B, D, F, H and J) shows images of cells immediately prior to their initiation of process retraction. The right column of photomicrographs (C, E, G and I) shows images of cells immediately prior to their cytokinesis. The position of the soma (black arrowheads) and process tips (white arrowheads) of the relevant cells are indicated. The sequence in photomicrographs G–I, for example, shows the following. (G) Cell no. 4 at 104 h 6 min after plating with its processes fully retracted and on the verge of dividing to give rise to Cell nos 6 and 7 (see lineage diagram in A). (H) Cell no. 6, one of cell no. 4’s daughters, 12 h 24 min after its birth and immediately prior to initiating rapid process retraction. At this time it has three processes (white arrowheads). (I) Cell no. 6 immediately prior to its cytokinesis to give rise to Cell nos 8 and 9. This image was collected just 12 min after the image in H. Together, photomicrographs B–I demonstrate the repeated extension and full retraction of elaborate processes over multiple generations of a single lineage of SVZa neuronal progenitor cells. Scale bar, 20 µm in J (applies to all panels).
Discussion
In the present study we focused on understanding the dynamics of process formation relative to proliferation. For these experiments, multiple microscopic fields within neonatal SVZa progenitor cell cultures were imaged every 20 or 60 s for up to 6 days using videomicroscopic methods. Although it has been suspected that SVZa-derived neuronal progenitor cells divide after extending processes, previous studies using BrdU or retroviral labeling followed by analysis of fixed tissue have only indirectly tested this hypothesis. Here our results directly demonstrate that cultured SVZa neuronal progenitor cells retract their processes and then undergo cell division, that their progeny extend new processes, and that the sequence of process extension, then process retraction followed by cell division is repeated generation after generation in SVZa-derived neuronal progenitor cell lineages.
A number of studies have analysed the migration of SVZa-derived cells and their progeny in the postnatal RMS using videomicroscopy and slice cultures (Suzuki & Goldman, 2003; Nguyen-Ba-Charvet et al., 2004; Ocbina et al., 2006). In an attempt to further investigate the dynamics of dividing SVZa-derived cells and process extension and retraction, immature neurons of the RMS were labeled by electroporation of an expression vector containing ds-Red (unpublished findings). Although we were able to observe ds-Red-positive immature neurons with processes in various parts of the RMS, due to their dynamic nature of migration, cells moved rapidly in and out of the focal plane, precluding the continuous tracking of process extension and retraction in this manner. Therefore, we could not follow SVZa-derived cell division and process dynamics over time in slices and opted for following individual cells in low-density dissociated cultures.
Process extension and then retraction precede every division of SVZa-derived neuronal progenitor cells. The processes are rapidly withdrawn, typically within 30 min of cytokinesis. The short interval between initiation of process retraction and cytokinesis suggests that the cell has committed to division long before process retraction begins, as S-phase, the stage of the cell cycle during which DNA is replicated, for SVZa progenitor cells in vivo lasts ~3 h (Smith & Luskin, 1998) and, following S-phase completion, additional time (the G2-phase) elapses before mitosis and cytokinesis. The rapid withdrawal of processes shortly before cytokinesis further suggests that, for these cells, withdrawal is mechanistically tied to mitosis and/or cytokinesis, the final events in each round of the cell cycle. The length of the cell cycle, between 3 and 6 DIV for SVZa progenitor cells in the cultures studied videomicroscopically (13–18 h), was similar to the length of the cell cycle for migrating SVZa-derived progenitor cells in the RMS in vivo (Smith & Luskin, 1998), raising the possibility that the kinetics of process withdrawal and extension in vivo may be similar to the times that we have measured in culture.
Direct analysis of proliferation supports previous interpretations based on BrdU data
In our previous in-vivo studies, BrdU, which is incorporated into the DNA of dividing cells during the S-phase of the cell cycle (Nowakowski et al., 1989), was used to determine the distribution of dividing cells along the neonatal rodent RMS (Menezes et al., 1995; Coskun & Luskin, 2001; Pencea & Luskin, 2003). A gradient of BrdU incorporation was observed along the RMS, with the highest density of labeled cells in the SVZa. However, even in the SEZ, the distal end of the RMS, some cells were labeled by a short pulse of BrdU. Importantly, along the entire posterior (SVZa)–anterior (SEZ) axis, the BrdU-labeled cells in the RMS were found to express neuron-specific tubulin and often observed to have a leading and trailing process (Menezes et al., 1995). Furthermore, our previous in vitro studies showed that, in cultures of SVZa progenitor cells fixed after 24 h of BrdU exposure, the labeled cells are neuron-specific tubulin-positive and have processes, which in older (5 DIV) cultures can be extensive (Luskin et al., 1997). We have interpreted these results as demonstrating that there is a gradient of proliferation along the RMS and that SVZa-derived progenitors bear processes within a few hours following division.
However, concern has been raised that, despite the large number of labeled cells observed along the RMS in 3H-thymidine (Altman & Das, 1966) and BrdU labeling experiments (Menezes et al., 1995; Coskun & Luskin, 2001; Pencea & Luskin, 2003), the labeling of process-bearing SVZa progenitor cells along the RMS and in culture (Luskin et al., 1997) may not reflect proliferation of neuronal progenitor cells. Furthermore, evidence has been presented by others that postmitotic cells undergoing apoptosis or DNA repair can take up BrdU (Selden et al., 1993; Kornack & Rakic, 2001b; Yang et al., 2001). To directly determine whether SVZa progenitor cells with neuronal processes undergo division, in the present study we used videomicroscopic analysis to assess the proliferation of SVZa progenitor cells in culture. Our results demonstrate the proliferation of cells that, within a few hours of division, display the same morphological characteristics as we have previously observed for cells that are both BrdU-positive and neuron-specific tubulin-positive in culture (Luskin et al., 1997). Furthermore, the videomicroscopic analysis demonstrates that the parental cell giving rise to daughters with a neuronal morphology (Schwartz & Westbrook, 2000) typically also has extensive processes in the hours prior to its division. These studies demonstrate that some, if not all, of the cultured SVZa neuronal progenitor cells that incorporate BrdU undergo mitosis.
Given the findings of the present study, that SVZa progenitor cells withdraw their processes preceding cytokinesis, the question arises as to why almost all SVZa progenitor cells observed to incorporate BrdU are process-bearing. A consideration of the time-course of process retraction, division and then process extension explains why, at any moment in time, there are few BrdU-positive cells that lack processes. Specifically, cultured SVZa progenitor cells have long and motile processes, except for a short time (< 30 min) prior to cytokinesis, cytokinesis (a few minutes) and a short time following cytokinesis (typically <1.5 h) (Falls, Lane, Coskun & Luskin, unpublished findings) (Figs 2 and 3). As we determined that the average cell cycle time of SVZa progenitor cells in culture is 13–18 h, then less than ~15% of the mitotically active cells will be without processes at any given time. Thus, the dynamics of process withdrawal and extension relative to cytokinesis account for why only a small proportion of SVZa neuronal progenitor cells lack processes despite their ongoing proliferation.
Comparison of SVZa neuronal progenitors to other neural progenitors with processes
Virtually all of the SVZa progenitor cells that we studied retracted their processes in conjunction with cytokinesis and their daughters extended new processes after cytokinesis. This uniform, complete withdrawal differentiates SVZa progenitor cells from several other process-bearing types of neural progenitors including cultured sympathetic neuroblasts, neonatal cerebellar neural progenitors, cerebral cortical radial glia and embryonic retinal progenitor cells that have been studied using time-lapse videomicroscopy. In the following paragraphs each of these cell types is briefly compared with SVZa neuronal progenitors with respect to the disposition of processes when the cell divides.
Cultured rodent peripheral nervous system sympathetic neuroblasts share with SVZa neuronal progenitor cells the characteristic that they express neuronal markers (DiCicco-Bloom et al., 1990). However, the disposition of processes relative to division is quite different for these sympathetic neuroblasts than for SVZa progenitors. Sibling sympathetic neuroblasts inherit the processes of their parent, with one neuroblast inheriting some of the processes and the other the remainder (DiCicco-Bloom et al., 1990; Wolf et al., 1996, 1999). In contrast, as described in the Results, nearly all SVZa progenitors fully retract their processes prior to dividing and the new daughter cells form processes that are different from the parent cell.
Rodent neonatal cerebellar neural progenitors (also known as cerebellar neuroblasts) in the external granule cell layer are variable in their morphological pattern. Not all cerebellar neural progenitors have processes prior to division and when process-bearing cerebellar neural progenitors do divide they may (i) pass on their processes to their progeny, (ii) withdraw or remodel their processes, or (iii) pass on some processes and withdraw others (Wolf et al., 1997). Unlike SVZa neuronal progenitors, cerebellar granule cell neural progenitors do not express neuron-specific tubulin (Zigova & Luskin, unpublished findings).
In the lineage of rodent cerebral cortical neurons, precursor proliferation and neuronal process formation have been considered as sequential events (Rakic, 1990; Jacobson, 1991). A videomicro-scopic study (Noctor et al., 2004) of organotypic embryonic cortical cultures has confirmed that many cortical projection neurons derive from asymmetric division of ventricular zone stem cells (now known to be radial glia; see Miyata et al., 2001; Noctor et al., 2001; Weissman et al., 2003) in which one daughter is a radial glial cell and the other is a postmitotic immature neuron, which is the first cell in these lineages to exhibit a bipolar migratory neuron-like morphology or to express neuronal markers such as neuron-specific tubulin (Noctor et al., 2004) and doublecortin (Gleeson et al., 1999).
Unlike SVZa progenitor cells, radial glial progenitors do not express neuronal markers. Similar to radial glia, but unlike SVZa neuronal progenitors, zebrafish embryonic retinal progenitor cells in vivo divide while retaining a basal process (Das et al., 2003). The basal process of these retinal progenitor cells may be inherited by only one of the daughters produced in the division or split between the two daughters. Thus, SVZa neuronal progenitor cells are distinct and can be distinguished from other types of neural progenitor cells by their ability to divide, even though they express properties unique to neurons.
Concluding remarks
In our efforts to resolve how SVZa progenitor cells undergo cell division, while expressing a neuronal phenotype, we previously proposed a model based on observations suggesting that the cells in the RMS do not become permanently postmitotic following their expression of the cell cycle inhibitor p19Ink4d (see Coskun & Luskin, 2002; Luskin & Coskun, 2002). This model posits that SVZa-derived progenitor cells in the RMS down-regulate p19Ink4d, undergo division and then re-express p19Ink4d in a cyclical manner, and that down-regulation of p19Ink4d expression is accompanied by de-differentiation, whereas up-regulation of p19Ink4d expression is accompanied by differentiation. In the current study, we provide additional support for our model by directly showing that a mitotically active SVZa neuronal progenitor cell retracts its processes prior to cytokinesis, indicative of morphological de-differentiation, and its progeny subsequently extend processes indicative of re-differentiation. The methods for videomicroscopic analysis of long-term cultures utilized in this study together with reporters of gene expression detectable in living cells will allow detailed analysis of the role of p19Ink4d expression and other potential mechanisms regulating the proliferation and differentiation of SVZa neuronal progenitor cells.
Supplementary Material
Video S1. Process extension and retraction coordinated with cytokinesis in cultured anterior subventricular zone progenitor cells.
Video S2. Illustration of methods for quantitative analysis of process retraction preceding cytokinesis.
Fig. S1. Two lineage diagrams showing the clonal progeny of two SVZa progenitor cells over five successive generations.
Acknowledgements
This work was supported by the National Institute on Deafness and Other Communication Disorders (grant RO1 DC03190 to M.B.L.). We thank Ms Kewa Mou for preparing the cultures and Ms Yuk T. Tso for assistance with data analysis.
Abbreviations
- bFGF
basic fibroblast growth factor
- BrdU
bromodeoxyuridine
- DIV
days in vitro
- EGF
epidermal growth factor
- RMS
rostral migratory stream
- SEZ
subependymal zone
- SVZa
anterior subventricular zone
Footnotes
Supplementary material
The following supplementary material may be found on www.blackwell-synergy.com
References
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J. Comp. Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience. 1994;62:291–305. doi: 10.1016/0306-4522(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Coskun V, Luskin MB. The expression pattern of the cell cycle inhibitor p19(INK4d) by progenitor cells of the rat embryonic telencephalon and neonatal anterior subventricular zone. J. Neurosci. 2001;21:3092–3103. doi: 10.1523/JNEUROSCI.21-09-03092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun V, Luskin MB. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J. Neurosci. Res. 2002;69:795–802. doi: 10.1002/jnr.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous sube-pendymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron. 2003;37:597–609. doi: 10.1016/s0896-6273(03)00066-7. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Townes-Anderson E, Black IB. Neuroblast mitosis in dissociated culture: regulation and relationship to differentiation. J. Cell Biol. 1990;110:2073–2086. doi: 10.1083/jcb.110.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Falls DL, Luskin MB. Neuronal progenitor cells of the mammalian neonatal anterior subventricular zone. In: Rao MS, editor. Stem Cells and CNS Development. 2nd Edn. New Jersey: Humana Press; 2005. [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. Developmental Neurobiology. New York: Plenum Press; 1991. [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl Acad. Sci. U.S.A. 2001a;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001b;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AK, Pencea V, Buck CR, Luskin MB. Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes. Dev. Biol. 1999;216:622–634. doi: 10.1006/dbio.1999.9498. [DOI] [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc. Natl Acad. Sci. U.S.A. 1990a;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil. Cytoskel. 1990b;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem. Senses. 1994;6:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Coskun V. The progenitor cells of the embryonic telencephalon and the neonatal anterior subventricular zone differentially regulate their cell cycle. Chem. Senses. 2002;27:577–580. doi: 10.1093/chemse/27.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol. Cell. Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- Mehes E, Czirok A, Hegedus B, Vicsek T, Jancsik V. Laminin-1 increases motility, path-searching, and process dynamism of rat and mouse Muller glial cells in vitro: implication of relationship between cell behavior and formation of retinal morphology. Cell Motil. Cytoskel. 2002;53:203–213. doi: 10.1002/cm.10062. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Smith CM, Nelson KC, Luskin MB. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol. Cell. Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Dizon ML, Shin L, Szele FG. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol. Cell. Neurosci. 2006;33:126–135. doi: 10.1016/j.mcn.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Pencea V, Luskin MB. Prenatal development of the rodent rostral migratory stream. J. Comp. Neurol. 2003;463:402–418. doi: 10.1002/cne.10746. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Reid CB, Liang I, Walsh CA. Clonal mixing, clonal restriction, and specification of cell types in the developing rat olfactory bulb. J. Comp. Neurol. 1999;403:106–118. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Lois C, Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J. Comp. Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- Schwartz JH, Westbrook GL. The cytology of neurons. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th Edn. New York: McGraw-Hill; 2000. pp. 67–87. [Google Scholar]
- Selden JR, Dolbeare F, Clair JH, Nichols WW, Miller JE, Kleemeyer KM, Hyland RJ, DeLuca JG. Statistical confirmation that immunofluorescent detection of DNA repair in human fibroblasts by measurement of bromodeoxyuridine incorporation is stoichiometric and sensitive. Cytometry. 1993;14:154–167. doi: 10.1002/cyto.990140207. [DOI] [PubMed] [Google Scholar]
- Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev. Dyn. 1998;213:220–227. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Zigova T, Luskin MB. Potassium currents in precursor cells isolated from the anterior subventricular zone of the neonatal rat forebrain. J. Neurophysiol. 1999;81:95–102. doi: 10.1152/jn.1999.81.1.95. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J. Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J. Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb. Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Wolf E, Black IB, DiCicco-Bloom E. Mitotic neuroblasts determine neuritic patterning of progeny. J. Comp. Neurol. 1996;367:623–635. doi: 10.1002/(SICI)1096-9861(19960415)367:4<623::AID-CNE11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wolf E, Wagner JP, Black IB, DiCicco-Bloom E. Cerebellar granule cells elaborate neurites before mitosis. Brain Res. Dev. Brain Res. 1997;102:305–308. doi: 10.1016/s0165-3806(97)00111-9. [DOI] [PubMed] [Google Scholar]
- Wolf E, Black IB, DiCicco-Bloom E. Mitotic sympathetic neuroblasts initiate axonal pathfinding in vivo. J. Neurobiol. 1999;40:366–374. [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in. Alzheimer’s disease. J. Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Newman MB. Transplantation into neonatal rat brain as a tool to study properties of stem cells. In: Zigova T, Sanberg PR, Sanchez-Ramos JR, editors. Neural Stem Cells: Methods and Protocols. Totowa, NJ: Humana Press; 2002. [DOI] [PubMed] [Google Scholar]
- Zigova T, Betarbet R, Soteres BJ, Brock S, Bakay RA, Luskin MB. A comparison of the patterns of migration and the destinations of homotopically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev. Biol. 1996;173:459–474. doi: 10.1006/dbio.1996.0040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Process extension and retraction coordinated with cytokinesis in cultured anterior subventricular zone progenitor cells.
Video S2. Illustration of methods for quantitative analysis of process retraction preceding cytokinesis.
Fig. S1. Two lineage diagrams showing the clonal progeny of two SVZa progenitor cells over five successive generations.