Abstract
Cell polarity plays key roles in tissue development, regeneration, and pathological processes. However, how the cells establish and maintain polarity is still obscure so far. In this study, by employing microfluidic techniques, we explored the influence of geometrical confinement and chemical stimulation on the cell polarity and their interplay. We found that teardrop shape-induced anterior/posterior polarization of cells displayed homogeneous distribution of epidermal growth factor receptor, and the polarity could be maintained in a uniform epidermal growth factor (EGF) solution, but be broken by a reverse gradient of EGF, implying different mechanism of geometrical and chemical cue-induced cell polarity. Further studies indicated that a teardrop pattern could cause polarized distribution of microtubule-organization center and nucleus-Golgi complex, and this polarity was weakened when the cells were released from the confinement. Our study provides the evidence regarding the difference between geometrical and chemical cue-induced cell polarity and would be useful for understanding relationship between polarity and directional migration of cells.
Cell migration is essential for many fundamental biological processes such as embryonic morphogenesis, tissue repair, and regeneration, and pathological processes such as inflammation, cancer metastasis, and immune disorders.1 Directional migration of cells induced by gradient concentration of small molecules, a process known as chemotaxis, plays a major role in innate immunity, development, and tumor metastasis.2 During chemotaxis, cells obtain the ability to maintain a stable asymmetric shape with a defined anterior and posterior, this process is called “polarization.”3,4 A polarized cell, such as neutrophil, can maintain its polarity in an uniform concentration of chemoattractant, and respond to change of the chemical gradient by performing a U-turn movement because of the asymmetric sensitivity of the anterior and posterior part of the cell.5,6 In the previous study, we7 used surface micropattern with functionalized self-assembled monolayers (SAM) on gold surface to constrain cells to teardrop shaped patterns, which allowed them to migrate toward a definite direction.8 This specific physical confinement defined relative anterior and posterior positions of the cells. Thus, an important question is whether the anterior/posterior polarity given by asymmetric geometries is the same as that given by chemotaxis. If patterned cells with definitive anterior/posterior polarity meet a reverse chemical gradient, which end they will use to respond?
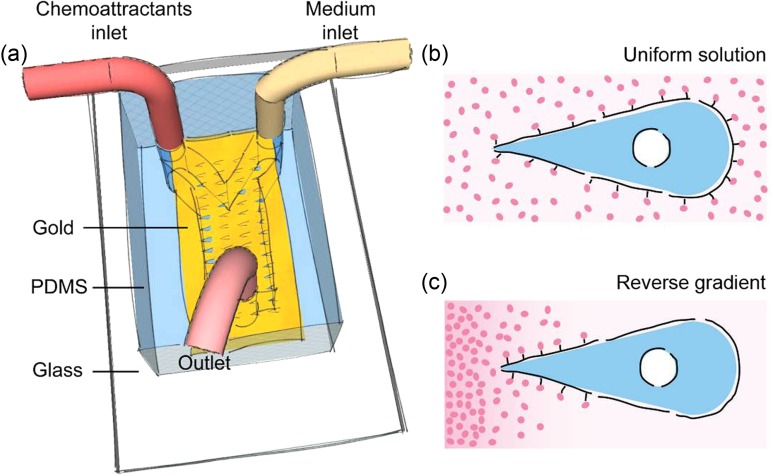
To explore this question, we designed a microchip (Fig. 1(a)) combining microcontact printing (μCP), microfluidic system, and electrochemical desorption (ECD) techniques to apply a gradient of chemical cues on cells released from designed shapes (Fig. 1(c)). The chemical gradient was generated by diffusion of epidermal growth factor (EGF) using a “Y”-shaped polydimethysiloxane (PDMS) channel. The substrate we used was a glass slide locally coated with a thin layer of gold. After modification with adhesive thiols groups (HS (CH2)14CH3) with desired geometries through μCP, the rest areas were modified with inert thiols (HS(CH2)14EG6). The substrate was bonded with the “Y”-shaped channel to form an enclosed microenvironment. We seeded MDA-MB-231 cells into the channel to let them attach to the gold surface and adopt defined geometry on the patterns. We used ECD to release the cells from the geometric confinement. We placed an anode in a droplet of solution that connects to the microchannel, and used the gold surface as the cathode. A potential of 1.2 V was applied for 30 s to electrochemically desorb only the SAMs exposed to the fluids in the microchannels. After the release of the cells from the geometric confinement by ECD, two flows of solution (medium with and without chemical cues) were connected to the “Y”-shaped channel and pushed by a syringe pump with a suitable flow speed. The chemical gradient was generated at the interface of the two streams, whose concentration changed according to the distance from the connection of “Y”-shaped channel.
FIG. 1.
The strategy for imposing a gradient of attractants on cells patterned with the shape of a teardrop. (a) We first inked the PDMS stamp with HS (CH2)14CH3 on the region of gold located on a glass. A “Y”-shaped channel was bonded to the surface of printed patterns using plasma treatment before which the region of gold was protected by a slide of PDMS membrane. Fibronectin and cells were filled into the channels, respectively. After attachment, cells were starved in DMEM with 0.2% serum overnight. We applied ECD and introduced two kinds of solutions into the microchip immediately via two inlets of the “Y” shaped channel: one is DMEM with 0.2% serum and the other is DMEM with 0.2% serum that also contained attractants. (b) The schematic shows the tear-shaped cell in the uniform solution of the chemical cue. (c) The schematic shows the tear-shaped cell in the solution with a reverse gradient of chemical cue.
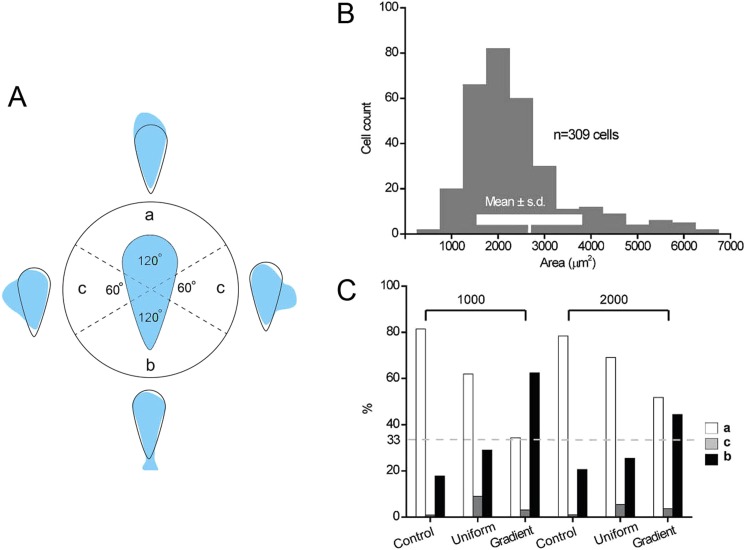
In our previous work,7 we traced the centroid of individual patterned cell to quantify its moving direction. In this paper, we recorded the region where patterned cells initially move from to identify the anterior portion they used.22 We classified the region of individual cell into three categories: the blunt end (a), the sharp end (b), and the sides (c) (Fig. 2(a)). To select suitable pattern size to confine the cells, we cultured MDA-MB-231 cells, a model tumor cell line that is sensitive to physical and chemical stimuli, on Petri dish freely and measured their spreading areas. The size of freely adhered MDA-MB-231 cells ranged from 2000 to 3000 μm2 (Mean ± s.d = 2674 ± 1128 μm2, Fig. 2(b)). Therefore, in this study, we chose the shape of teardrop with area of 1000 and 2000 μm2 to confine cells.
FIG. 2.
(a) The statistics of the ends from which teardrop shape confined cells initially move. a, b, and c represent cells moving from: the blunt end, sharp end, or the sides of the teardrop, respectively. (b) The areas of individual MDA-MB-231 cells cultured freely on culture dish were measured by phase contrast microscopy and calculated by Image-Pro Plus. Within the histogram, the mean area ± one standard deviation is shown. (c) The polarity of teardrop shape confined cells in different chemical environments. Cell numbers counted from left to right was 133, 155, 27, 102, 110, and 32, respectively. All the data were collected from at least 3 individual experiments.
During chemotaxis, cells can respond directionally to very shallow chemoattractant gradients. For example, eukaryotic cells can respond to differences in chemoattractant concentration that are as small as 2%–10% between the front and the back of the cell.9 Jeon et al.10 used a microfluidic chamber to generate a non-linear gradient of EGF to induce the chemotaxis of MDA-MB-231 cells. In their system, the cells showed a stronger response in the 0–50 ng/ml gradient of EGF. We analyzed the difference of chemical concentration in our “Y”-shaped channel.22 The average difference in concentration in the region of ±100 μm away from the mid line of the “Y”-shaped channel was calculated. The concentration differences were larger than 20% along the length of 60 μm and 90 μm (the length of cell along their long symmetric axis with the patterned area of 1000 μm2 and 2000 μm2 is 60 μm and 90 μm, respectively). This gradient can fulfill enough difference in concentration between the anterior and the posterior of the cell.
We compared the maintenance of anterior and posterior polarity of the cells confined in a teardrop shape between the environment with uniform concentration of EGF and a reverse gradient (the concentration decreases from the sharp end to the blunt end) of EGF (Fig. 2(c)). The cells confined in a teardrop shape were starved in Dulbecco's Modified Eagle's medium (DMEM) with 0.2% serum overnight. After applying ECD, the cells were cultured in DMEM with 0.2% serum (control), and DMEM with 0.2% serum containing EGF (50 ng/ml) (uniform), respectively. The tendency of teardrop confined cells to move out of the blunt end was still predominant (62%, 1000 μm2; 2000 μm2, 69%) when the cells were stimulated in uniform EGF solution (50 ng/ml) (Fig. 2(c)). The result indicated that the axis of front-rear of the cells caused by geometric confinement could be maintained in uniform EGF environment. The gradient experiment was performed in “Y” channel and generated by DMEM with 0.2% serum and DMEM with 0.2% serum containing EGF (50 ng/ml). The speed of flow was 2 μl/min. When we applied a reverse gradient of EGF on cells confined in 1000 μm2 patterns using the “Y”-shaped microchannel, the percentage of cells moved out of the blunt end deceased (34%) with the increase of the percentage of the cells moved from the sharp end (62%) and sides (4%). Under the reverse EGF gradient, the percentage of cells with the patterning area of 2000 μm2 moved out of the blunt end deceased (52%) with the increase of the percentage of the cells moved from the sharp end (45%) and sides (3%). So, the anterior/posterior polarity of the teardrop shape confined cells, no matter in 1000 μm2 or 2000 μm2 patterns, changed their polarity by switching their front ends from the blunt ends to the sharp ends when they encounter a reverse chemical gradient (Fig. 2(c)). We also found that the change of the polarity of the cells with 1000 μm2 area was large than that of the cells with 2000 μm2. This may be due to the decreased epidermal growth factor receptor (EGFR) density on the larger cells, the detailed mechanism need further study. We also patterned cells in the “Y”-shaped channel, and released and observed them under the flow of DMEM with 0.2% serum (the speed of flow was 2 μl/min) as a control. The results in the “Y”-shaped channel showed no obvious difference from that released in culture dish, which excludes the influence of flow rate. Therefore, the anterior/posterior polarity generated by geometric confinement can not be maintained in the stimulation of a reverse EGF gradient.
According to the previous report, in EGF-triggered chemotaxis, the receptors of EGF (EGFR) are homogeneously distributed on the plasma membrane, while the receptor internalization was accumulated on the side of the cell exposed to upstream of the chemical gradient.11 The differential internal signaling mechanism may amplify the signaling difference between receptors on the near and far sides of the cell to build polarity of the cell which moves toward the source of EGF.11,12 We transfected NIH 3T3 cells with green fluorescent protein (GFP) labelled EGFR and observed them under confocal microscope to identify the distribution of EGFR on cells patterned with the shape of a teardrop. We found that the distribution and endocytosis of EGFR was homogeneous in patterned cells without release under uniform EGF stimulation,22 indicating that asymmetric physical confinement can not induce asymmetric distribution of the receptors and subsequent asymmetric sensitivity of the cells to uniform chemoattractants. Thus, we presumed that the internal axis caused by the asymmetric shape may dominate the migration direction of the cells in a uniform EGF environment; and the reverse EGF gradient (Fig. 1(c)) may lead to the accumulation of EGFR internalization near the sharp end of the patterned cell, and subsequently re-polarize the cells to move from the sharp end (Fig. 2(c)). Therefore, as mentioned above, the anterior/posterior polarity caused by physical confinement can not be maintained in a reverse attractant gradient.
It is generally recognized that the relative position of microtubule-organization center (MTOC) and the relative position of nucleus in migrating cells are good indicators of the orientation of cell polarity axis.13,14 This kind of orientation of cell polarity occurred in many cell types in two-dimensional culture15 and wound healing assays.16 However, several observations indicated that the MTOCs are not always located between the leading edge and nucleus in migrating cells, and the location of MTOCs highly depends on the nature of the adhesive substrate17 and the physical environment cell constrained.18 Bornens et al.19 proposed that in immobile cells, adhesion geometry governs the assemblies and distribution of cytoskeleton, and finally polarizes the nucleus-centrosome-Golgi apparatus.
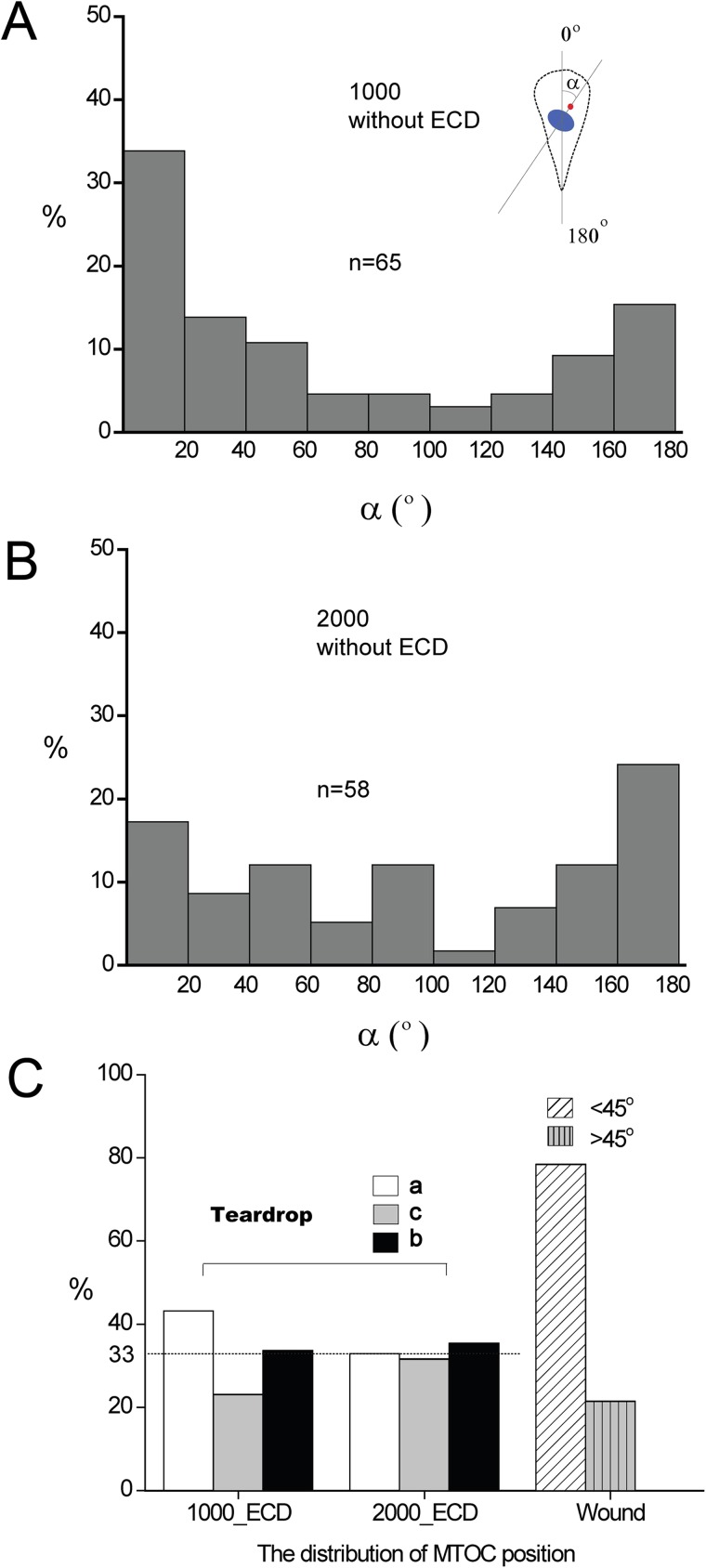
To explore the role of subcellular organelles in patterned cell polarity, we first analyzed the angle between the nucleus–MTOC axis and the symmetry axis of the teardrop (Figs. 3(a) and 3(b)). We defined the angle between the nucleus–MTOC axis orienting toward the blunt end and the symmetric axis of the teardrop as 0°. The angle ranging from 0° to 60°indicates a preferential positioning of the MTOC in front of the nucleus in the direction of the blunt end. 120°–180° represents a position of the MTOC behind the nucleus, and 60°–120° represents the position of MTOC located on the sides of the nucleus. In immobile teardrop patterned cells, the nucleus–MTOC axis mainly pointed to the blunt end and the sharp end, these two ends accounts for almost 80% of orientation (Figs. 3(a) and 3(b)). With the size of 1000 μm2, the distribution of angles showed a predominant tendency of toward the blunt end (58%) over that of toward the sharp end (29%) (Fig. 3(a)). When cells were confined to the size of 2000 μm2, the distribution of angles had no obvious difference toward the blunt end (38%) and toward the sharp end (43%) (Fig. 3(b)). We also measured the distribution of MTOC in our three classified regions a, b, and c after releasing the teardrop shape confined cells by ECD (Fig. 3(c)). The results showed that when the patterned cells were released from the physical confinement, the distribution of MTOC position in a, b, and c is 43%, 34%, and 23% with the size of 1000 μm2, and 33%, 35%, and 32% with the size of 2000 μm2, respectively, indicating that the distribution of MTOC in region a (representing the anterior side) is not dominant after cell releasing. Furthermore, we measured the distribution of MTOC using wound healing mode as a control.20 MDA-MB-231 cells were cultured freely on culture dish. After cell grew into a monolayer, we scratched the cell layer to make an artificial “wound” using a pipette tip. Then cultured the cells in DMEM with 10% serum for 30 min, fixed the cells and stained them with pericentrin antibody and Hoechst 33342. The cells were analyzed by measuring the angle between the MTOC–nucleus axis and an axis perpendicular to the wound. For a polarized cell orienting toward the wound, this angle is <±45°. For a randomly oriented cell, this angle is >±45°. Statistic data showed that 80% of the cells on the wound model were polarized (Fig. 3(c)). Compared with the control (wound model), the nucleus–MTOC axis was considered to be incapable of indicating the moving direction of cells patterned with the shape of a teardrop.
FIG. 3.
Internal polarity of the teardrop shape confined cells. (a) and (b) Distribution of the angle between the nucleus-MTOC axis and the symmetry axis of the teardrop in patterned MDA-MB-231 cells without applying ECD in different sizes: (a) 1000 μm2 and (b) 2000 μm2); n represents the counting number of cells. (c) The distribution of MTOC position in MDA-MB-231 cells patterned with the shape of a teardrop in different sizes after releasing by ECD, and MTOC position in the wound model as a control. The released cells were incubated in DMEM with 10% serum for 10 min, then fixed and stained with pericentrin antibody and Hoechst 33342. a, b, and c represents cells move from: the blunt end, sharp end, or the sides of the teardrop, respectively. Cell numbers counted in Figure 3(c) (from left to right) were 95, 79, and 107, respectively. All the data were collected from at least 3 individual experiments.
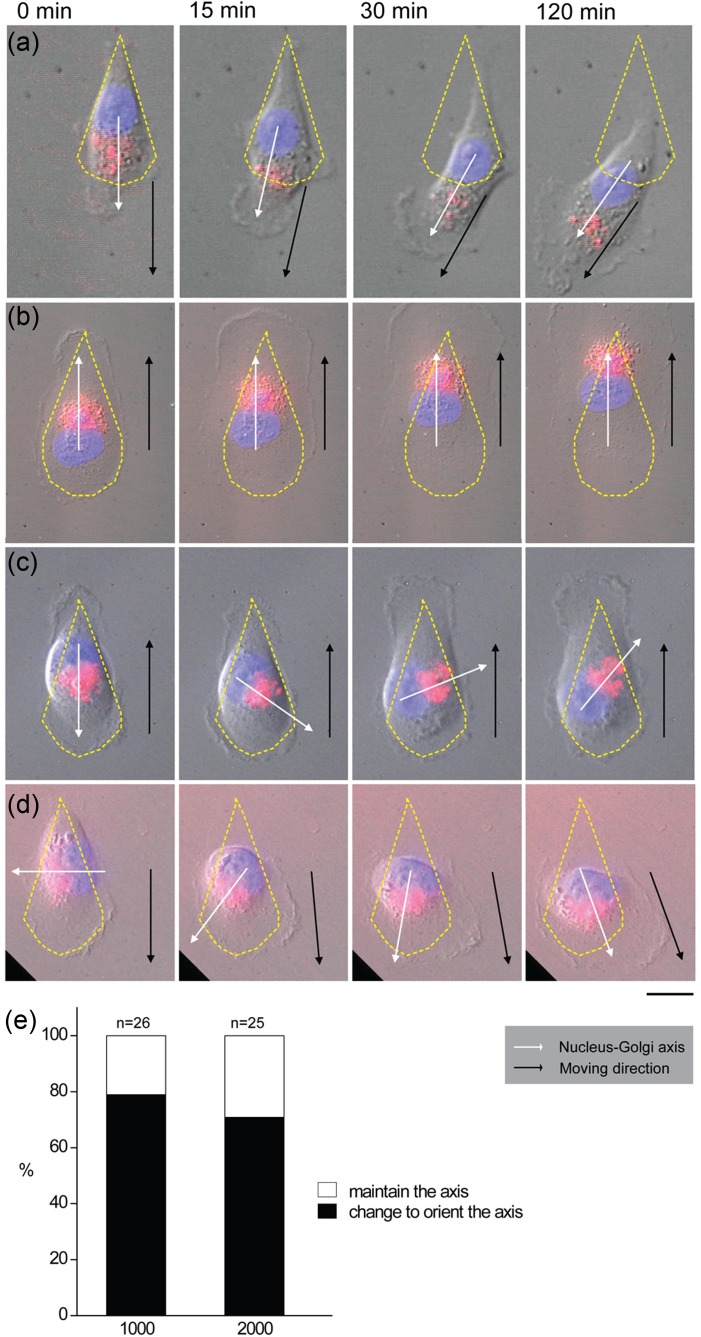
We also explored the relationship between the position of nucleus-Golgi complex and the migration direction of the released cells from the asymmetrical confinement. When we released the teardrop shape confined cells, two different phenomena appeared, one is that nucleus-Golgi complex axis can indicate the direction of migration, no matter the given anterior (the blunt end, Fig. 4(a)) or posterior (the sharp end, Fig. 4(b)). The other is that the initial nucleus-Golgi complex axis cannot represent the direction of cell movement. We observed that if the initial nucleus-Golgi complex axis is not consistent with the direction of migration, the nucleus-Golgi complex axis prefer to reorient to establish a new nucleus-Golgi complex axis before moving. For example, the initial nucleus-Golgi complex axis pointing to the blunt end can change and point to the sharp end, and finally move out from it (Fig. 4(c)). The initial nucleus-Golgi complex axis which pointed to the sides tended to reorient and form a new nucleus-Golgi complex axis toward the blunt end (Fig. 4(d)). Statistical data indicated that the reorientation of nucleus-Golgi complex axis happened in about 80% cells, whose initial nucleus-Golgi complex axis were not consistent with their final moving direction (Fig. 4(e)). This implies that although the geometrical confinement can direct the distribution of the nucleus-Golgi complex, the release of the confinement is a signal for the cell to migrate freely, compromising the polarity of the cells built by the physical confinement.
FIG. 4.
The dynamical changes of the nucleus-Golgi complex axis and moving direction. MDA-MB-231 Cells were patterned with the shape of a teardrop with the size of 2000 μm2. Cells were treated with CellLight Golgi-RFP and Hoechst 33342 as the description in Fig 4. ECD was applied before observation under microscopy. (a) The nucleus-Golgi complex axis was consistent with the direction of cell migration in patterned cells moving from the blunt end. (b) The nucleus-Golgi complex axis was consistent with the direction of cell migration in patterned cells moving from the sharp end. (c) The nucleus-Golgi complex axis changed and finally consistent with the direction of cell migration in patterned cells moving from the sharp end. (d) The nucleus-Golgi complex axis changed and finally consistent with the direction of cell migration in patterned cells moving from the blunt end. The yellow dotted line indicates the outline of a patterned cell. The black line and arrow indicate the moving direction. The white line and arrow indicate the direction of nucleus-Golgi complex axis. (e) Statistics of the orientation of nucleus-Golgi complex axis before moving out of the physical confinement; n represents the counting number of cells; scale bar, 20 μm. All the data were collected from at least 3 individual experiments.
In this paper, we explored the influence of cell geometry and chemical stimulation on anterior/posterior polarity of cells. Teardrop, the asymmetric geometry used to confine cells in this study, induced anterior/posterior polarity of the cells. This polarity could be broken by a reverse EGF gradient. Differ from chemical cue-induced polarity, the asymmetric physical confinement could not induce asymmetric distribution of the EGF receptors on the membrane, implying that certain intracellular mechanism may determine the geometrical confinement-induced cell polarity. By exploring the role of MTOC and Golgi complex in the establishment and maintenance of cell polarity, we found that both MTOC and nucleus-Golgi complex displayed some extent of polarized distribution when the cells were physically confined to an asymmetrical geometry (teardrop). However, when the cells were released from the confinement, both the distribution of MTOC and nucleus-Golgi complex lost their polarity and subsequently led to the random migration of the cells. This is in part in agreement with the previous report21 that the position of MTOC in patterned cells alone cannot determine the direction of cell movement. The nucleus-Golgi complex axis, which can orient dynamically to be consistent with the axis of the geometry that confines the cell, can reorient to direct the random migration of the cells when the cells were released from the teardrop confinement. We presume that the physical confinement can exert mechanical stress to the cells. In a highly elongated cell shape, such as a teardrop, cell is restricted to an unnatural shape which may influence the distribution of the mechanics-related subcellular structures such as MTOC and nucleus-Golgi complex and lead to the polarity of the cell. But, once the external force (physical confinement) disappears, the polarity of the cell based on the confinement will be weakened and subsequently disappear. In fact, our study showed that the polarity of the cells in 2000 μm2 pattern was not obvious as that in 1000 μm2 pattern (Fig. 2(c)), demonstrating that the larger the confining area, the more free space for the cell to adopt near-natural state. The release of the cells from confinement equals to giving the cells full space to spread and migration, the polarity built by the confinement will disappear. This may be the reason for the inability of MTOC and nucleus-Golgi complex to direct the directional migration of the released cells like their confined counterpart.
In summary, we found that the mechanisms underlying the geometrical confinement and chemical cue-induced cell polarity are different: chemical cues are mainly through the redistribution of receptors in the cell whereas geometrical confinement depends on the organization of MTOC and/or nucleus-Golgi complex. Based on this paper, the interplay between the geometrical confinement and chemical cue in determining cell polarity and directional migration will be further investigated.
Acknowledgments
The financial support was provided by the Ministry of Science and Technology of China (2011CB933201, 2011AA030308, 2012AA022703), National Science Foundation of China (31170905, 81361140345, 51373043, 21025520, and GZ614), and the Beijing Natural Science Foundation (2122058 and 2122060).
References
- 1.Rorth P., Annu. Rev. Cell Dev. Biol. 25, 407 (2009). 10.1146/annurev.cellbio.042308.113231 [DOI] [PubMed] [Google Scholar]
- 2.Van Haastert P. J. M. and Devreotes P. N., Nat. Rev. Mol. Cell Biol. 5, 626 (2004). 10.1038/nrm1435 [DOI] [PubMed] [Google Scholar]
- 3.Huttenlocher A., Nat. Cell Biol. 7, 336 (2005). 10.1038/ncb0405-336 [DOI] [PubMed] [Google Scholar]
- 4.Iijima M. and Devreotes P., Cell 109, 599 (2002). 10.1016/S0092-8674(02)00745-6 [DOI] [PubMed] [Google Scholar]
- 5.Zigmond S. H., Levitsky H. I., and Kreel B. J., J. Cell Biol. 89, 585 (1981). 10.1083/jcb.89.3.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J. S., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., and Bourne H. R., Cell 114, 201 (2003). 10.1016/S0092-8674(03)00555-5 [DOI] [PubMed] [Google Scholar]
- 7.Jiang X., Bruzewicz D. A., Wong A. P., Piel M., and Whitesides G. M., Proc. Natl. Acad. Sci. U.S.A. 102, 975 (2005). 10.1073/pnas.0408954102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery M., J. Cell Sci. 123, 4201 (2010). 10.1242/jcs.075150 [DOI] [PubMed] [Google Scholar]
- 9.Zigmond S. H., J. Cell Biol. 75, 606 (1977). 10.1083/jcb.75.2.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadi W., Wang S. J., Lin F., and Jeon N. L., Biomed. Microdevices 8, 109 (2006). 10.1007/s10544-006-7706-6 [DOI] [PubMed] [Google Scholar]
- 11.Bailly M., Wyckoff J., Bouzahzah B., Hammerman R., Sylvestre V., Cammer M., Pestell R., and Segall J. E., Mol. Biol. Cell 11, 3873 (2000). 10.1091/mbc.11.11.3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent C. A. and Devreotes P. N., Science 284, 765–770 (1999). 10.1126/science.284.5415.765 [DOI] [PubMed] [Google Scholar]
- 13.Anda F. C. de, Pollarolo G., Silva J. S. Da, Camoletto P. G., Feiguin F., and Dotti C. G., Nature 436, 704 (2005). 10.1038/nature03811 [DOI] [PubMed] [Google Scholar]
- 14.Nabi I. R., J. Cell Sci. 112, 1803 (1999); available at http://jcs.biologists.org/content/112/12/1803 [DOI] [PubMed] [Google Scholar]
- 15.Nemere I., Kupfer A., and Singer S. J., Cell Motil. Cytoskeleton 5, 17 (1985). 10.1002/cm.970050103 [DOI] [PubMed] [Google Scholar]
- 16.Euteneuer U. and Schliwa M., J. Cell Biol. 116, 1157 (1992). 10.1083/jcb.116.5.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutze K., Maniotis A., and Schliwa M., Proc. Natl. Acad. Sci. U. S. A. 88, 8367 (1991). 10.1073/pnas.88.19.8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouthas F., Girard P., Lecaudey V., Ly T. B. N., Gilmour D., Boulin C., Pepperkok R., and Reynaud E. G., J. Cell Sci. 121, 2406 (2008). 10.1242/jcs.026849 [DOI] [PubMed] [Google Scholar]
- 19.Thery M., Racine V., Piel M., Pepin A., Dimitrov A., Chen Y., Sibarita J.-B., and Bornens M., Proc. Natl. Acad. Sci. U.S.A. 103, 19771 (2006). 10.1073/pnas.0609267103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupin I., Camand E., and Etienne-Manneville S., J. Cell Biol. 185, 779–786 (2009). 10.1038/nrn2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B., Kumar G., Co C. C., and Ho C. C., Sci. Rep. 3, 2827 (2013). 10.1038/srep02827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.See supplementary material at http://dx.doi.org/10.1063/1.4898209E-BIOMGB-8-021492 for the time-lapse imaging of cell migration after their release from the tear-drop confinement; analysis of EGF gradient generated in the Y shaped microchannel; the distribution of EGFR on 3T3-EGFR-GFP cells patterned with the shape of a teardrop under the stimulation of uniform EGF-Alexa Fluor 555 solution.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4898209E-BIOMGB-8-021492 for the time-lapse imaging of cell migration after their release from the tear-drop confinement; analysis of EGF gradient generated in the Y shaped microchannel; the distribution of EGFR on 3T3-EGFR-GFP cells patterned with the shape of a teardrop under the stimulation of uniform EGF-Alexa Fluor 555 solution.