Abstract
We reported a new microfluidic system integrated with worm responders for evaluating the environmental manganese toxicity. The micro device consists of worm loading units, worm observing chambers, and a radial concentration gradient generator (CGG). Eight T-shape worm loading units of the micro device were used to load the exact number of worms into the corresponding eight chambers with the assistance of worm responders and doorsills. The worm responder, as a key component, was employed for performing automated worm-counting assay through electric impedance sensing. This label-free and non-invasive worm-counting technique was applied to the microsystem for the first time. In addition, the disk-shaped CGG can generate a range of stepwise concentrations of the appointed chemical automatically and simultaneously. Due to the scalable architecture of radial CGG, it has the potential to increase the throughput of the assay. Dopaminergic (DAergic) neurotoxicity of manganese on C. elegans was quantitatively assessed via the observation of green fluorescence protein-tagged DAergic neurons of the strain BZ555 on-chip. In addition, oxidative stress triggered by manganese was evaluated by the quantitative fluorescence intensity of the strain CL2166. By scoring the survival ratio and stroke frequency of worms, we characterized the dose- and time-dependent mobility defects of the manganese-exposed worms. Furthermore, we applied the microsystem to investigate the effect of natural antioxidants to protect manganese-induced toxicity.
I. INTRODUCTION
C. elegans, a small soil nematode, has been widely used as a powerful model organism because of a number of beneficial properties including tiny size, optical transparency, short life cycle, and genetic tractability. In particular, the conservation of many genes and biological mechanisms between C. elegans and humans, together with the simplicity and ease of their maintenance, can make C. elegans amenable in the field of biomedical and environmental toxicology.1 However, the painstaking manual handling of worms such as repeatedly seeding, cleaning, and transferring poses technical obstacles toward the throughput of behavioral assay. Recently, the advances of microfluidics and micro-electromechanical systems (MEMS) technology offer technical possibilities to effectively overcome these challenges.2 The benefits of microfluidic device in manipulating these worms are plenteous including biocompatible material, compatible dimension between tiny worm and microfluidic channel, precise delivery of external stimuli, high-throughput of optical imaging and automated screening.2–5
Microfluidics-based worm researches have been introduced intensively since the pioneer work was reported by Bargmann in 2004.6 Up to now, the application of a large body of novel components and functional units has facilitated the microfluidics-based worm studies, such as droplet-based microfluidic platform,7,8 electric-based microfluidic device,9–13 and micro pillars-based worms sorting chip and locomotion patterns analysis microfluidic device.14,15 In addition, microfluidic device has also been used to toxicity analysis.16–19 Currently, complete worm immobilization can be achieved by applying mechanical forces,20–24 cooling the worm's body,25 creating a CO2 micro-environment,21 using Pluronic F127 as an immobilization agent,26 or optical immobilization.27 Although these recent innovations have promoted the related process of the researches using C. elegans, few reports were concentrated on the delivery of the exact amount of worms into the microsystem.28,29 Due to their small size and rapid movement, the corresponding technical challenges also exist.30
In this work, we proposed a unique microfluidic platform to encapsulate the exact number of worms into individual chamber and investigate the diverse behavioral responses to manganese. Manganese is the twelfth most prevalent element in the Earth's crust.31 It is also one out of seven essential metals for human normal growth and development.32 Manganese as a catalyzer has been widely used in some industries such as mining and the manufacturing of dry batteries, steel, aluminum, and welding metals.33 Its presence in gasoline additives and fungicides such as Maneb or Mancozeb also increases the risk of environmental manganese exposure to public health.34,35 It has long been appreciated that excess accumulation of manganese in the brain can result in a neurological syndrome resembling Parkinson's disease (PD) with a characteristic of dopaminergic (DAergic) neurodegeneration.36 To better understand the specificity of DAergic neurodegeneration, we studied manganese toxicity in a novel microfluidic platform by monitoring three endpoints of mobility defects, DAergic neurodegeneration, and oxidative stress in C. elegans. The microfluidic platform can simultaneously fulfill the following functions. First, our on-chip electrical worm-counting mechanism based on electric impedance sensing can load a certain number of worms into all the chambers in a parallel manner at once. Second, the worms dispensed into the chambers can be uniformly exposed to the chemical stimuli of well-defined concentration gradient. At last, the toxicity assessment and rescue assay can be performed on-chip. The results showed that manganese can cause obvious mobility defects, oxidative stress and DAergic neurodegeneration, and natural antioxidants such as Vitamin E, resveratrol, and quercetin can improve the viability of the exposed worms effectively.
II. EXPERIMENTAL
A. Microfluidic device design
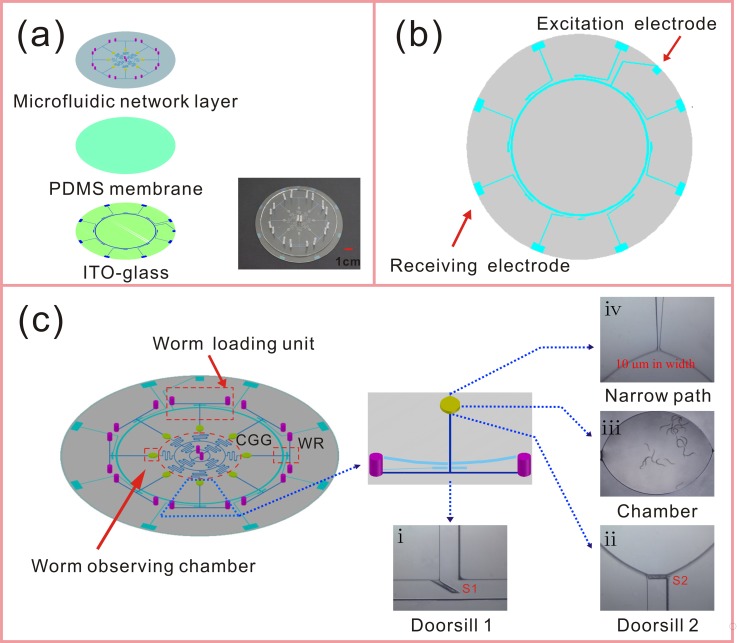
The design of the microfluidic platform is shown in Fig. 1. The structure of the chip is composed of three layers, the microfluidic network layer, thin polydimethylsiloxane (PDMS) membrane, and indium tin oxide (ITO)-coated glass [Fig. 1(a)]. The microfluidic network layer contains the structures of concentration gradient generator (CGG, 200 μm width and 60 μm height) and eight uniform units which are composed of eight T-shape channels (280 μm width and 60 μm height) and eight ellipse observing chambers (3 mm minor axis length × 4 mm major axis length and 500 μm height). The CGG is a radial micro-channel network which was designed to generate a combinatorial, quantitative, and predictable concentration gradient of eight stepwise concentrations.37 Two central chemicals inlets (2 mm diameter and 2 mm height) were designed to facilitate the addition, removal, or exchange of chemical stimuli of eight chambers rapidly. Each T-shape loading channel has been connected with a chamber and two separate inlets (2 mm diameter and 2 mm height). One inlet (I1) is used for loading worms and another inlet (I2) for media exchange. A 100 μm-thick PDMS membrane was spin coated onto the ITO conductive electrodes, which were fabricated by screen printing and chemical etching methods.38 The thin PDMS membrane can protect the worms against the injury of the electric current in the microfluidic channel.
FIG. 1.
Schematics and photographs of the microfluidic platform for manganese toxicity assessment and rescue assay. (a) The structure of the chip is composed of three layers, the microfluidic network layer, thin PDMS membrane (100 μm in thickness), and ITO-glass. The inset is a photograph of the fabricated microfluidic chip. (b) The layout of the ITO electrodes for worm responder. (c) Schematics of the microfluidic chip. Our chip contains the structures of CGG and eight uniform loading units which are composed of eight T-shape channels and eight ellipse observing chambers. There are four functional units: worm loading units, worm observing chambers, CGG, and worm responders (WRs), which have been highlighted using red dashed outline. The magnified photomicrographs of the unique components, (i) doorsill S1 (in the cross-sectional area of T-shape channel), (ii) doorsill S2 (before the ellipse observing chamber), (iii) the chamber within 20 worms, and (iv) a narrow path (at the exit of the observing chamber) have been located in the schematics.
The four functional units of the microfluidic chip, worm loading units, worm observing chambers, CGG, and worm responders have been highlighted using red dashed outline in Fig. 1(c). The unique components, worm responders, and doorsills can enable automated loading of the exact amount of worms, convenient observation of multiple behavioral responses to chemicals and confined microenvironment of chemical stimuli for worms. For every worm loading unit of the device, we designed two doorsills which are both 45–μm high (there is a gap of 15 μm from each doorsill to the bottom of the micro channel). One is located in the cross-sectional area of T-shape channel [S1, Fig. 1(c-i)] and it can ensure that most of the worms from I1 would enter the chamber. Another lies before the ellipse observing chamber [S2, Fig. 1(c-ii)], and there is another gap of 15 μm between the wall of the micro channel and the doorsill. During the worms loading process, large fluid pressure from the inlet I2 can temporarily allow these two gaps to expand enough for worms passing through freely. After this process, the size of these gaps would recover and the L4 worms would be housed in the chamber [Fig. 1(c-iii)]. Hence, a confined microenvironment of chemical stimuli for worms was formed. In addition, these ingenious microstructures can allow the laying eggs and L1 worms to be flushed out of the device immediately, without interfering with the observation of adult worms. A design of 10 μm-wide narrow path [Fig. 1(c-iv)] also effectively prevents the worms from escaping and allows fluid flowing through the chamber at the same time. As the highlight of this device, worm responders at the cross-sectional zones of T-shape micro channels can non-invasively detect the worms passing through the measurement units accurately. The electric impedance sensing based technique has been used to detect the capacitance change when C. elegans is placed in the sensing space.39 In order to count the number of worms into the device, the auxiliary equipment including a power of excitation voltage (80 kHz at 30 Vpp), a signal amplifier, and an A/D converter should be connected with the device. The signals from the current pathway were amplified by the amplifier, and the enhanced signals were converted into voltage signals by an A/D converter which was connected to a computer. As shown in Fig. 2, when worms flowed through the measurement unit one by one, the voltage signals were obtained serially.
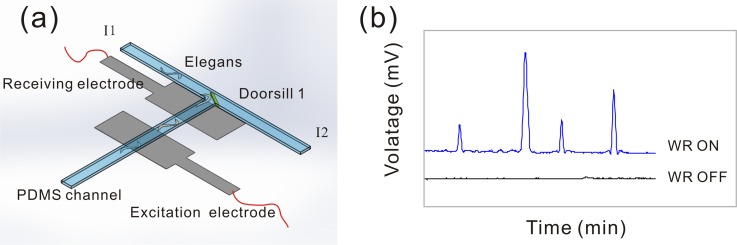
FIG. 2.
(a) The worm responder (WR) of the microfluidic device, which is composed of excitation electrode, receiving electrode and other auxiliary equipment. The two ITO electrodes of each worm responder are 0.8-mm wide, 1350 Å ± 150 Å in thickness with 0.8 mm spacing which are embedded under the cross-sectional area of T-shape micro channel. An optimal voltage signal (80 kHz at 30 Vpp) is applied to the excitation electrode. Worms are driven through the measurement area one by one at the flow rate of 5 μl min−1. (b) The schematic diagram of the voltage profile. Four peaks correspond to 4 worms passing through the measurement zone serially.
B. Device fabrication
The microfluidic network layer was fabricated in PDMS (Sylgard 184, Dow Corning, Midland, Michigan) by the cast molding technique. The SU-8 (Microchem Corp, Newton, Massachusetts) mold was fabricated by a method based on multiple coating and exposure steps and a single development step.40 The detailed fabrication process of the three-layer SU-8 mold including the unique microstructures of doorsills has been introduced in the supplementary material.41 To form a 2 mm-thick PDMS layer of microfluidic network structure, PDMS base and curing agent were mixed thoroughly with 10:1 ratio by mass, degassed under vacuum, and poured onto the mold. The PDMS layer was cured at 80 °C for 30 min. After cooling, the PDMS replica was gently peeled off from the mold. Then the microfluidic network layer was cut into round shape, and access holes were punched. The 1.1 mm-thick ITO-glass was obtained from Leybold Optics Corp. (Shenzhen, China). The layout of the ITO electrodes for worm responder was designed by CorelDraw software, which was shown in Fig. 1(b). The ITO electrodes were fabricated by a silk-screen and wet chemical etching procedure which has been introduced in detail in the previous reports.38 Next, the 100 μm-thick PDMS membrane was spin coated on the ITO glass with the side of conductivity electrodes, and then cured at 80 °C for 30 min. After oxygen plasma treatment for 3 min (PDC-32G plasma cleaner, Harrick Plasma, USA), the microfluidic network layer and PDMS coated ITO-glass were aligned and irreversibly bonded together at 80 °C for at least 3 h which constitute the multiple functional microfluidic system. The electrodes of the assembled microchip were connected to the detector by conducting silver resin. The syringe pumps (LongerPump, Bao-ding, China) were used as the driven power for the worm delivery and medium exchange. The signal amplifier and A/D converter should be connected to the device for worms-counting assay. Prior to experiments, the micro channels were incubated with K buffer (0.929 g NaCl, 0.716 g KCl in 300 ml water)42 mixed with 0.02 wt% Triton Х100 for 24 h.
C. Worm strains and maintenance
C. elegans strains used in this work were wild-type N2, transgenetic strains CL2166 (dvIs19 [pAF15 (gst-4::GFP::NLS)]), and BZ555 (egIs1 [dat-1p::GFP]). The transgenic strain CL2166 was used for estimating manganese induced oxidative stress which can express an oxidative stress-induced green fluorescence protein (GFP) under stress condition. The transgenic strain BZ555 was used for estimating manganese induced DAergic neurodegeneration which can express bright GFP in the dopamine neuronal soma. All the strains were cultured at 20 °C on Nematode Growth Medium (NGM) plates seeded with OP50. All experiments were performed using synchronized L4 worms which were obtained using an established method.43 The worms used in the on-chip manganese toxicity assessment and rescue assay survived without food OP50.
D. Microfluidic device operation
First, the liquid suspension of worms was transported from worm inlet I1 [Fig. 2(a)]. In this work, the density of the worm suspension was adjusted to about one worm every microliter to ensure that worms were counted by the worm responder one by one. After 20 worms passed through the detection unit which locates in the intersection region of T-shape channel at the flow rate of 5 μl min−1, the chip was quickly pressurized via external fluid pressure from the inlet I2 [Fig. 2(a)]. This rapid water flushed away the rest of the worms in the T-shape channel towards the inlet I1. Then, the 20 worms in front of doorsill 2 would be driven into the chamber by another external fluid pressure from the inlet I2. The pressure temporarily allowed the size of the gaps between the doorsill 2 and the wall of the micro channel to expand enough for worms entering the chamber. Here, the quick pressurization was generated by the operator pushing the syringe manually (pressure value not shown). After worms loading, two streams of the original solutions from the inlets (O1 and O2) were simultaneously delivered into the eight chambers at a rate of 20 μl min−1 through micro syringe pumps. The eight well-refined concentrations of chemicals were generated, and the device was maintained at 20 °C during the experiment.
E. Worm cultivation and treatment
Assisted by the worm responder, about 20 synchronous worms of L4 stage (N2, CL2166, or BZ555) were loaded into each chamber using the syringe pumps. When all the worms in the eight chambers were stabilized, 100 mM MnCl2 and buffer (K solution) were simultaneously perfused into the CGG to generate multiple concentrations for on-chip toxicity assessment. The duration of exposure to manganese was 48 h for N2, 24 h for CL2166 or BZ555. For on-chip rescue assay, natural antioxidants (Vitamin E, resveratrol, or quercetin) were used. After the worms were exposed to 100 mM MnCl2 for 10 h, the solution in the whole device was exchanged with the buffer. Then natural antioxidant (100 μM) and K solution were simultaneously infused into the CGG. Therefore, the rescue effect of natural antioxidants can be investigated at multiple levels. MnCl2 was directly dissolved in the K solution, while resveratrol and quercetin were dissolved in the K solution with 1% DMSO, and Vitamin E was also prepared in the K solution using 1% ethyl alcohol as solubilizer. The device was incubated in the dark at 20 °C and 85% relative humidity. For the long time assay, worms were incubated without the food OP50.
F. Survival ratio and fluorescence data acquisition
The movements of the worms in the chambers were observed using a stereomicroscope (SMZ-T4, Optec, Chongqing, China) with a digital camera (DV200) at 40× magnification. For the on chip assay of oxidative stress and DAergic neurodegeneration, fluorescent images of CL2166 and BZ555 were acquired through a fluorescent microscope (Nikon TS100F, Japan) with a Nikon DCFi1c cooled CCD camera at 200× or 400× magnification. The excitation wavelength is 470–495 nm and detection wavelength is 510–550 nm. The integral optical density (IOD) of the images was calculated with the image processing and analysis software (Image-Pro Plus, Media Cybernetics, USA), and the data were analyzed further using the data analysis software (Origin, Electronic Arts, Inc, USA).
III. RESULTS AND DISCUSSION
A. The mechanism of worm responders
Every T-shape micro channel corresponds to a single worm responder. The worm responder, as a key component in the microfluidic device, can guarantee the uniform number of worms in eight chambers. It is composed of excitation electrode, receiving electrode, and other auxiliary equipment. Fig. 1(b) shows the structure of ITO conductive electrodes. Since the receiving electrodes of eight detection units are independent while the excitation electrode is shared, eight worm responders can work without interfering with each other. Together with independently controlled worm responder and worm loading unit (including T-channel or doorsills), we can control the number of the worms in each chamber individually.
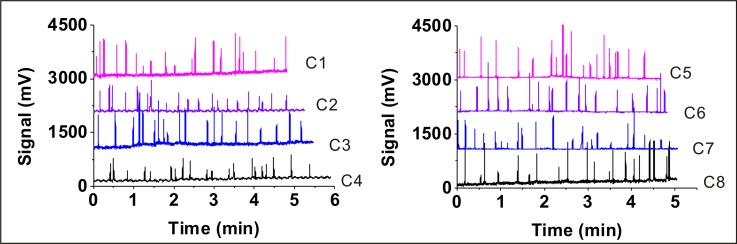
As shown in Fig. 2(a), the two ITO electrodes of each worm responder are 0.8-mm wide, 1350 Å ± 150 Å in thickness with 0.8 mm spacing which are embedded under the cross-sectional area of T-shape micro channel. An optimal voltage signal (80 kHz at 30 Vpp) was applied to the excitation electrode. The electrical resistivities of the worm's body and the deionized water (medium in the microfluidic channel) are 350 Ω cm (Ref. 44) and 18.3 MΩ cm, respectively. Based on the difference of the resistivity value, as shown in Fig. 2 and movie SII which has been introduced in the supplementary material,41 when worms approach and pass the receiving electrode, the change of the conductivity causes the increase of current in the signal pathway. Fig. 3 shows eight voltage profiles which correspond to the eight worm responders. Each voltage profile indicates the signals of time series with 20 worms passing through the measurement zone at the flow rate of 5 μl min−1. The magnitude of the signal is correlated to the worm's shape and speed of the worm passing through the worm responder.
FIG. 3.
The experimental data of the worm responders. Eight voltage profiles correspond to the eight worm responders. After 20 worms pass through the measurement area at the flow rate of 5 μl min−1, the voltage profile would be obtained. The worms finally were captured into the chamber 1–8 (C1-8).
Over 32 independent operations of different units, the number of worms in all chambers was from 20 to 24. Since the data processing is based on the condition that worms pass through the worm responder one by one, it cannot record the rare event of several worms going through the detection unit together. As shown in the supplementary material,41 we illustrated this disability of the microsystem clearly. The device enabled the encapsulation of average 20 animals into a chamber with 96.1% probability. Compared to the previous work in our research group,29 our on-chip electrical counting mechanism possesses obvious advantages. First, our loading mechanism has higher probability to allow the exact number of worms in the chambers. Second, in the previous research, the number of the worms was limited by the length of the micro channels, so it is impossible to load a large number of worms. Theoretically, our counting mechanism is superior to their mechanism. Last, our counting process is serial and semi-automatic. Compared to the random loading method by Yang et al.,29 it could be recommendable for the operators to load a certain number of worms into all the chambers in a parallel manner at once.
The excellent optical qualities of PDMS and ITO-glass allow direct observation via microscopy. Due to the feature of non-contact between electrodes and medium in the micro channel, this technique can detect the worms through a nondestructive and noninvasive method. In addition, this unique design of the worm responder is simple and low-cost, and it does not require any specialized or expensive off-chip components.
B. Characterization of concentration gradient generation
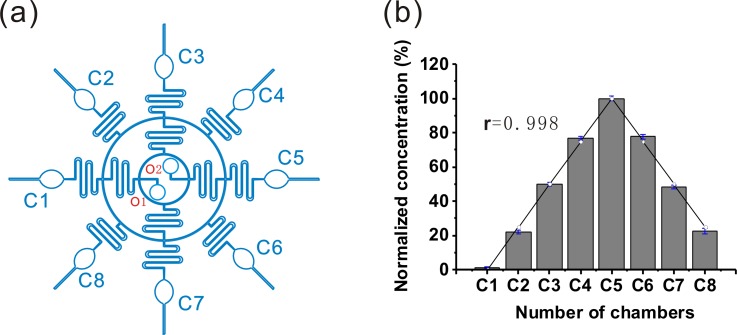
The chemical concentration gradient between adjacent chambers was realized by repeated splitting and mixing of the source solutions in a disk-shaped CGG structure [Fig. 4(a)], which was similar to the method reported previously.45 Fluorescein isothiocyanate (FITC) was chosen as the indicator to evaluate the on-chip chemical concentration gradient because it has a comparable molecular weight to the tested chemicals in the work and its fluorescence intensity is proportional to its concentration.29 We evaluated the effect of CGG by the correlation factor between the experimental data and the theoretical estimations. The high correlation factor shows high quality of CGG which can guarantee the distribution of the chemicals similar to the estimated value.
FIG. 4.
Microfluidic chemical concentration gradient generation (CGG) in a single device for on-chip toxicity assessment and rescue assay. (a) The construction of CGG in the device. O1 and O2 indicate the two central chemicals inlets. Cn means the nth chamber (1 ≤ n ≤ 8). (b) Quantitative comparison of the formed chemical concentrations in the terminal chambers between the actual and estimated (○) experiments. The actual concentration was inferred from fluorescence intensity of FITC in the eight chambers. The correlation coefficient between the experimental data and theoretic prediction is 0.998.
In this work, two types of model chemicals (O1 K solution; O2 100 μM FITC) were simultaneously infused at the same flow rate into the dilution networks by syringe pumps. Based on the principle of laminar flow, the fluid mixing in the serpentine channels and fluid splitting at the channel cross points in our device can generate a good chemical gradient.45 Eight well-refined chemical concentrations spanning the two inlet concentrations (0 and 100 μM) in the eight chambers can be quantified by the fluorometer. Furthermore, the performance of CGG was estimated by comparing the experimental results with the theoretical calculations. According to the dilution network of CGG, the theoretical calculations of the gradient concentrations at exit chambers fit Eq. (1) described by Yang et al.,37
| (1) |
where the concentration value at the nth chamber (Cn) has been given, n means the code number of the chambers (1 ≤ n ≤ 8), and C0 is the initial concentration of chemical. The code number of the chambers has been marked in Fig. 4(a).
The prerequisite for well-defined concentration gradient generation is complete mixing of fluids in the branch micro channels. Obviously, large length of the branch channels and low velocity can facilitate the diffusive mixing. As a balance between the generation time of concentration gradient and thorough mixing, the flow rate was optimized as 20 μl min−1. As shown in Fig. 4(b), the correlation factor between the experimental data and the theoretical estimations was 0.998. The results showed that the change trends of the chemical concentrations between the terminal chambers were the same, in other words, the chemical concentration gradient was successfully constructed across this device. It should be noted that due to the symmetrical design, this CGG can generate two similar gradients. In Secs. III C (“On-chip toxicity assessment”) and III D (“On-chip rescue assay),” all the results reported are for five chambers to avoid repeatability.
C. On-chip toxicity assessment
To obtain better understanding of manganese-induced toxicity on-chip, a strategy of consistent exposure was performed. Prior to the test, synchronized L4 worms (N2, CL2166, or BZ555) in deionized water were delivered into the chip. With the worm responder, we controlled the amount of the worms into the chambers (∼20 worms each chamber). After worms settled down in the chambers, the chemical solution (MnCl2) and buffer were infused into the chambers from the two central chemicals inlets together. The exposed concentration of Mn is from 0 to 100 mM. To guarantee the uniformity of the chemical concentration, the flow of chemical solution was continuous for 48 h.
1. Manganese induced mobility defects
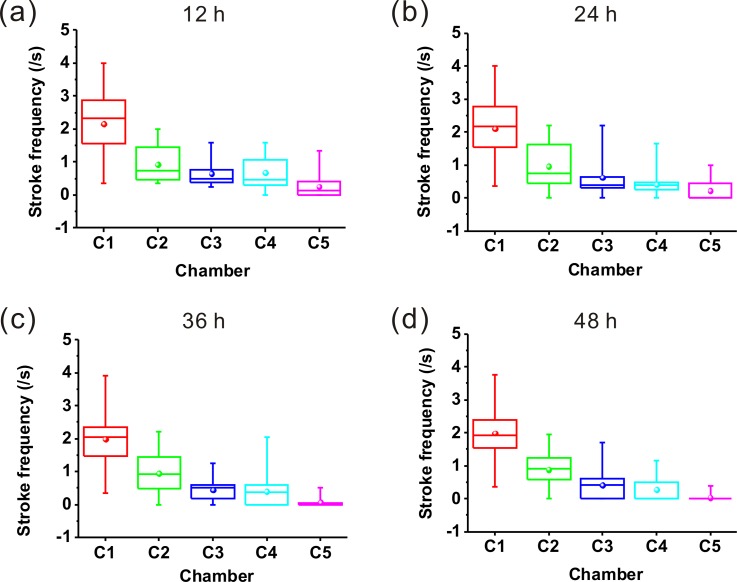
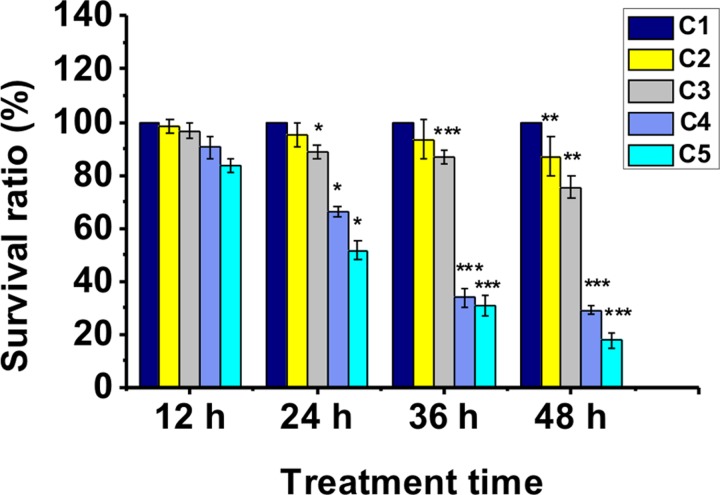
In this work, we investigated the mobility behavior of worms every 12 h. It was found that the untreated worms exhibited free movements with sine wave-shape and C-shape at the test days. However, the treated worms began to appear different mobility defects, such as curly- or tetanic-state. For further understanding manganese-induced mobility defects, the stroke frequencies (number per second) of worms after treatment with or without MnCl2 in chambers 1–5 were recorded (Fig. 5). We plotted the stroke frequency as a boxplot representation. The bottom and top of each box correspond to the first and third quartiles, respectively, while the horizontal line inside the box denotes the median. The ends of whiskers are the minimum and maximum values of the data set. And the sphere indicates the mean. Fig. 5(a) showed the stroke frequencies of worms exposed to MnCl2 at 12 h. As a control comparison, we can see that the stroke frequencies of the worms in chamber 1 were relatively higher. However, the stroke frequencies of worms in chambers 2–5 gradually decreased. Figs. 5(b)–5(d) also demonstrated the decreased stroke frequencies with the increasing concentration of MnCl2. In most cases, worms in chamber 5 exhibited low stroke frequency and their movements often “frozen” in the curly- or tetanic-state. Fig. 6 demonstrated that the worms were sensitive to high dose of MnCl2. The survival rates of the treated worms decreased significantly with the increase of chemical concentration from chamber 1 to chamber 5 and with the increase of incubation time. We also summarized the survival rates of the strains BZ555 and CL2166, the results were similar to N2 (data shown in the supplementary material41). There is no doubt that manganese induced an obvious change of the worm's mobility behavior in a dose- and time-dependent manner.
FIG. 5.
Stroke frequencies of worms in chamber 1–5 exposed to MnCl2 for (a) 12 h, (b) 24 h, (c) 36 h, (d) 48 h. The bottom and top of each box correspond to the 25th and 75th percentiles, respectively, while the horizontal line inside the box denotes the median. The ends of whiskers are the minimum and maximum values of the data set. And the sphere indicates the mean. 20 worms used in each chamber.
FIG. 6.
The survival rates of the worms (N2) in the chamber 1–5 after exposure to MnCl2 (the largest concentration of MnCl2 in chamber 5 can achieve about 100 mM). The asterisks indicate a statistically significant difference from the control group (chamber 1, *P < 0.05, **P < 0.01, and ***P < 0.001). 20–24 worms used in each assay, 48 h of exposure time and 3 independent assays under each condition. Error bars denote the SD.
2. Manganese induced DAergic neurodegeneration
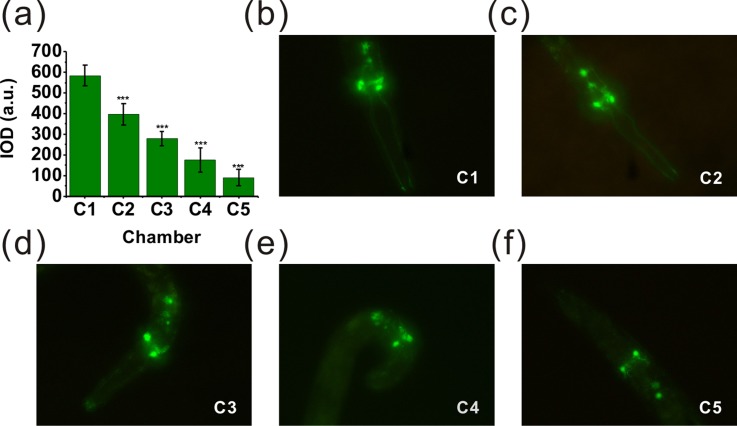
It is reported that intrastriatal Mn injections can result in loss of DAergic neurons, and oxidative stress plays a significant role in this process.1,32,46 For further investigation, we estimated the possible DAergic neurodegeneration induced by manganese using C. elegans. The transgenic strain BZ555, as an ideal model system, can be used to estimate this effect. All DAergic neurons of BZ555 are tagged with GFP. Here, fluorescence intensity was analyzed only in the nerve ring of the head, which contains GFP-tagged DAergic neurons. After all the loaded worms were stabilized in the chambers, 1M sodium azide (NaN3) was used to immobilize the worms for fluorescence image analysis. As shown in Fig. 7, the representative fluorescence images of GFP expression of the worms in different chambers were obtained. Fig. 7(a) showed that treatment with manganese at different concentrations (0–100 mM) for 24 h resulted in a statistically significant reduction in fluorescence intensity compared to the control group in chamber 1 (***P < 0.001). Fig. 7(b) demonstrated the L4 worms in chamber 1 expressed intact and strong GFP. However, the worms in chamber 5 [Fig. 7(f)] showed a significant decrease of GFP expression after treatment with about 100 mM MnCl2, and this corresponded to a shrinkage of the soma of DAergic neurons. All those confirmed that the obvious effects of DAergic neurodegeneration were triggered by manganese.
FIG. 7.
The on-chip treatments of BZ555 nematodes. Photomicrographs (400× magnification) are from the worms in chamber 1–5 after exposure to MnCl2. The IOD was determined as described in the “Experimental” section (Sec. II). (a) Data were presented as mean IOD ± SD of the DAergic neurons in the nerve ring of the worms treated with MnCl2. (b) Photomicrograph of the worm treated with MnCl2 in chamber 1 (control group). (c)–(f) Photomicrographs of the worms treated with MnCl2 in the chamber 2–5. All the photomicrographs showed the decreased sizes of DAergic neurons compared to the control group. The asterisks indicate a statistically significant difference from the control group (***P < 0.001). 20–24 worms used in each assay, 24 h of exposure time and 3 independent assays under each condition.
3. Manganese induced oxidative stress
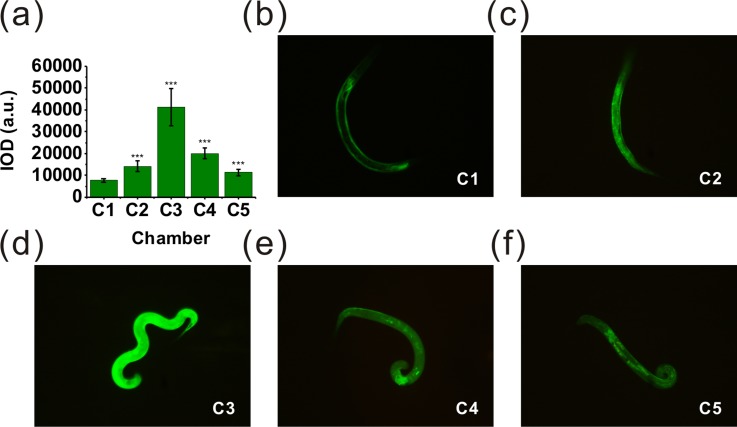
With respect to the probable connection between DAergic neurodegeneration and the increased generation of reactive oxygen species, we also used worm strain CL2166 to identify this probability on the microfluidic platform. CL2166 can express an oxidative stress-induced GFP under stress condition. The fluorescence intensity of GFP in CL2166 showed the level of inner oxidative stress under different stress condition. Fig. 8 showed the representative fluorescence images of GFP expression of the L4 worms in different chambers. As shown in Fig. 8(a), following a 24 h treatment with manganese at multiple levels, a significant enhancement in fluorescence intensity was noted in gst-4::GFP. The fluorescence intensity of worms in chamber 3 increases 5.3 fold compared to the control group (***P < 0.001). The observed fluorescence intensity became much brighter after treatment with manganese at different concentrations, which showed the increased generation of inner reactive oxygen species of C. elegans. Otherwise, the fluorescence intensity of worms in chamber 4 [Fig. 8(e)] and chamber 5 [Fig. 8(f)] decreased more than that in chamber 3 [Fig. 8(d)], which can be speculated that the manganese of high concentration can cause cell death of the worms.47,48
FIG. 8.
The on-chip treatments of CL2166 nematodes. Photomicrographs (200× magnification) are from the worms in chamber 1–5 after exposure to MnCl2. The IOD was determined as described in the “Experimental” section (Sec. II). (a) Data were presented as mean IOD ± SD of the oxidative stress fluorescence of the strain CL2166 treated with MnCl2. (b) Photomicrograph of the worm treated with MnCl2 in chamber 1 (control group). (c)–(f) Photomicrographs of the worms treated with MnCl2 in chambers 2–5. All the photomicrographs showed MnCl2 can induce much stronger oxidative stress than the control group. The fluorescence intensity of the worms in chamber 3 is the maximum value. With the increasing concentration of MnCl2, the fluorescence intensity decreases which can be explained that Mn of high concentration can cause the apoptosis of the somatic cell of the worms. The asterisks indicate a statistically significant difference from the control group (***P < 0.001). 20–24 worms used in each assay, 24 h of exposure time and 3 independent assays under each condition.
D. On-chip rescue assay
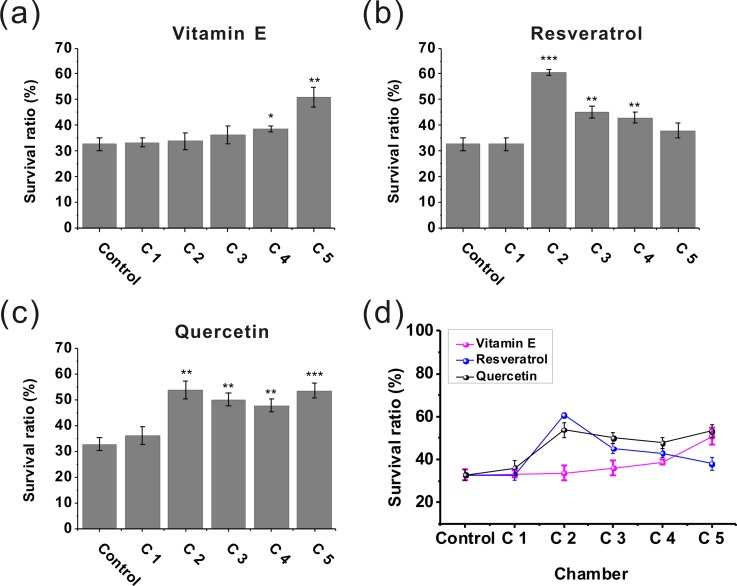
We then applied the developed platform to assess the effect of natural antioxidants (Vitamin E, resveratrol, and quercetin) to protect manganese-induced toxicity. When all the worms in the eight chambers were stabilized, 100 mM MnCl2 was infused into the microfluidic device from the two inlets of the CGG. After the worms were pretreated with 100 mM MnCl2 for 10 h, the natural antioxidant (100 μM) and K solution were simultaneously infused into the CGG. The rescue effect of natural antioxidants can be investigated at multiple levels after 7 days. Fig. 9 showed the survival rates of the exposed worms (100 mM MnCl2 for 10 h) after the treatment with natural antioxidants (Vitamin E, resveratrol or quercetin) for 7 days at different concentration (the maximum concentration in Chamber 5 about 100 μM). As shown in Fig. 9(a), the treatment effect of Vitamin E (chamber 5) was statistically significant (**P < 0.01) compared to the control group without any treatment (chamber 1). Quercetin also had significant effect of rescuing the exposed worms in chamber 5 [***P < 0.001, Fig. 9(c)]. Among these natural antioxidants, resveratrol can increase the worm survival rates by about 2-fold than the control group [chamber 2, Fig. 9(b)]. However, the treatment effect of resveratrol decreased with the increasing concentration, so we speculated that because the resveratrol of high concentration has certain toxicity for rescuing manganese-induced neurotoxicity.49 Above all, the antioxidants could rescue the exposed worms to varying degrees. As a conclusion, the natural antioxidants (Vitamin E, resveratrol, and quercetin) are effective to rescue the worms after exposure to manganese.
FIG. 9.
The performances of the microfluidic device for estimating the effects of natural antioxidants to protect manganese-induced toxicity. First, the worms were exposed to MnCl2 (100 mM) for 10 h. The survival rates of the exposed worms after treatment with different natural antioxidants of different concentrations in chamber 1–5 were investigated. (a) Vitamin E, (b) Resveratrol, and (c) Quercetin. The asterisks indicate a statistically significant difference from the control group (*P < 0.05, **P < 0.01, and ***P < 0.001). (d) Comparison of the treatment effects of vitamin E, resveratrol, and quercetin. The treatment effect of resveratrol did not rise with the increasing of drug concentration, and we speculated that was because resveratrol of high concentration has certain toxicity for treating Mn-induced neurotoxicity. 20–24 worms used in each assay, 7 days of treatment time, and 3 independent assays under each condition. Error bars denote the SD.
IV. CONCLUSIONS
An integrated microfluidic system has been developed to evaluate the environmental manganese toxicity. Assisted by the key components worm responders and doorsills, the device can load about 20 worms into each chamber noninvasively. The on-chip electrical counting process based on electric impedance sensing is serial and semi-automatic. It could be recommendable for the operators to load a certain number of worms into all the chambers in a parallel manner at once. More importantly, the unique microfluidic component doorsill retains the worms in the chambers tactfully, which enables the confined microenvironment of chemical stimuli for worms and convenient observation of multiple behavioral responses of worms to chemicals. In addition, the excellent optical qualities of PDMS and ITO-glass which compose the microfluidic system allow direct observation via microscopy. Because the concentration gradient system was a radial channel network, an increasing number of concentric circular channels and branch channels can expand the number of the downstream ends of the serpentine branch channels, which further improve the throughput. To demonstrate these features of our device, we then employed this device for the on-chip toxicity assessment and on-chip rescue assay in term of manganese-induced neurodegenerative disease. It was found that the worms exhibit obvious mobility defects, oxidative stress, and DAergic neurodegeneration after exposure to manganese. Natural antioxidants can rescue the exposed worms effectively. The established device is low-cost, biocompatible, and multifunctional. We believe that the trend of lab-on-a-chip for C. elegans will focus on the high-throughput manipulation and automation. There would be more significant work on microfluidics-based assay for C. elegans or other multicellular organisms (Danio rerio and Drosophila melanogaster) for toxicity evaluation or drug screening.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 21375152). The authors thank Professor Zhong Pei, Neurology Department of the First Affiliated Hospital of Sun Yat-Sen University for providing the CL2166 and BZ555 C. elegans strains.
References
- 1.Leung M. C. K., Williams P. L., Benedetto A., Au C., Helmcke K. J., Aschner M., and Meyer J. N., Toxicol. Sci. 106, 5 (2008). 10.1093/toxsci/kfn121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chronis N., Lab Chip 10, 432 (2010). 10.1039/b919983g [DOI] [PubMed] [Google Scholar]
- 3.Hulme S. E. and Whitesides G. M., Angew. Chem. Int. Ed. 50, 4774 (2011). 10.1002/anie.201005461 [DOI] [PubMed] [Google Scholar]
- 4.Shi W., Wen H., Lin B., and Qin J., Top. Curr. Chem. 304, 323 (2011). 10.1007/128_2011_145 [DOI] [PubMed] [Google Scholar]
- 5.Crane M. M., Chung K., Stirman J., and Lu H., Lab Chip 10, 1509 (2010). 10.1039/b927258e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray J. M., Karow D. S., Lu H., Chang A. J., Chang J. S., Ellis R. E., Marletta M. A., and Bargmann C. I., Nature 430, 317 (2004). 10.1038/nature02714 [DOI] [PubMed] [Google Scholar]
- 7.Shi W., Qin J., Ye N., and Lin B., Lab Chip 8, 1432 (2008). 10.1039/b808753a [DOI] [PubMed] [Google Scholar]
- 8.Shi W., Wen H., Lu Y., Shi Y., Lin B., and Qin J., Lab Chip 10, 2855 (2010). 10.1039/c0lc00256a [DOI] [PubMed] [Google Scholar]
- 9.Han B., Kim D., Ko U. H., and Shin J. H., Lab Chip 12, 4128 (2012). 10.1039/c2lc40209b [DOI] [PubMed] [Google Scholar]
- 10.Rezai P., Salam S., Selvaganapathy P. R., and Gupta B. P., Lab Chip 12, 1831 (2012). 10.1039/c2lc20967e [DOI] [PubMed] [Google Scholar]
- 11.Rezai P., Siddiqui A., Selvaganapathy P. R., and Gupta B. P., Lab Chip 10, 220 (2010). 10.1039/b917486a [DOI] [PubMed] [Google Scholar]
- 12.Rezai P., Salam S., Selvaganapathy P. R., and Gupta B. P., Biomicrofluidics 5, 044116 (2011). 10.1063/1.3665224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr J. A., Parashar A., Gibson R., Robertson A. P., Martin R. J., and Pandey S., Lab Chip 11, 2385 (2011). 10.1039/c1lc20170k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai X., Zhuo W., Liang Q., McGrath P. T., and Lu H., Lab Chip 14, 1746 (2014). 10.1039/c3lc51334c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johari S., Nock V., Alkaisi M. M., and Wang W., Lab Chip 13, 1699 (2013). 10.1039/c3lc41403e [DOI] [PubMed] [Google Scholar]
- 16.Jung J., Nakajima M., Tajima H., Huang Q., and Fukuda T., J. Micromech. Microeng. 23, 085008 (2013). 10.1088/0960-1317/23/8/085008 [DOI] [Google Scholar]
- 17.Jung J., Nakajima M., Tajima H., Huang Q., and Fukuda T., J. Micromech. Microeng. 24, 035012 (2014). 10.1088/0960-1317/24/3/035012 [DOI] [Google Scholar]
- 18.Saldanha J. N., Parashar A., Pandey S., and Powell-Coffman J. A., Toxicol. Sci. 135, 156 (2013). 10.1093/toxsci/kft138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lycke R., Parashar A., and Pandey S., Biomicrofluidics 7, 064103 (2013). 10.1063/1.4829777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng F., Rohde C. B., and Yanik M. F., Lab Chip 8, 653 (2008). 10.1039/b804808h [DOI] [PubMed] [Google Scholar]
- 21.Chokshi T. V., Ben-Yakar A., and Chronis N., Lab Chip 9, 151 (2009). 10.1039/b807345g [DOI] [PubMed] [Google Scholar]
- 22.Wen H., Shi W., and Qin J., Biomed. Microdevices 14, 721 (2012). 10.1007/s10544-012-9652-9 [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Feng X., Du W., and Liu B. F., Anal. Chim. Acta 701, 23 (2011). 10.1016/j.aca.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 24.Hulme S. E., Shevkoplyas S. S., Apfeld J., Fontana W., and Whitesides G. M., Lab Chip 7, 1515 (2007). 10.1039/b707861g [DOI] [PubMed] [Google Scholar]
- 25.Chung K., Crane M. M., and Lu H., Nat. Methods 5, 637 (2008). 10.1038/nmeth.1227 [DOI] [PubMed] [Google Scholar]
- 26.Krajniak J. and Lu H., Lab Chip 10, 1862 (2010). 10.1039/c001986k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang H., Krajniak J., Matsunaga Y., Benian G. M., and Lu H., Lab Chip 14, 3498 (2014). 10.1039/C4LC00697F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghorashian N., Gökçe S. K., Guo S. X., Everett W. N., and Ben-Yakar A., PloS One 8, e74480 (2013). 10.1371/journal.pone.0074480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J., Chen Z., Ching P., Shi Q., and Li X., Lab Chip 13, 3373 (2013). 10.1039/c3lc50264c [DOI] [PubMed] [Google Scholar]
- 30.Chung K., Zhan M., Srinivasan J., Sternberg P. W., Gong E., Schroeder F. C., and Lu H., Lab Chip 11, 3689 (2011). 10.1039/c1lc20400a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olanow C. W., Ann. N.Y. Acad. Sci. 1012, 209 (2004). 10.1196/annals.1306.018 [DOI] [PubMed] [Google Scholar]
- 32.Benedetto A., Au C., and Aschner M., Chem. Rev. 109, 4862 (2009). 10.1021/cr800536y [DOI] [PubMed] [Google Scholar]
- 33.Aschner M., Erikson K. M., Hernández E. H., and Tjalkens R., Neuromol. Med. 11, 252 (2009). 10.1007/s12017-009-8083-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedetto A., Au C., Avila D. S., Milatovic D., and Aschner M., PLoS Genet. 6, e1001084 (2010). 10.1371/journal.pgen.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negga R., Stuart J. A., Machen M. L., Salva J., Lizek A. J., Richardson S. J., Osborne A. S., Mirallas O., McVey K. A., and Fitsanakis V. A., Neurotox. Res. 21, 281 (2012). 10.1007/s12640-011-9274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilarte T. R., Environ. Health Perspect. 118, 1071 (2010). 10.1289/ehp.0901748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C. G., Wu Y. F., Xu Z. R., and Wang J. H., Lab Chip 11, 3305 (2011). 10.1039/c1lc20123a [DOI] [PubMed] [Google Scholar]
- 38.Zhao J., Chen Z., Li X., and Pan J., Talanta 85, 2614 (2011). 10.1016/j.talanta.2011.08.029 [DOI] [PubMed] [Google Scholar]
- 39.Jung J., Nakajima M., Kojima M., Ooe K., and Fukuda T., J. Micro-Nano Mechatron. 7, 3 (2012). 10.1007/s12213-011-0036-7 [DOI] [Google Scholar]
- 40.Mata A., Fleischman A. J., and Roy S., J. Micromech. Microeng. 16, 276 (2006). 10.1088/0960-1317/16/2/012 [DOI] [Google Scholar]
- 41.See supplementary material at http://dx.doi.org/10.1063/1.4896663E-BIOMGB-8-014405 for details on fabrication process of the three-layer SU-8 mold, voltage signal for several worms passing through the worm responder together, comparison of the sensitivities of the used strains in respond to manganese on chip and introduction for the movies.
- 42.Williams P. L. and Dusenbery D. B., Environ. Toxicol. Chem. 9, 1285 (1990). 10.1897/1552-8618(1990)9[1285:ATTUTN]2.0.CO;2 [DOI] [Google Scholar]
- 43.Stiernagle T., “Maintenance of C. elegans” (February 11, 2006), in WormBook, edited by The elegans C. Research Community (WormBook, 2006), pp. 1–11, http://www.wormbook.org 10.1895/wormbook.1.101.1 [DOI] [Google Scholar]
- 44.Liu P., Martin R. J., and Dong L., Lab Chip 13, 650 (2013). 10.1039/c2lc41174a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Liu W., Wang Y., Wang J. C., Tu Q., Liu R., and Wang J., Lab Chip 13, 695 (2013). 10.1039/c2lc40661f [DOI] [PubMed] [Google Scholar]
- 46.Jenner P., Ann. Neurol. 53, S26 (2003). 10.1002/ana.10483 [DOI] [PubMed] [Google Scholar]
- 47.Roth J. A. and Garrick M. D., Biochem. Pharmacol. 66, 1 (2003). 10.1016/S0006-2952(03)00145-X [DOI] [PubMed] [Google Scholar]
- 48.Choi C. J., Anantharam V., Saetveit N. J., Houk R. S., Kanthasamy A., and Kanthasamy A. G., Toxicol. Sci. 98, 495 (2007). 10.1093/toxsci/kfm099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cottart C.-H., Nivet-Antoine V., Laguillier-Morizot C., and Beaudeux J. L., Mol. Nutr. Food Res. 54, 7 (2010). 10.1002/mnfr.200900437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4896663E-BIOMGB-8-014405 for details on fabrication process of the three-layer SU-8 mold, voltage signal for several worms passing through the worm responder together, comparison of the sensitivities of the used strains in respond to manganese on chip and introduction for the movies.