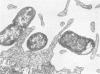
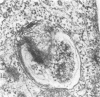
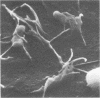
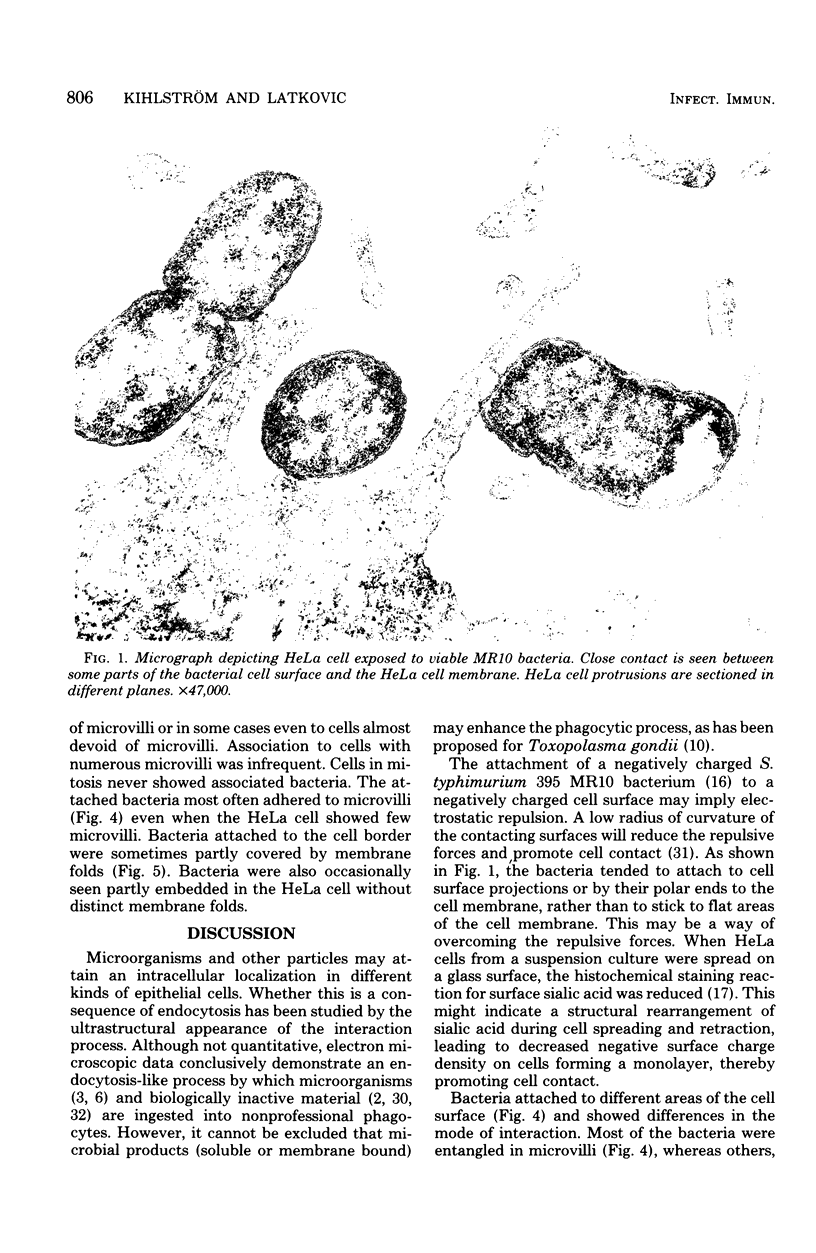
Abstract
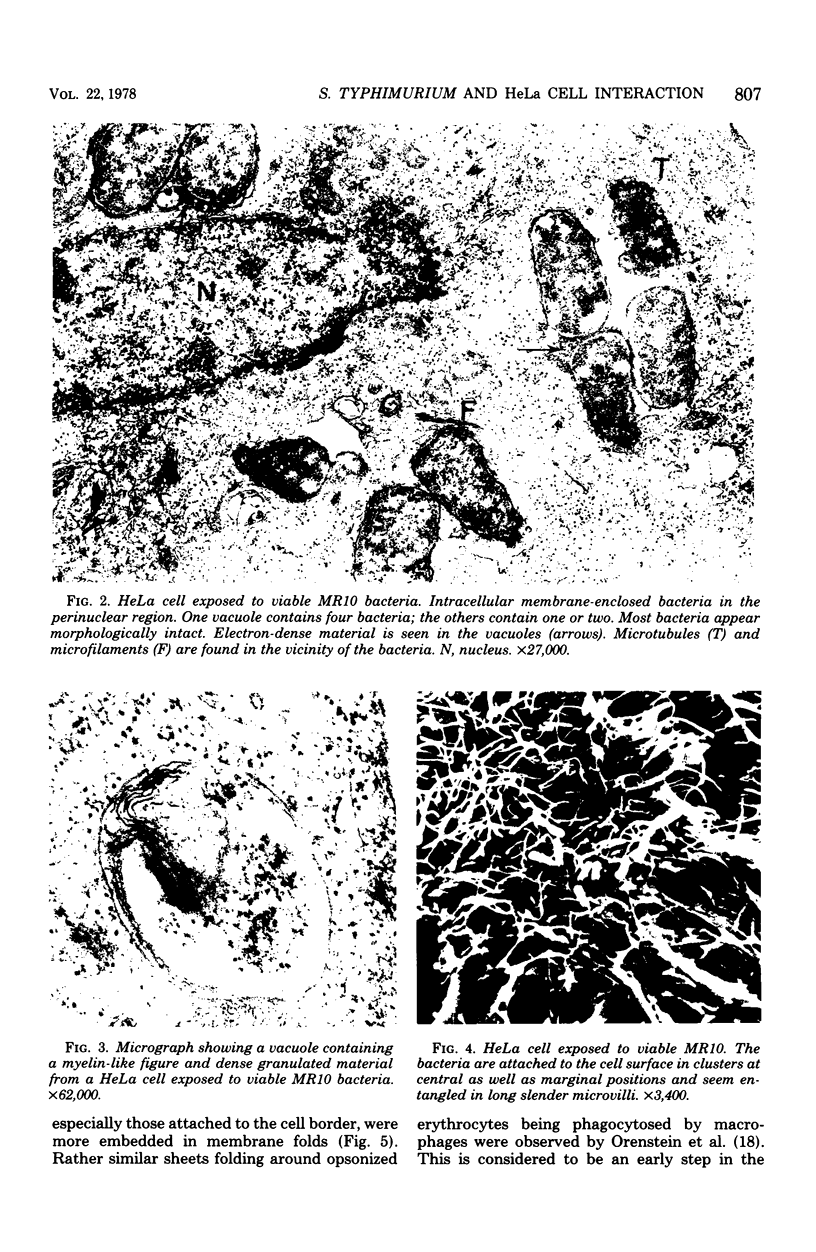
The interaction of Salmonella typhimurium 395 MS and its rough Rd-mutant 395 MR10 with HeLa cells was studied by transmission and scanning electron microscopy. The bacteria attached to central as well as more marginal positions of the HeLa cell surface. Bacteria associated preferentially to HeLa cells with a relatively low number of microvilli, in which they often were entangled. Bacteria attached to the cell border were sometimes surrounded by membrane folds, possibly as a response to their attachment. Infected cells had longer and more slender microvilli compared with noninfected cells. Some parts of the attached bacteria were in close contact with the HeLa cell membrane, whereas other parts were separated from the latter by a gap. Bacteria adhered preferentially to microvilli without obvious membrane damage. Most of the intracellular bacteria were surrounded by a membrane, often appearing as a vacuole, which sometimes contained more than one bacterium. Intracellular bacteria seemed to be morphologically intact. We propose that S. typhimurium enter HeLa cells by a process of phagocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Jauregui H. O., Chang J., Trump B. F. Studies on cellular autophagocytosis. Relationship between heterophagy and autophagy in HeLa cells. Lab Invest. 1971 Feb;24(2):162–174. [PubMed] [Google Scholar]
- Bovallius A., Nilsson G. Ingestion and survival of Y. pseudotuberculosis in HeLa cells. Can J Microbiol. 1975 Dec;21(12):1997–2007. doi: 10.1139/m75-287. [DOI] [PubMed] [Google Scholar]
- Cunningham R. K., Söderström T. O., Gillman C. F., van Oss C. J. Phagocytosis as a surface phenomenon. V. Contact angles and phagocytosis of rough and smooth strains of Salmonella typhimurium, and the influence of specific antiserum. Immunol Commun. 1975;4(5):429–442. doi: 10.3109/08820137509057331. [DOI] [PubMed] [Google Scholar]
- Edebo L., Normann B. Virulence and immunogenicity of mutant strains of Salmonella typhimurium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):75–84. doi: 10.1111/j.1699-0463.1970.tb04271.x. [DOI] [PubMed] [Google Scholar]
- Evans B. A. Ultrastructural study of cervical gonorrhea. J Infect Dis. 1977 Aug;136(2):248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- FOGH J., FOGH H. A METHOD FOR DIRECT DEMONSTRATION OF PLEUROPNEUMONIA-LIKE ORGANISMS IN CULTURED CELLS. Proc Soc Exp Biol Med. 1964 Dec;117:899–901. doi: 10.3181/00379727-117-29731. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Edebo L. Association of viable and inactivated Salmonella typhimurium 395 MS and MR 10 with HeLa cells. Infect Immun. 1976 Oct;14(4):851–857. doi: 10.1128/iai.14.4.851-857.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977 Aug;17(2):290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Tagesson C., Edebo L., Johansson G. The tendency of smooth and rough Salmonella typhimurium bacteria and lipopolysaccharide to hydrophobic and ionic interaction, as studied in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Jun;85(3):212–218. doi: 10.1111/j.1699-0463.1977.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Mareel M., De Ridder L., Deman J. Histochemical study of the distribution of sialic acid on the surface of HeLa cells in culture. Histochemie. 1972;32(4):335–341. doi: 10.1007/BF00305767. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Shelton E. Membrane phenomena accompanying erythrophagocytosis. A scanning electron microscope study. Lab Invest. 1977 Apr;36(4):363–374. [PubMed] [Google Scholar]
- Osada Y., Ogawa H. Phagocytosis stimulation by an extracellular product of virulent Shigella flexneri 2a. Microbiol Immunol. 1977;21(1):49–55. doi: 10.1111/j.1348-0421.1977.tb02807.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Perers L., Andåker L., Edebo L., Stendahl O., Tagesson C. Association of some enterobacteria with the intestinal mucosa of mouse in relation to their partition in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):308–316. doi: 10.1111/j.1699-0463.1977.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Fonte V., Weiss G. A scanning microscope study of the topography of HeLa cells. Cancer Res. 1974 Jun;34(6):1385–1394. [PubMed] [Google Scholar]
- Rowley A. F., Ratcliffe N. A. An ultrastructural study of the in vitro phagocytosis of Escherichia coli by the hemocytes of Calliphora erythrocephala. J Ultrastruct Res. 1976 May;55(2):193–202. doi: 10.1016/s0022-5320(76)80066-4. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Magnusson K. E., Tagesson C., Cunningham R., Edebo L. Characterization of mutants of Salmonella typhimurium by counter-current distribution in an aqueous two-polymer phase system. Infect Immun. 1973 Apr;7(4):573–577. doi: 10.1128/iai.7.4.573-577.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo M. Partition of Salmonella typhimurium in a two-polymer acqueous phase system in relation to liability to phagocytosis. Infect Immun. 1973 Jul;8(1):36–41. doi: 10.1128/iai.8.1.36-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K., Konrad K. Phagocytosis of latex beads by epidermal keratinocytes in vivo. J Ultrastruct Res. 1972 May;39(3):262–280. doi: 10.1016/s0022-5320(72)90022-6. [DOI] [PubMed] [Google Scholar]