Abstract
Because of the complex causal factors leading to depression, epigenetics is of considerable interest for the understanding effect of stress in depression. Dopamine is a key neurotransmitter important in many physiological functions, including motor control, mood, and the reward pathway. These factors lead many drugs to target Dopamine receptors in treating depressive disorders. In this review, we try to portray how chronic stress as an epigenetic factor changes the gene regulation pattern by interrupting Dopamine signaling mechanism.
Keywords: epigenetics, chronic stress, dopamine signaling, depression
Introduction
Stress is a normal physical response to events that make a person feel threatened or upset their balance in some way. When danger is sensed—whether it’s real or imagined—the body’s defenses kick into high gear in a rapid, automatic process known as the “fight-or-flight” reaction, or the stress response. Stress is therefore good for a person. It keeps one alert, motivated, and primed to respond to danger. As anyone who has faced a work deadline or competed in a sport can attest, stress mobilizes the body to respond, thereby improving performance. Yet too much stress or chronic stress may lead to major depression in susceptible people.
The Global Burden of Disease Study predicted major depression to become the second leading cause of disability by 2020. Lifetime prevalence was estimated to be approximately 17% in the United States, with similar rates being reported on the European level.1 Major depressive disorders (MDD) displays a variety of psychopathological symptoms and diverse clinical manifestations, with depressed mood and/or a loss of interest or pleasure, as core symptoms. Additional symptoms encompass changes to weight, appetite, sleep, psychomotor, as well as thinking disturbances that include excessive worrying, guilt, and possibly suicidal ideation.2 Modulating the brain’s reward and motivation circuits, governed primarily by Dopamine (a monoamine neurotransmitter), has therefore become one of the most attractive targets for treating depressive disorders.3
The neurotransmitter Dopamine was first identified as a potential neurotransmitter in the brain in the late 1950s by Carlsson.4 Dopamine plays a key role in the regulation of various physiological functions of a normal brain, including reward, locomotion, behavior, learning, and emotion. The brain contains two major groups of dopamine neurons. One is located in the arcuate nucleus of the hypothalamic median eminence and is involved in neuroendocrine regulation. The other is located in the ventral mesencephalon and projects to the forebrain.5
Dopamine exerts its effects on neurons through five known subtypes of dopamine receptors, which are grouped into two classes D1-like (D1, D5) and D2-like (D2, D3, D4). All Five subtypes of dopamine receptors (D1R-D5R) belonging to the G-protein coupled receptor (GPCR) superfamily have been cloned, through which dopamine transduces its various effects. D1 receptors couple to the adenylate cyclase stimulatory G protein Gαs and increase cAMP levels, thereby activating a cAMP-dependent protein kinase (PKA). This enzyme transfers a phosphate group from adenosine triphosphate to several specific protein substrates, modifying their properties in many ways. D2 receptors on the other hand are coupled to heterotrimeric G proteins Gαi/o, which decrease cAMP levels and alter the permeability of different ion channels. While D1 and D2 receptors have opposite effects at the molecular level, they often have a synergistic action.5 Neurons in the midbrain project their axons to the striatum and release dopamine, which modulates cAMP production by activating D1 and D2 receptors expressed by striatal neurons. Striatal neurons also receive input from neurons located the cortex that release the excitatory neurotransmitter glutamate. This results in stimulation of AMPA (2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid) and NMDA (N-Methyl-D-aspartic acid or N-Methyl-D-aspartate) ligand-gated ion channels and increases the intracellular concentration of Ca2+, leading to activation of signaling pathways dependent upon this second messenger. Among the downstream effectors of cAMP and Ca2+ are DARPP-32 (a dopamine- and cAMP-regulated phosphoprotein with a molecular mass of 32 kDa) and RCS (a regulator of calmodulin signaling), both of which integrate signals from both of these second messengers.6
Although there exist numerous hints toward a neurobiological understanding of depression,7 the epigenetic perspective may add new insights into the gene-environment interaction (G × E), findings that were shown to be applicable to depression. Caspi et al8 were the first to suggest that it might be applicable to depression. They showed that childhood maltreatment and later stressful life events predicted the onset of depressive symptoms only in genetically predisposed individuals with a short (s) allele of the serotonin transporter promoter polymorphism (5-HTTLPR), while the long allele carriers were more resilient to depression after adverse life events. Moreover, incidences of childhood maltreatment were found to be predictive for adult depression only among short allele carriers.
Epigenetics
In 1942, CH Waddington was the first to describe epigenetics when genetics was still flourishing. Waddington referred to epigenetics as an amalgam of genetics and epigenesis, related epigenetics to embryonic development, and put forward the idea that the epigenetics is not entirely due to the “program” encoded in DNA, but also depends on environmental influences.9 Literally the prefix epi means “over or above”; therfore, epigenetics is the science of “control above genetics”. It refers to a variety of processes that affect gene expression independent of actual DNA sequence. Epigenetic information provides instruction on how, where, and when genetic information will be used. Hence, the importance of epigenetic information is that it regulates gene expression.
How Epigenetics Changes Gene Function
Besides the sequence of nucleobases in the genome, gene expression in different types of cells and tissues is modulated by two major mechanisms that are currently crucial for the understanding of epigenetics in psychiatric disorders.
The first mechanism is DNA methylation. In DNA methylation, methyl groups are added to the DNA, which normally takes place at the cytosine bases.10 Methylation is involved in both initiation of transcription and silencing of genes, depending on the type of methylation and the gene that is methylated.10 Repression caused by DNA methylation can happen directly or elaborately. The direct way is when the methyl groups inhibit the transcription factors from binding to the promoter region. The elaborate way represses DNA expression with the use of other chromatin modifying factors, which bind to methylated CpGs.11 The methylation of promoter cytosines in repetitive dinucleotide sequences of cytosines and guanines (CpG) allows further methyl-CpG binding proteins, like methyl CpG-binding protein 2 (MeCP2), to bind and repress expression of the gene.12
The second mechanism is histone modifications. Histones are proteins that order and pack DNA into structural units called nucleosomes. Typically, a nucleosome is composed of two copies of each of the four core histones, H2A, H2B, H3 and H4, with 146 base pairs of DNA wrapped around to form an octamer. Histone modifications are changes in the properties of the histones, such as charge, shape13 and size.14 The state of the chromatin is by and large controlled by covalent modifications of histone tails. The major modifications are acetylation, phosphorylation, methylation and ubiquitylation,15 which affect the net charge, shape or other properties of the histones.13 Histone acetylation involves the attachment of an acetyl group from acetyl-CoA to the α-amino group of the specific lysine (K) side chains15 and is carried out by the enzyme histone acetyltransferase (HAT).16 The reverse, deacetylation, catalyzed by histone deacetylases (HDAC),16 removes the acetyl groups. Another form of histone modification is phosphorylation, which influences processes such as transcription, DNA repair, apoptosis and chromatin condensation.17 Histone methylation is catalyzed by the histone methyltransferases (HMTs), which transfer a methyl group from the methyl donor S-adenosyl-L-methionine (SAM) to the residues. Depending on the residue getting methylated, histone methylation can either enhance or repress transcriptional expression.
There are also other types of epigenetic mechanisms, including RNA-based mechanisms and polycomb proteinmediated chromatin remodeling.5,18 However, these are yet to be characterized in the brain and are thus of less relevance to this review.
Dopamine Signaling and Depression
Although the cause of depression is multifaceted, it is generally agreed upon that psychological stress leads to depression by influencing the metabolism of the monoamine neurotransmitter system. The monoamine hypothesis, describing deficiency or imbalance of the monoamine systems as a cause, has been a central topic of research.19–21 This hypothesis was generated and supported by the fact that most antidepressants share the property of acutely modifying the serotonin or noradrenaline levels at the synapse.22–25 However, treatment with 5-HT/NE reuptake inhibitors or monoamaine oxidase inhibitors elicited poor therapeutic effects on more than 30% of depressed patients with a number of residual symptoms associated with dopaminergic malfunction, including loss of motivation, attention, and pleasure. Thus, a dopaminergic dysfunction subtype of depression was proposed clinically and it was suggested that the dopaminergic system might play a crucial role in the pathogenesis of depression; however, the exact molecular mechanism remains unclear.26
The prostate apoptosis response-4 (Par-4) protein is expressed in striatal neurons along with DRD2 and interacts with DRD2 in neural cells. Par-4 normally enhances DRD2 signaling and thereby inhibits dopamine/DRD2-mediated neurotransmission. Interestingly, an autopsy study of patients with major depression revealed a 67% decrease in Par-4 expression in postmortem temporal cortex; knockout of Par-4 gene led to depression-like behavior in mice.26
In the issue of Cell (Volume 122, Issue 2), Park et al27 and Beaulieu et al28 reveal interactions of D2 receptors with two new and unexpected signaling pathway components. In their study, Park and colleagues demonstrate that the proapoptotic protein Par-4 (prostate apoptosis response 4) interacts with the third intracellular loop of the long isoform of human D2 receptor (D2i3). Furthermore, this interaction, which involves the leucine zipper domain of Par-4, is essential for Gαi/o mediated inhibition of cAMP activity. The region of the D2 receptor that interacts with Par-4 contains a calmodulin binding domain and Ca2+-activated calmodulin competes with Par-4 for this site. This discovery is important, as the authors demonstrate that Gαi/o-dependent D2 regulation of gene expression dictated by the transcription factor CREB depends on equilibrium between binding of Par-4 and binding of calmodulin to the D2 receptor. Increases in the intracellular Ca2+ concentration, possibly in response to activation of the D2 receptor, could result in displacement of Par-4 and uncoupling of the D2 receptor from Gαi/o, thereby providing negative feedback on D2 mediated cAMP attenuation.
It has been reported previously that calmodulin binding to D2i3 negatively regulates D2DR by interfering with the coupling of the Gi-protein in a non-competitive manner.29 Maternal deprivation or chronic mild stress increases the level of Ca2+ influx in striatum of mouse model.26 Increased concentration of Ca2+ interferes with the interaction of Par-4 with D2i3, indicating displacement of Par-4 from D2i3. Thus, an equilibrium shift from the Par-4/D2DR interaction to the calmodulin/D2DR interaction by augmented Ca2+ concentrations results in a downregulation of D2DR efficacy. This in turn relieves the inhibitory tone on dopamine-mediated cAMP signaling.30 On the other hand, disruption of the Par-4/D2DR interaction may facilitate calmodulin/D2DR complex formation upon Ca2+ and hence an upregulation of dopamine-cAMP-CREB (cAMP-responsive element binding protein) signaling. cAMP-responsive element binding protein (CREB) is a downstream transcription factor whose activity is regulated by the cAMP-PKA signaling pathway. CREB associate with the histone acetyltransferase (HAT) CREB-binding protein (CBP). CBP, in turn, acetylates nearby histones and thereby promotes transcriptional activation (epigenetical modification). Hence, upregulation of dopamine-cAMP-CREB signaling occurs. Thus, this signaling pathway may serve as a critical point for increased dopamine concentration, which may in turn contribute to the increased intensity of depression-like behaviors in the multiple behavioral paradigms tested in patients with depression.31
Conclusion
Although there is much work to do, epigenetics and the epigenome deserve consideration for investigation to analyze the linkages between chronic stress and onset of depression. Epigenetics may have the potential to understand the underlying molecular processes of G × E, with both hopeful benefits for future personalized diagnostics and therapies for depression and other psychiatric disorders. In this review we tried to present maternal separation and chronic stress effects on epigenetics and dopamine signaling, in the case of depression. While we do not claim that other neurotransmitters or signaling mechanisms are not involved on the onset of depression, calmodulin-mediated downregulation of D2 receptor efficacy is relatively specific.29 Work is now needed to investigate other brain regions that have been implicated in depression and its treatment. Additionally, there is a need to investigate the involvement of other genes in mediating the long-term effects of stress. Understanding the mechanisms by which such changes are brought about would not only advance our knowledge of the basic neurobiology of this illness, but might also provide new therapeutic avenues for depression.
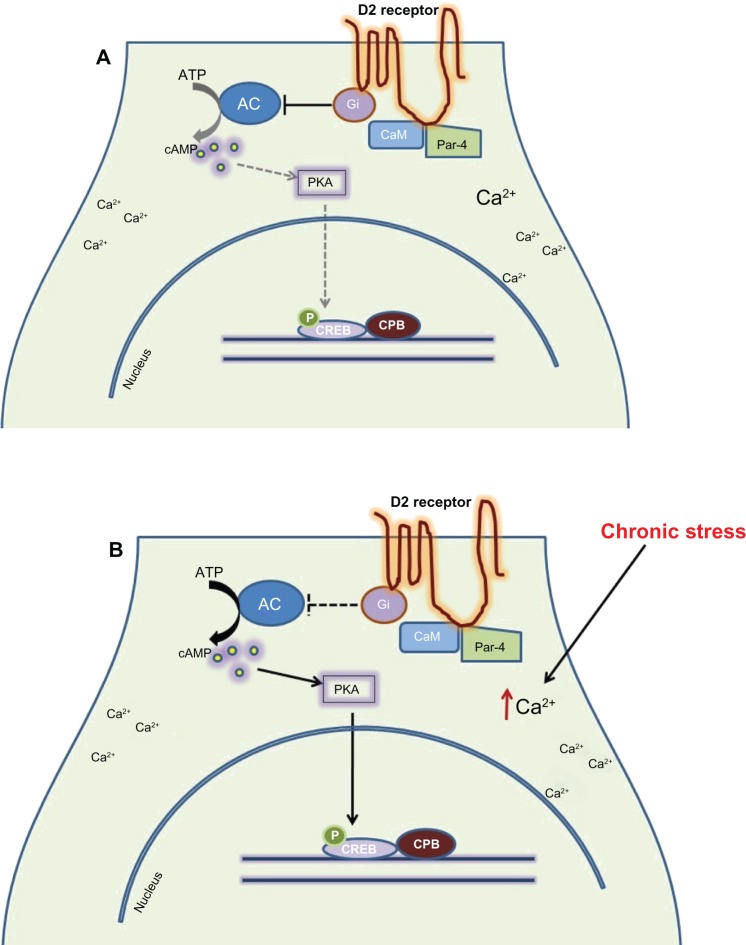
Figure 1.
A model for the epigenetic regulation of involvement of Par-4 in the Ca2+ dependent Regulation of Dopamine D2 receptor (D2DR) Signaling. (A) Par-4/D2DR complex formation is necessary to maintain an inhibitory tone on dopamine-mediated cAMP signaling generated by D2DR in the low Ca2+ condition. (B) Chronic stress disrupts Par-4/D2DR interaction and may facilitate calmodulin/D2DR complex formation upon Ca2+ influx; hence, an upregulation of dopamine-cAMP-CREB signaling, which may contribute to the increased intensity of depression-like behaviors.
Note: Identification of Par-4/D2DR interaction potentially reveals a mechanism for crosstalk between Ca2+ signaling and dopamine-mediated cAMP signaling.
Acknowledgments
We thank Edward Korzus, Department of Psychology, University of California Riverside, Frank Masterpasqua, Widener University for helpful suggestions. We also thank Sally Thomas, Business Manager, Mountain of Love Productions, Inc. for giving a free copy of the book “Spontaneous Evolution”.
Footnotes
Author Contributions
All authors equally contributed for preparation of the initial draft of the manuscript. All authors read and approved the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Greden JF. The burden of disease for treatment-resistant depression. Clin Psychiatry. 2001;62(Suppl 16):26–31. [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 1994. (DSM-IV). [Google Scholar]
- 3.Kinney JL. Nomifensine maleate: a new second-generation antidepressant. Clin Pharm. 1985;4:625–36. [PubMed] [Google Scholar]
- 4.Carlsson A. The occurance, distribution and Physiologiacal role of Catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–3. [PubMed] [Google Scholar]
- 5.Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol. 2006;7:612–6. doi: 10.1038/nrm1946. [DOI] [PubMed] [Google Scholar]
- 6.Rakhilin SV, Olson PA, Nishi A, et al. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AP. Phenotypic plasticity and the epigenetics of human diseases. Nature. 2007;447:433–40. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 10.Tost J. Epigenetics. 1st ed. Caister Academic Press; 2008. [Google Scholar]
- 11.Bogdanovic O, Veenstra G. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118(5):549–65. doi: 10.1007/s00412-009-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 13.Lehninger AL, Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 5th ed. New York: WH Freeman; 2008. [Google Scholar]
- 14.Allis CD, Jenuwein T, Reinberg D, Caparros M, editors. Epigenetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2007. [Google Scholar]
- 15.Tollefsbol TO. Cancer Epigenetics. Boca Raton: CRC Press/Taylor & Francis Group; 2009. [Google Scholar]
- 16.Chung D. Histone modification: the ‘next wave’ in cancer therapeutics. Trends Mol Med. 2002;8(Suppl 4):S10–11. doi: 10.1016/s1471-4914(02)02303-1. [DOI] [PubMed] [Google Scholar]
- 17.Grant PA. A tale of histone modifications. Genome Biol. 2001 2001 Apr 5;2(4):REVIEWS0003. doi: 10.1186/gb-2001-2-4-reviews0003. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity. 2010;105:4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 19.Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–94. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 20.Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;11:1237–64. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- 21.Schildkraut JJ, Gordon EK, Durell J. Catecholamine metabolism in affective disorders. I. Normetanephrine and VMA excretion in depressed patients treated with imipramine. J Psychiatr Res. 1965;3:213–28. doi: 10.1016/0022-3956(65)90003-8. [DOI] [PubMed] [Google Scholar]
- 22.Delay J, Laine B, Buisson JF. Note concernant l’action de l’isoamino hydrazide dans le traitement des etats depressifs. Ann Med Psychol. 1952;110:689–692. [PubMed] [Google Scholar]
- 23.Fuller RW. Serotonin uptake inhibitors: uses in clinical therapy and in laboratory research. Prog Drug Res. 1995;45:167–204. doi: 10.1007/978-3-0348-7164-8_5. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn R. The treatment of depressive states with G 22355(imipramine hydrochloride) Am J Psychiatry. 1958;115:459–64. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- 25.Leonard BE. Mianserin, an antidepressant with a unique aponeuropharmacological profile. Acta Psychiatr Belg. 1978;78:770–80. [PubMed] [Google Scholar]
- 26.Zhu X, Peng S, Zhang S, Zhang X. Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behavioural Brain Research. 2011;223:329–35. doi: 10.1016/j.bbr.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Park SK, Nguyen MD, Fischer A, et al. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–87. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Raul RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–80. doi: 10.1074/jbc.M002780200. [DOI] [PubMed] [Google Scholar]
- 30.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:5515–20. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bibb JA. Decoding dopamine signaling. Cell. 2005;122(2):153–5. doi: 10.1016/j.cell.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Girault J-A, Greengerd P. The neurobiology of Dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 33.Kundu TK, Dasgupta D. Chromatin and Disease—Subcellular Biochemistry. New York: Springer Science; 2007. [Google Scholar]
- 34.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]