Abstract
The field of epigenetics requires that traditional divisions between scientific disciplines give way to cross-fertilization of concepts and ideas from different areas of investigation. Such is the case with research in autoimmunity. Recent discoveries of stimuli that induce autoimmunity reveal that epigenetic marks of autoantigens are recognized by autoreactive B and T cell receptors. Thus, insights into the initiation of autoimmunity, its prevention and therapy will arise from understanding the biochemistry, cell biology and microbiology of autoantigen epigenetics. Here, we highlight potential benefits from the inhibition of a histone modifying enzyme and the administration of a phosphorylated, spliceosome-derived peptide, in the treatment of autoimmunity.
Keywords: Lupus, autoimmunity, post-translational modifications, deimination, phosphorylation, clinical trial, neutrophil extracellular traps, histones, spliceosome, U1-70, Lupuzor
Introduction
One of the essential tasks of the immune system is to distinguish self from non-self. An almost daily encounter with outside attacks, ranging from infectious agents to environmental toxins and other stresses, can alter the self-components of our tissues and cells and therefore affect the well-controlled state of our immune system. In normal conditions, the immune system deploys an impressive array of molecules and cellular tools to quickly eliminate the intruders and correct environmentally-induced self-modifications. Under adverse conditions (“at-risk” genetic background, weakened immune system due to a massive infection or immunosuppressive treatment, or profound damage of tissues and cells, for example), the immune system can wrongly identify self as non-self and initiate misdirected immune responses. An autoimmune disease can thus emerge and affect individuals after an asymptomatic period of a few months or even years.
Self-components (tissues, cell membranes) are not static but instead continuously undergo physiological modifications. Thus, during the cell cycle, the protein/phospholipid ratio fluctuates, the composition of constituents varies, and the organization of plasma membrane rafts changes. Post- translational modifications (PTM) of proteins play a central role in this permanent motion, allowing or not allowing ligands or co-receptors to bind their signaling partners along highly regulated pathways. Numerous physiological PTMs also occur during the cell cycle, notably in nuclear components (proteins, DNA and RNA). These programmed cascades of events that naturally modify basic cell constituents are key elements of cell maintenance and system failure (over- or under- regulation) can cause malfunctioning of cells and ultimately lead to their death.
The immune system normally does not react against self-components. This immune tolerance is naturally established, as immune cells are educated very early during development to ignore them. However, specific modifications (due to incompletely defined causes) can turn a normal self-constituent into an autoantigen. Thus, an autoantigen is defined as an antigen that—despite being a normal tissue constituent—becomes the target of an immune response. Modifications of autoantigens occur in nucleic acids or proteins that may be targeted by immune B and T cells. In recent years, accumulated evidence has demonstrated that in addition to genetic characteristics that favor the establishment of autoimmunity in certain predisposed individuals, epigenetics plays a critical role in the pathogenesis and development of autoimmune diseases.1–13
Epigenetic Modifications of Autoantigens
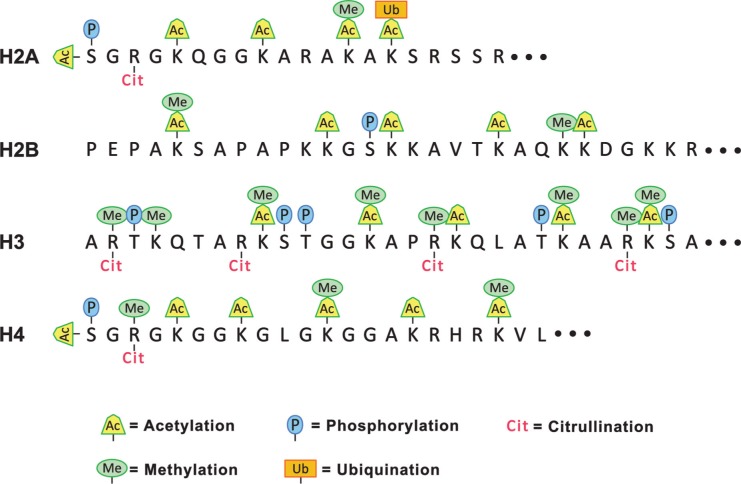
The analysis of epigenetic modifications in autoantigens has attracted much attention as it provides a new and richer understanding of the central dogma of molecular biology. Proteins modify DNA, RNA and other proteins in a combinatorial manner that exponentially expands the information content in the cell. An excellent example is provided by the large number of PTMs in histones.14–18 Even though the primary sequence of the 4 core histones (H2A, H2B, H3 and H4) is highly conserved in all eukaryotes, the multitude of PTMs generates an almost infinite number of subtle conformational changes leading to functional variations (Fig. 1). The enzymology of histone PTMs reflects the complexity of several families of enzymes that introduce, recognize or erase these “molecular sign posts”.19–21 Similar diversity exists in ribonucleoproteins, where PTMs regulate splicing, transport and localized translation of mRNA.16,22
Figure 1.
Diversity of histone amino-terminal PTMs. The amino acid sequences at the amino termini of the four core histones are given in the single letter code and possible PTMs are indicated by symbols above and below the sequence. The PTMs are identified at the bottom of the diagram. Lysine residues can be mono-, di- or tri-methylated, or mono- or di-acetylated. Arginine residues can be mono-, or di-methylated and the dimethyl arginine can exist in the ‘syn’ or ‘trans’ conformation.
Numerous cell components can be the target of autoantibodies. Over 100 different self-molecules act as autoantigens in different autoimmune disorders.23–25 Many or even most of these combine a number of different epigenetic marks. Examples are poly(ADP-ribose) polymerase (PARP) that, in addition to auto-poly(ADP-ribosyl)ation, can be phosphorylated, acetylated, ubiquitinylated and sumoylated,26 heat-shock proteins (HSPs) such as HSP90 that can be phosphorylated, acetylated or citrullinated27 and HSPA8/HSC70 that can be acetylated and tyrosine-phosphorylated,28,29 and lysosomal-associated membrane protein-2 (LAMP-2), which contains O- and N-glycosylation sites (some of the 16 N-linked glycans are polylactosaminoglycans).30 Many of these modifications are recognized by autoantibodies occurring in systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and other autoimmune diseases.31–37
Certain PTMs arise as part of the normal developmental program, whereas others may fluctuate during normal physiological activity, such as the regulated progression of the cell cycle. An important set of autoantigen modifications arise in dying cells. These may differentiate between different forms of cell death, such as apoptosis, necrosis, anoikis, or pyroptosis. Additional modifications arise in cell death that results from infections or the associated inflammation. Each of these diverse, cell death-associated PTMs may have unique functions, such as to assist in the elimination of microbes, to orchestrate the innate or adaptive immune response, or to facilitate clearance of the dead cell remnants. Specific stimulation of autoantibodies may follow if physiological clearance pathways are insufficient and modified autoantigens accumulate. If the burden of dead cells increases, these autoantibodies may serve the beneficial purpose to assist in clearance of “debris”. Clearly, some of the autoantibodies may evolve functions that are detrimental to the host organism.
The general principle that PTMs can induce autoantibody (B cell) responses is demonstrated by the deimination of arginine residues to citrulline residues in histones.36 For that, it is necessary to provide some background. Although different cell types deiminate arginine residues in proteins, this reaction is most rapid and extensive in neutrophils,38 which are the most abundant white blood cells. These cells are exquisitely sensitive to chemokines and bacterial chemoattractants and they arrive quickly at a site of an infection.39 Neutrophils attach to the blood vessel endothelium and leave the blood stream in order to enter tissues. There, they actively migrate toward the infectious agents. This remarkable cellular transformation requires a coordinated switch in gene expression. In their fight against microbes, neutrophils are effective phagocytes that inactivate and degrade the intruders.39 In addition, recent studies have identified an unexpected antimicrobial mechanism that is available to neutrophils at the site of an infection.40
As first observed by Brinkmann and colleagues, neutrophils externalize chromatin that acts as an extracellular “trap” to immobilize bacteria, fungi and viruses.41 The chromatin in these so-called neutrophil extracellular traps (NETs) is associated with bactericidal enzymes and peptides that can damage the bacterial membranes. Importantly, the release of NET chromatin (NETosis) depends on the activity of peptidylargininedeiminase IV (PAD4).42,43 It has been suggested that conversion of arginine residues in histones to citrulline residues weakens the interactions between histones and DNA. This loosening of the condensed structure of chromatin may be a precondition for NET release.44
NETs are prime suspects in the events that stimulate the adaptive immune system to produce autoantibodies directed against histones (and other chromatin antigens), as is common in lupus and related disorders.36 The arguments implicating NETs in the induction of autoimmunity are summarized in Table 1. In brief, NETs represent an innate mechanism that is induced in the presence of pro-inflammatory cytokines and externalizes chromatin. In an infection, NET chromatin associates with bacterial, fungal, or viral components that may act as adjuvants to stimulate an immune response. The complexes of chromatin and microbes arise under circumstances that promote phagocytosis and, likely, antigen presentation to helper T cells. Because NETs contain histones with a distinguishing PTM, the hypothesis that NETosis may induce an autoimmune reaction could be tested by searching for autoantibodies to deiminated histones.
Table 1.
Arguments for NETosis as a direct stimulus for autoimmunity.
| 1. NETosis is induced in the presence of pro-inflammatory stimuli |
| 2. NETosis is a physiological circumstance that leads to chromatin externalization |
| 3. NET chromatin tightly associates with microbial (foreign) antigens |
| 4. The “jumble” of NET chromatin and microbes is likely taken up by phagocytes, processed and presented to the adaptive immune system. |
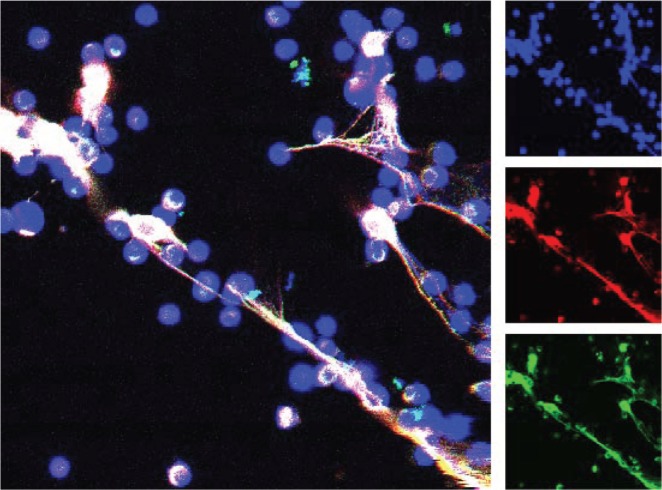
Indeed, sera from SLE, RA and, most often, from patients with Felty’s syndrome contain autoantibodies that preferentially react with deiminated histones. These antibodies could be identified by ELISA and Western blots using in vitro deiminated histones.45 The structure of NETs induced by neutrophil stimulation with calcium ionophore is shown in Figure 2. The image suggests that the extranucleosomal, linker histone H1 is associated with the extracellular chromatin. Thus, our work extends the growing list of autoantigens that contain citrulline residues. Arthritic disorders in general are characterized by the presence of autoantibodies to citrullinated protein antigens (ACPA).46 Over a decade ago, RA sera were shown to react specifically with citrullinated peptides derived from filaggrin.47 Pratesi et al confirmed our results by identifying autoantibodies to deiminated histone H4 in a majority of RA patient sera.48
Figure 2.
Detection of histone H1 on neutrophil extracellular chromatin “traps”. Human neutrophils were purified and incubated with A23187 ionophore for 2 hours before processing for confocal microscopy with anti-linker histone H1 antibody. The main image shows three color confocal laser scanning micrograph that combines staining of DNA (blue) and anti-H1 antibody (detected by two different secondary antibodies, shown in green and red). Linker histone H1 remains associated with neutrophil extracellular traps.
Additional studies support the idea that neutrophil activation is a key factor in the stimulation of autoantibodies. Separate studies indicated that neutrophils from juvenile SLE patients show an enhanced response to anti-ribonucleoprotein (RNP) immune complexes49 and that RA neutrophils are more susceptible than control neutrophils to spontaneous NET release.50 Moreover, in lupus, NETs may resist degradation because the patients’ autoantibodies protect them from nuclease degradation.51 Persistent NETs may aggravate inflammation by stimulating plasmacytoid dendritic cells and causing type I interferon release.52
Interestingly, RA sera also contain anti-PAD4 autoantibodies that activate the deiminase and lower its requirement for calcium,53 thus suggesting a possible extracellular role of PAD4. In light of this observation, it is reasonable to assume that PAD4, externalized during NETosis, deiminates extracellular matrix proteins such as filaggrin, fibrinogen and collagen and thereby contributes to generate highly specific RA autoantigens.54 Deimination of myelin basic protein, most likely by PAD2 or PAD4, may participate in the pathogenesis of neuro-degenerative disorders.55 The recurrent involvement of deimination in the activation of autoimmune responses implies a major role for PAD4 in enhancing the immunogenicity of autoantigens.
How to Turn a Self, Non-antigenic Molecule into an Immunogen?
Immunological tolerance is an active mechanism that detects, suppresses, and eliminates B and T cells with autoreactive antigen receptors. During lymphocyte development, B and T cells that encounter self-antigens are inactivated by so-called “central” tolerance.56 The autoimmune regulator (AIRE) transcription factor allows thymic epithelial cells to express low levels of antigens found in other tissues.57 As a result, developing T cells with self-reactive receptors are eliminated by enforced apoptosis. Central tolerance mechanisms remain largely intact in overt autoimmune diseases.58 How could PTMs assist in overcoming tolerance and converting self-components into autoantigens?
Immune cells should not “see” self-components with PTMs that exist under physiological conditions (cell cycle, development, and cell death). It is plausible that PTMs that can transform a self-component into an autoantigen may be different from the ones that are normally expressed during physiological conditions (qualitatively, by involving different sites, or quantitatively) or that they are expressed with a kinetics that differ from the “norm”. Delocalization of naturally-modified antigens might also play an important role.59–61
Importantly, some antigens are recognized by autoantibodies, only when they carry specific PTMs. These same antigens may not be targeted by antibodies and B/T cells when modifications are naturally absent or have been experimentally erased. The first evidence of the involvement of phosphorylation in the recognition of autoantigen by naturally-occurring antibodies was reported by Stetler and his colleagues in the mid-80’s who showed that dephosphorylation of RNA polymerase I abolishes its interaction with antibodies from patients and very young lupus-prone MRL mice.62–64 Satoh et al65 also identified a subset of SLE autoantibodies that react specifically with the transcriptionally active (phosphorylated) form of RNA polymerase II. Phosphorylation of serine/arginine-rich (SR) proteins, which constitute a family of essential pre-messenger RNA splicing factors associated with the U1 snRNP complex, also provide an important component of the autoreactivity in SLE. Interestingly, upon dephosphorylation and depending on each patient’s response, a decrease in SR proteins reactivity was observed, suggesting the existence of PTM-reactive antibody sub-populations in lupus patients.66 It is still unclear whether these PTMs are directly involved in the breakdown of tolerance and initiation of the autoimmune response. However, this possibility and its many potential implications in the etiology of autoimmune diseases are important to explore.
Epigenetic Modifications as Targets for Therapeutics?
The involvement of PTM in the induction of autoimmunity can be tested by focusing on a particular form of cell death. Assuming that NETosis yields favorable circumstances for the induction of systemic autoimmunity, it may be possible to inhibit autoimmune reactions by blocking NETosis. Incidentally, NETosis may be a suitable therapeutic target because it is non-essential for innate immune responses to infections. NETosis can be prevented by using inhibitors of PAD4 deiminase.67 An inhibitor of PAD4, Cl-amidine, has been tested in mouse models of lupus, arthritis, colitis and multiple sclerosis. In each case, disease manifestations greatly improved.68–71
In mice with collagen-induced arthritis, Cl-amidine administration greatly improved disease manifestations, thus inviting further experimentation.71 PAD4 inhibition also showed promise in the treatment of induced colitis, whose consequences could be blunted by treatment with Cl-amidine.68 In these mice, Cl-amidine stimulated apoptosis of inflammatory cells in the colon. In a spontaneous mouse model of lupus, Cl-amidine was administered over a period of 3 months at levels that could inhibit PAD4.69 The drug showed no adverse effects, and the treatment inhibited NET formation and anti-DNA antibody production without affecting overall serum antibody isotypes. In addition, there was reduced myeloperoxidase and immune complex deposition in the kidneys, and endothelial cell function and vasorelaxation were improved. In a fourth mouse model, neurodegeneration resembling that of multiple sclerosis was prevented by the administration of a newly developed deiminase inhibitor70 Strikingly, even thrombosis, a complication in many autoimmune disorders, was significantly blunted in the absence of PAD4.72 Together, these findings suggest that autoantigen deimination plays a fundamental role in auto-inflammatory reactions and that prevention of deimination has great potential in the therapy of autoimmune disorders.
A fascinating example of how autoantigen PTM could be turned into starting points for autoimmune therapy is provided by the 21-mer peptide P140/Lupuzor™ that has been tested in clinical trials involving lupus patients. This phosphopeptide, encompassing residues 131–151 of the spliceosomal U1-70K protein, has successfully completed phase I, phase IIa and phase IIb clinical trials and will enter soon into a multi-center, double-blind, placebo-controlled phase III clinical trial, the final testing phase.
Lupuzor’s phase I study, which took place in 2006, did not show any side effects. The Phase IIa study was run in 2007 in lupus patients and was a proof of concept, dose ranging, safety, multi-centre European study.73 The proof of concept was assessed by measuring the decrease of anti-dsDNA antibodies as a surrogate marker for efficacy and IL-10, to ascertain its mechanism of action. The drug was administered 3 times by subcutaneous injections 2 weeks apart at doses of 200 μg and 1000 μg and the patients were monitored 1 month after treatment ceased. Lupus patients who received 200 μg Lupuzor as a lupus treatment on only 3 occasions, 3 weeks apart, demonstrated a significant clinical improvement in their condition in addition to the decrease of their biomarkers. Therefore, the Phase IIa trial of Lupuzor met its primary end-points. A phase IIb clinical trial started dosing of patients in February 2008 in Europe and Latin America, comparing Lupuzor to placebo in patients with SLE. An interim analysis demonstrated statistically significant superiority of Lupuzor over placebo.74 The interim analysis was performed and reviewed by an independent Data Monitoring Committee according to the so-called International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (better known as ICH guidelines). This analysis was conducted after 125 randomized patients with clinical SLEDAI-2K scores >6 and no Bilag A score had completed the 12-week treatment period, half of them having also completed the additional 12-week follow-up (week 24). The primary efficacy measure was a ‘SLEDAI response’, defined as a decrease of at least 4 points in the SLEDAI score, a scale generally accepted by physicians as an assessment of the clinical activity of lupus patients, a lower score representing lower disease activity. The analysis of the data demonstrated that the 200 μg dose of Lupuzor administered every 4 weeks was statistically significantly superior to placebo (p = 0.015; 62% responders versus 37% responders in the placebo group). Lupuzor was generally well tolerated with no significant drug related adverse events.
P140 peptide does not behave as an immunosuppressor. Its underlying mechanism of action involves autophagy,75 more specifically a selective form of autophagy called chaperone-mediated autophagy (CMA; Cuervo AM and Muller S, unpublished). This lysosomal mechanism is responsible for the degradation of approximately 30% of cytosolic proteins in tissues (liver, kidney) and is mainly activated in conditions of stress such as nutrient deprivation or exposure to different toxin compounds. In contrast to macroautophagy, which ensures the synthesis, degradation and recycling of damaged cell organelles or unused proteins, all substrate proteins targeted for CMA pathway contain a motif biochemically related to the pentapeptide KFERQ involved in their selective recognition by a cytosolic chaperone, the heat shock cognate protein HSPA8/HSC70. The interaction between the chaperone and the substrate in the cytosol targets the complex to the lysosomal membrane, where it binds to the lysosome associated membrane protein type 2A that acts as a receptor and a limiting factor for this pathway. Lys-HSPA8, a lysosomal form of HSPA8 that is present within the lumen, is required for the complete translocation of the substrate protein into the lysosomal matrix where it is completely degraded by the lysosomal proteases. We discovered that P140 reduces autophagic process that we found to be abnormally enhanced in T lymphocytes from lupus mice and patients.76 We also discovered that the P140 inhibitory effect on CMA results from its ability to alter the integrity of the HSPA8/HSP90 heterocomplex of lysosomal chaperones. Since autophagy has also been identified as a route by which cytoplasmic and nuclear antigens are delivered to MHC class II molecules (MHCII) for presentation to CD4+ T cells,77 we postulated that destabilization of the HSPA8/HSP90 heterocomplex by P140 peptide may alter the endogenous (auto)antigen processing, which is enhanced in lupus, and inhibit peptide loading onto MHCII molecules. As a consequence, P140 may lead to a lower activation of autoreactive T helper cells. Without signal from these T helper cells, autoreactive B cells cannot differentiate into antibody-producing plasma cells and therefore the levels of secreted antibodies is dramatically reduced as was shown in mice and patients with lupus.73,78
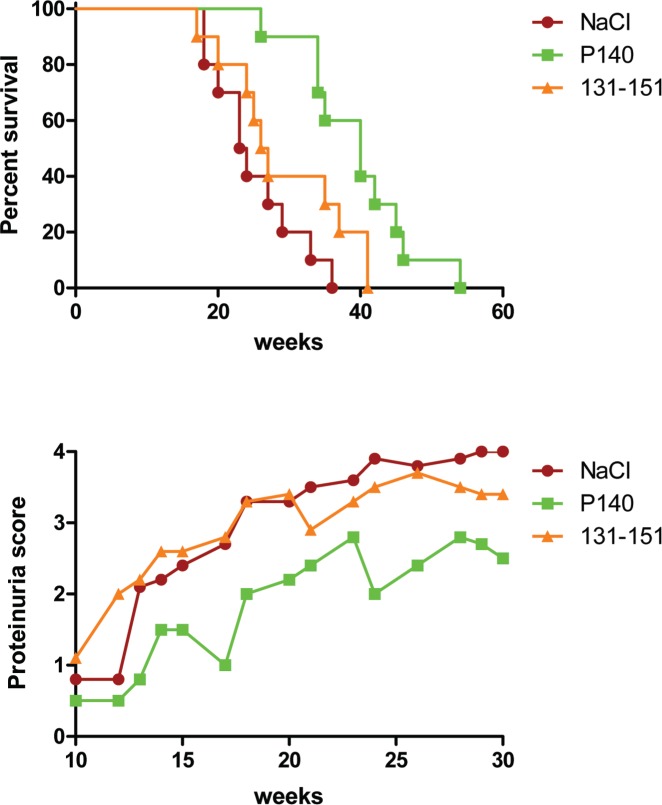
What is interesting in the P140 peptide behavior is that although the non-phosphorylated sequence 131–151 is readily recognized by T cells from 2 distinct strains of lupus-prone mice [MRL/lpr and (NZB/NZW) F1 mice] and patients with lupus and behaves as a promiscuous epitope with regard to murine and human MHC molecules, it displays no protective activity against the disease (Fig. 3). This absence of effect on the course of the lupus disease is in sharp contrast with the strong protective effect displayed by the peptide containing a phosphoserine residue at position 140 (hence P140 peptide). In this protective process, therefore, the phosphoSer140 residue appears central.79 In fact, this modification was found later to exist naturally in early apoptotic Jurkat cells.59 It was discovered that in the early stages of apoptosis the basal phosphorylation of the Ser140 residue increases very significantly and, in parallel, a caspase-dependent, PP1-mediated dephosphorylation of other serine residues occurs in a subset of U1-70K proteins, which are heavily phosphorylated.80 We found that the hypo-phosphorylated U1-70K protein carrying a phosphoSer residue at position 140 is then clustered in heterogeneous ectopic RNP-derived structures, which are finally extruded as apoptotic bodies. It is not yet known whether this material displays particular antigenic and/or immunogenic properties and if the same pathway is activated in vivo in lupus cells. The kinase that phosphorylates serine residue 140 also remains to be identified.
Figure 3.
The P140 phosphorylated peptide but not the non-phosphorylated sequence 131–151 of the U1-70K protein is protective against lupus disease in lupus mice. Ten 5-week-old MRL/lpr mice were administered intravenously with either P140 peptide in saline or with the peptide 131–151 in saline or with saline only. Mice were then subjected to three further administrations (weeks 7, 9, 12) in the same conditions. Their viability and the mean proteinuria score are shown. Survival of control and peptide-treated female MRL/lpr mice was analyzed by the Kaplan–Meier method, and the significance of differences was determined by the log-rank test. Median survival: 23.5 weeks (NaCl), 26.5 weeks (peptide 131–151) and 40 weeks (P140); p = 0.0002 (P140 treatment vs. NaCl) and p = 0.137 (peptide 131–151 vs. NaCl). Proteinuria (2-way ANOVA test): p = 0.0004 (P140 treatment vs. NaCl) and p = 0.8353 (peptide 131–151 vs. NaCl). Modified from.79
Conclusions
We anticipate that the two treatments described in this brief review will be the prototypes of directed therapies for lupus and other autoimmune disorders. Regions of antigens carrying a PTM of interest or enzymes involved in specific antigen modifications represent attractive targets for immunotherapy. For therapeutics development, a valuable approach would be therefore to carefully identify pathogenesis-related PTMs and pathways that are deregulated or altered in autoimmune disease. Currently, intense translational research is focused on enzymes that modulate epigenetics. For example, histone deacetylase inhibitors (HDACi), such as hydroxyl-butyrate, suberoylanilidehydroxamic acid (SAHA), or Trichostatin A (TSA) decrease inflammatory cytokine production by splenocytes and reduce kidney disease in both the MRL/lpr and (NZB/NZW) F1 mice lupus mouse models, and are currently in various stages of development.81–83 It is worth noting that several histone peptides with PTMs that are specifically linked to apoptosis, particularly acetylated and methylated residues, have been identified.84–86 They likely represent valuable tools for therapeutics development in lupus, as has been done in the P140/lupuzor program.
Footnotes
Author Contributions
MR and SM jointly wrote the manuscript. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
FUNDING: Research in MR’s laboratory is financially supported by The Lupus Research Institute of New York and the ORR Fund of Memphis, Tennessee. Research in SM’s laboratory is financially supported by the French Centre National de la Recherche Scientifique, Région Alsace, the Laboratory of Excellence Medalis, Initiative of Excellence (IdEx), Strasbourg University, and ImmuPharma France.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
REFERENCES
- 1.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004;16(6):753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Lührmann R. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem. 2000;275(22):17122–17129. doi: 10.1074/jbc.M000300200. [DOI] [PubMed] [Google Scholar]
- 3.Brooks WH, Le Dantec C, Pers JO, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J Autoimmun. 2010;34(3):J207–J219. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Cloos PA, Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology. 2004;5(3):139–158. doi: 10.1023/B:BGEN.0000031152.31352.8b. [DOI] [PubMed] [Google Scholar]
- 5.Dieker J, Muller S. Epigenetic histone code and autoimmunity. Clin Rev Allergy Immunol. 2010;39(1):78–84. doi: 10.1007/s12016-009-8173-7. [DOI] [PubMed] [Google Scholar]
- 6.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol. 2012;24(1):112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatto M, Zen M, Ghirardello A, et al. Emerging and critical issues in the pathogenesis of lupus. Autoimmun Rev. 2013;12(4):523–536. doi: 10.1016/j.autrev.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Grolleau-Julius A, Ray D, Yung RL. The role of epigenetics in aging and autoimmunity. Clin Rev Allergy Immunol. 2010;39(1):42–50. doi: 10.1007/s12016-009-8169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein K, Gay S. Epigenetic modifications in rheumatoid arthritis, a review. Curr Opin Pharmacol. 2013;13(3):420–425. doi: 10.1016/j.coph.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q. The critical importance of epigenetics in autoimmunity. J Autoimmun. 2013;41:1–5. doi: 10.1016/j.jaut.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Szyf M. Epigenetic therapeutics in autoimmune disease. Clin Rev Allergy Immunol. 2010;39(1):62–77. doi: 10.1007/s12016-009-8172-8. [DOI] [PubMed] [Google Scholar]
- 12.Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2(2):101–114. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Boekel MA, van Venrooij WJ. Modifications of arginines and their role in autoimmunity. Autoimmun Rev. 2003;2(2):57–62. doi: 10.1016/s1568-9972(02)00128-3. [DOI] [PubMed] [Google Scholar]
- 14.Cline AM, Radic MZ. Apoptosis, subcellular particles, and autoimmunity. Clin Immunol. 2004;112(2):175–182. doi: 10.1016/j.clim.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Dieker J, Muller S. Post-translational modifications, subcellular relocation and release in apoptotic microparticles: apoptosis turns nuclear proteins into autoantigens. Folia Histochem Cytobiol. 2009;47(3):343–348. doi: 10.2478/v10042-009-0068-1. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann MH, Trembleau S, Muller S, Steiner G. Nucleic acid-associated autoantigens: pathogenic involvement and therapeutic potential. J Autoimmun. 2010;34(3):J178–J206. doi: 10.1016/j.jaut.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Tropberger P, Schneider R. Scratching the (lateral) surface of chromatin regulation by histone modifications. Nat Struct Mol Biol. 2013;20(6):657–661. doi: 10.1038/nsmb.2581. [DOI] [PubMed] [Google Scholar]
- 18.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20(3):259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee C, Muir TW. Chemical approaches for studying histone modifications. J Biol Chem. 2010;285(15):11045–11050. doi: 10.1074/jbc.R109.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Patel DJ. Combinatorial readout of dual histone modifications by paired chromatin-associated modules. J Biol Chem. 2011;286(21):18363–18368. doi: 10.1074/jbc.R111.219139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Will CL, Luhrmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3(7) doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QZ, Zhou J, Lian Y, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159(3):281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34(2):501–537. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Yeste A, Quintana FJ. Antigen microarrays for the study of autoimmune diseases. Clin Chem. 2013;59(7):1036–1044. doi: 10.1373/clinchem.2012.194423. [DOI] [PubMed] [Google Scholar]
- 26.Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly (ADP-ribose) and PARP-1. Genes Dev. 2012;26(5):417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson SE. Hsp90: structure and function. Top Curr Chem. 2013;328:155–240. doi: 10.1007/128_2012_356. [DOI] [PubMed] [Google Scholar]
- 28.Egerton M, Moritz RL, Druker B, Kelso A, Simpson RJ. Identification of the 70 kD heat shock cognate protein (Hsc70) and alpha-actinin-1 as novel phosphotyrosine-containing proteins in T lymphocytes. Biochem Biophys Res Commun. 1996;224(3):666–674. doi: 10.1006/bbrc.1996.1082. [DOI] [PubMed] [Google Scholar]
- 29.Stricher F, Macri C, Ruff M, Muller S. HSPA8/HSC70 chaperone protein: structure, function and chemical targeting. Autophagy. 2013;9(12) doi: 10.4161/auto.26448. [DOI] [PubMed] [Google Scholar]
- 30.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem. 1999;274(43):31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 31.Deane KD, Nicolls MR. Developing better biomarkers for connective tissue disease-associated interstitial lung disease: citrullinated hsp90 autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2013;65(4):864–868. doi: 10.1002/art.37878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869–879. doi: 10.1002/art.37881. [DOI] [PubMed] [Google Scholar]
- 33.Hayem G, De Bandt M, Palazzo E, et al. Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58(5):291–296. doi: 10.1136/ard.58.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minota S, Cameron B, Welch WJ, Winfield JB. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988;168(4):1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller S, Briand JP, Barakat S, et al. Autoantibodies reacting with poly(ADP-ribose) and with a zinc-finger functional domain of poly(ADP-ribose) polymerase involved in the recognition of damaged DNA. Clin Immunol Immunopathol. 1994;73(2):187–196. doi: 10.1006/clin.1994.1187. [DOI] [PubMed] [Google Scholar]
- 36.Radic M, Marion TN. Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. Semin Immunopathol. 2013;35(4):465–480. doi: 10.1007/s00281-013-0376-6. [DOI] [PubMed] [Google Scholar]
- 37.Stinton LM, Eystathioy T, Selak S, Chan EK, Fritzler MJ. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes, and GW bodies. Clin Immunol. 2004;110(1):30–44. doi: 10.1016/j.clim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylargininedeiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277(51):49562–49568. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 39.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6(7):e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982–992. doi: 10.1002/art.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willemze A, Trouw LA, Toes RE, Huizinga TW. The influence of ACPA status and characteristics on the course of RA. Nat Rev Rheumatol. 2012;8(3):144–152. doi: 10.1038/nrrheum.2011.204. [DOI] [PubMed] [Google Scholar]
- 47.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratesi F, Dioni I, Tommasi C, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202765. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakkim A, Fürnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med. 2013;5(186):186ra65. doi: 10.1126/scitranslmed.3005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56(11):3541–3553. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 55.Wood DD, Ackerley CA, Brand Bv. et al. Myelin localization of peptidylargininedeiminases 2 and 4: comparison of PAD2 and PAD4 activities. Lab Invest. 2008;88(4):354–364. doi: 10.1038/labinvest.3700748. [DOI] [PubMed] [Google Scholar]
- 56.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6(10):728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 57.Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 2010;107(29):13016–13021. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panigrahi AK, Goodman NG, Eisenberg RA, Rickels MR, Naji A, Luning-Prak ET. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205(13):2985–2994. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieker J, Cisterna B, Monneaux F, et al. Apoptosis-linked changes in the phosphorylation status and subcellular localization of the spliceosomalautoantigen U1-70K. Cell Death Differ. 2008;15(4):793–804. doi: 10.1038/sj.cdd.4402312. [DOI] [PubMed] [Google Scholar]
- 60.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol. 1990;94(1):77–85. doi: 10.1111/1523-1747.ep12873930. [DOI] [PubMed] [Google Scholar]
- 62.Stetler DA, Cavallo T. Anti-RNA polymerase I antibodies: potential role in the induction and progression of murine lupus nephritis. J Immunol. 1987;138(7):2119–2123. [PubMed] [Google Scholar]
- 63.Stetler DA, Jacob ST. Phosphorylation of RNA polymerase I augments its interaction with autoantibodies of systemic lupus erythematosus patients. J Biol Chem. 1984;259(22):13629–13632. [PubMed] [Google Scholar]
- 64.Stetler DA, Signorelli D, Neil J, Brady S, Engler R, Brown JC. Anti-RNA polymerase I antibodies in the sera of MRL lupus mice at the initial stages of disease are directed primarily against phosphorylation-dependent epitopes. Autoimmunity. 1992;12(1):29–36. doi: 10.3109/08916939209146127. [DOI] [PubMed] [Google Scholar]
- 65.Satoh M, Ajmani AK, Ogasawara T, et al. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest. 1994;94(5):1981–1989. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neugebauer KM, Merrill JT, Wener MH, Lahita RG, Roth MB. SR proteins are autoantigens in patients with systemic lupus erythematosus. Importance of phosphoepitopes. Arthritis Rheum. 2000;43(8):1768–1778. doi: 10.1002/1529-0131(200008)43:8<1768::AID-ANR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 67.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chumanevich AA, Causey CP, Knuckley BA, et al. Suppression of colitis in mice by Cl-amidine: a novel peptidylargininedeiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G929–G938. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knight JS, Zhao W, Luo W, et al. Peptidylargininedeiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123(7):2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L, Wasilewski E, Chakka SK, Bello AM, Moscarello MA, Kotra LP. Novel inhibitors of protein arginine deiminase with potential activity in multiple sclerosis animal model. J Med Chem. 2013;56(4):1715–1722. doi: 10.1021/jm301755q. [DOI] [PubMed] [Google Scholar]
- 71.Willis VC, Gizinski AM, Banda NK, et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186(7):4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylargininedeiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110(21):8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller S, Monneaux F, Schall N, et al. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early phase II clinical trial. Arthritis Rheum. 2008;58(12):3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 74.Zimmer R, Scherbarth HR, Rillo OL, Gomez-Reino JJ, Muller S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis. 2013;72(11):1830–1835. doi: 10.1136/annrheumdis-2012-202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Page N, Gros F, Schall N, et al. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann Rheum Dis. 2011;70(5):837–843. doi: 10.1136/ard.2010.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gros F, Arnold J, Page N, et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8(7):1113–1123. doi: 10.4161/auto.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182(6):3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur J Immunol. 2003;33(2):287–296. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 79.Schall N, Page N, Macri C, Chaloin O, Briand JP, Muller S. Peptide-based approaches to treat lupus and other autoimmune diseases. J Autoimmun. 2012;39(3):143–153. doi: 10.1016/j.jaut.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Woppmann A, Will CL, Kornstädt U, Zuo P, Manley JL, Lührmann R. Identification of an snRNP-associated kinase activity that phosphorylates arginine/serine rich domains typical of splicing factors. Nucleic Acids Res. 1993;21(12):2815–2822. doi: 10.1093/nar/21.12.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111(4):539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reilly CM, Mishra N, Miller JM, et al. Modulation of renal disease in MRL/lpr mice by suberoylanilidehydroxamic acid. J Immunol. 2004;173(6):4171–4178. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 83.Reilly CM, Regna N, Mishra N. HDAC inhibition in lupus models. Mol Med. 2011;17(5–6):417–425. doi: 10.2119/molmed.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dieker JW, Fransen JH, van Bavel CC, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007;56(6):1921–1933. doi: 10.1002/art.22646. [DOI] [PubMed] [Google Scholar]
- 85.van Bavel CC, Dieker J, Muller S, et al. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol. 2009;47(2–3):511–516. doi: 10.1016/j.molimm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 86.van Bavel CC, Dieker JW, Kroeze Y, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011;70(1):201–207. doi: 10.1136/ard.2010.129320. [DOI] [PubMed] [Google Scholar]