Abstract
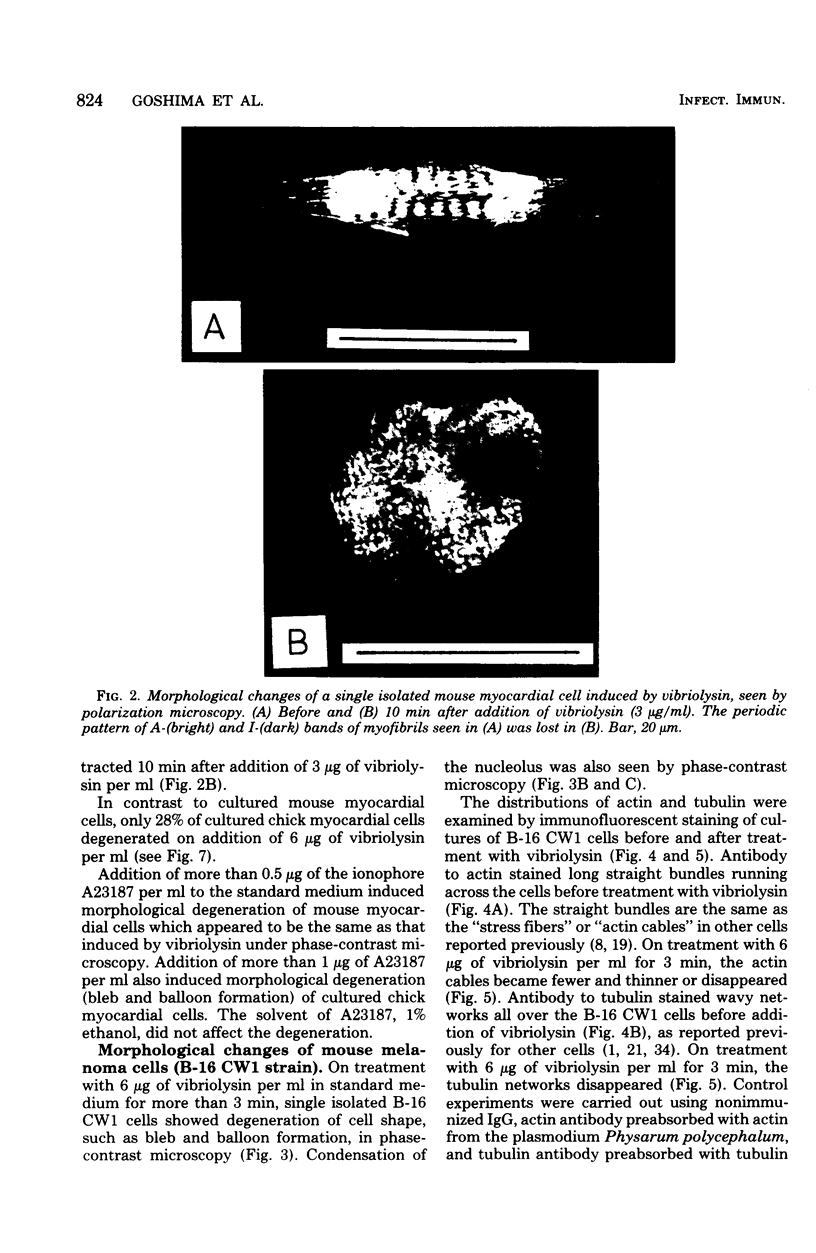
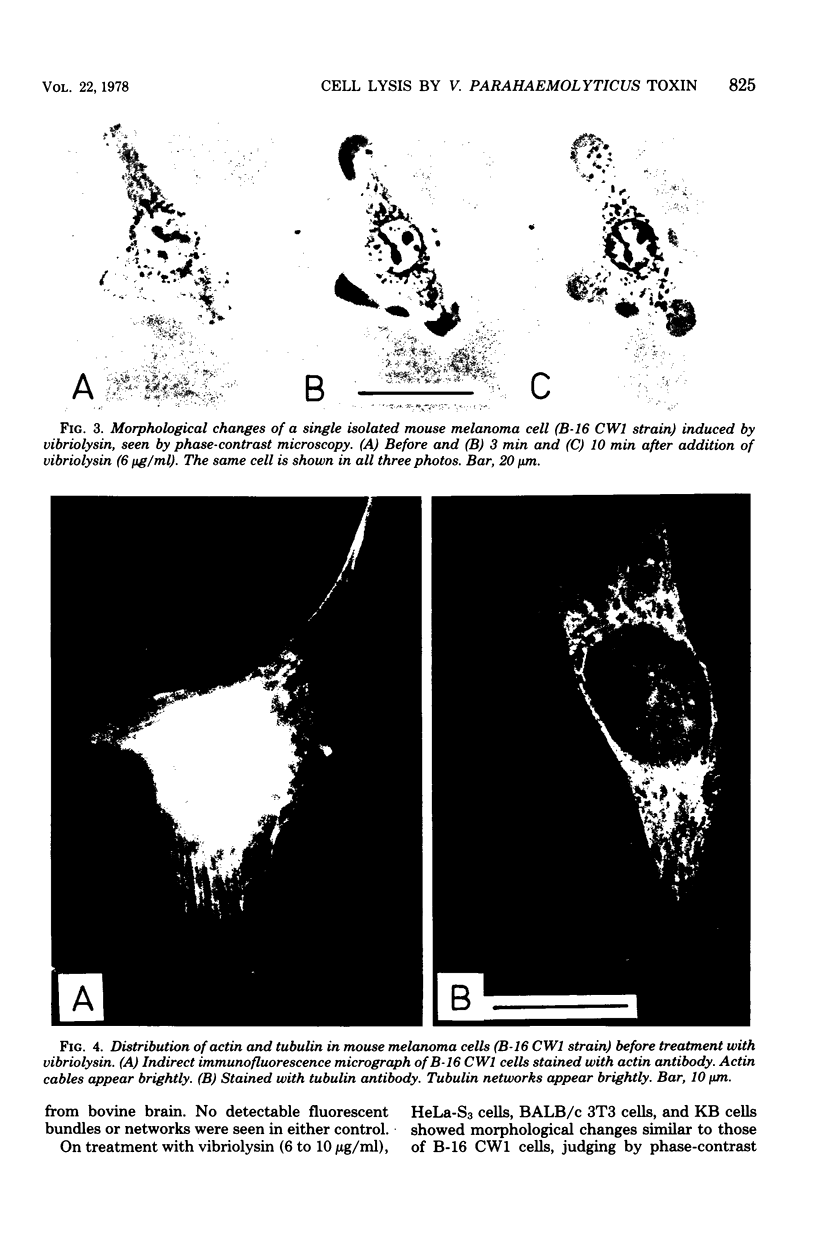
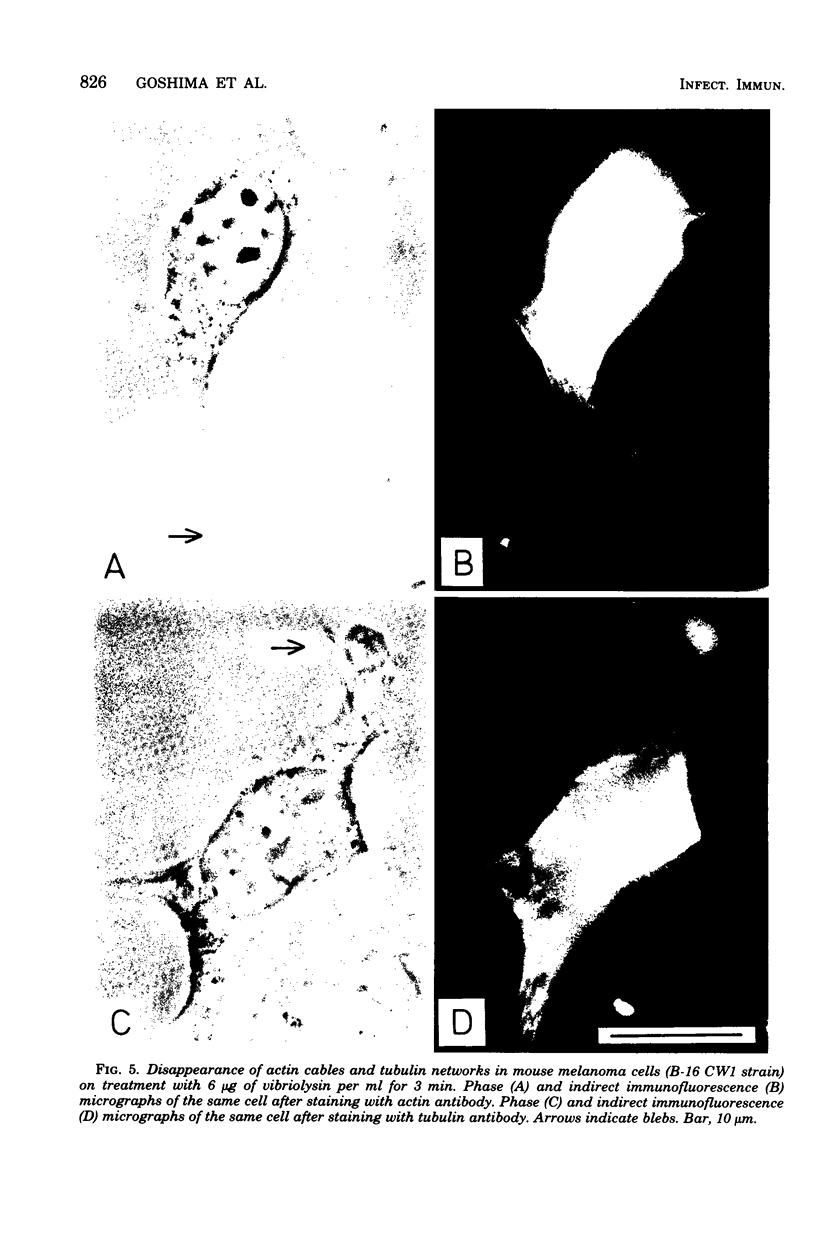
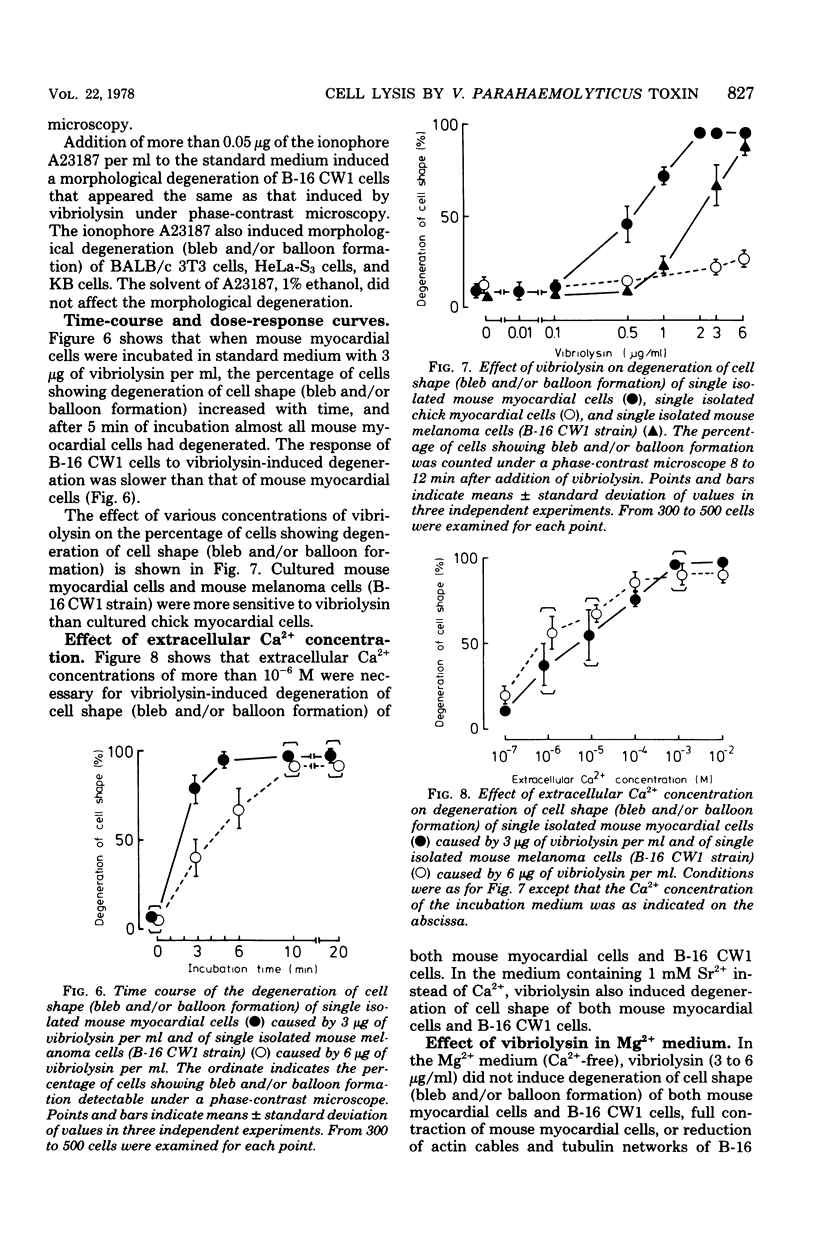
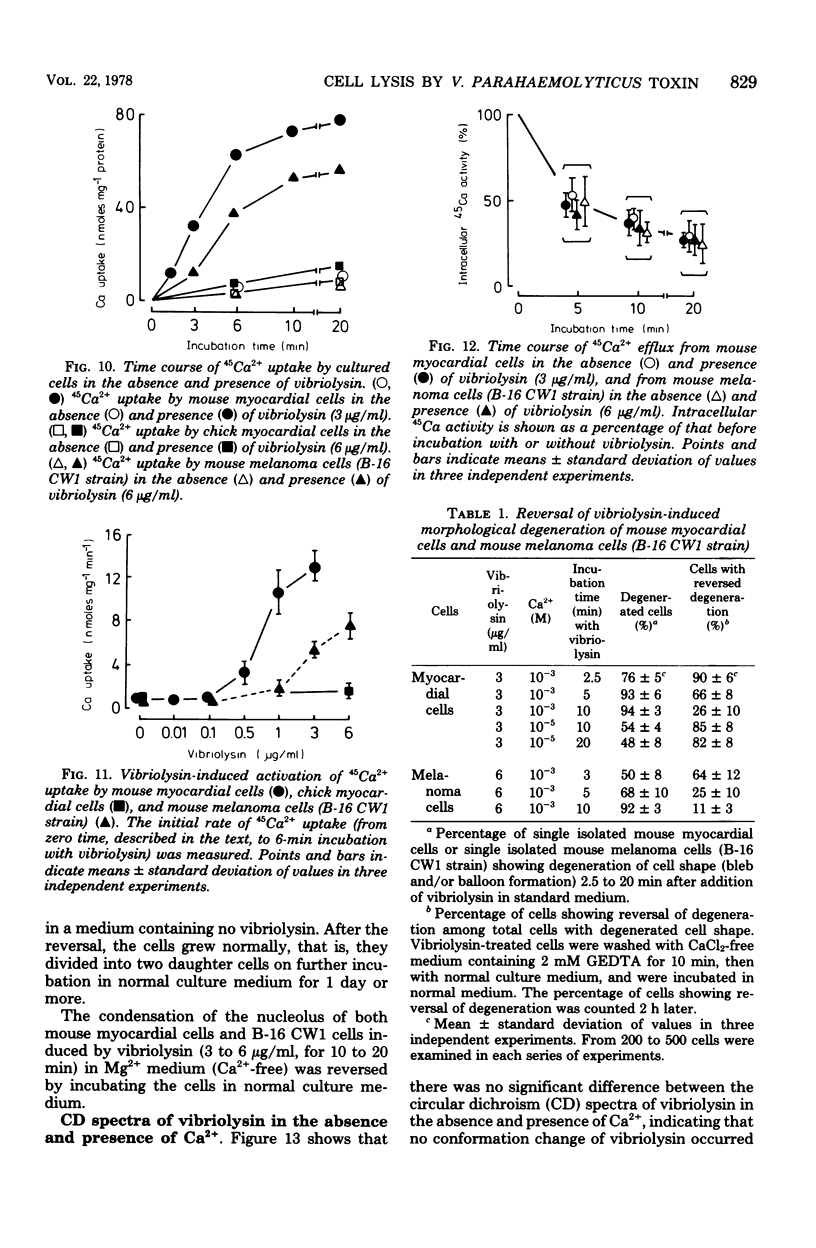
A highly purified toxin (vibriolysin) from Vibrio parahaemolyticus caused degeneration of cell shape, such as bleb and balloon formation, of mouse myocardial cells and mouse melanoma cells in culture. An extracellular Ca2+ concentration of more than 10(-6) M was necessary for the degeneration of cell shape, but extracellular Mg2+, Na+, and K+ were not necessary. In the presence of extracellular Ca2+, vibriolysin also caused full contraction of myofibrils of mouse myocardial cells and reduction of both actin cables and tubulin networks of mouse melanoma cells. Vibriolysin also caused excess uptake of Ca2+ from the incubation medium by mouse myocardial cells and mouse melanoma cells. Chick myocardial cells, which show neither degeneration of cell shape nor full contraction of myofibrils, did not take up excess 45Ca2+ in the presence of vibriolysin. These findings suggest that the vibriolysin-induced degeneration of cell shape of mouse myocardial cells and mouse melanoma cells is due to excess uptake of Ca2+ from the incubation medium by the cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- DeHaan R. L. The potassium-sensitivity of isolated embryonic heart cells increases with development. Dev Biol. 1970 Oct;23(2):226–240. doi: 10.1016/0012-1606(70)90096-5. [DOI] [PubMed] [Google Scholar]
- Doi E., Jirgensons B. Circular dichroism studies on the acid denaturation of gamma-immunoglobulin G and its fragments. Biochemistry. 1970 Mar 3;9(5):1066–1073. doi: 10.1021/bi00807a003. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Barry J., Lenoir M. C., Lazdunski M. Analysis of molecular aspects of Na+ and Ca2+ uptakes by embryonic cardiac cells in culture. J Biol Chem. 1977 Sep 10;252(17):6112–6117. [PubMed] [Google Scholar]
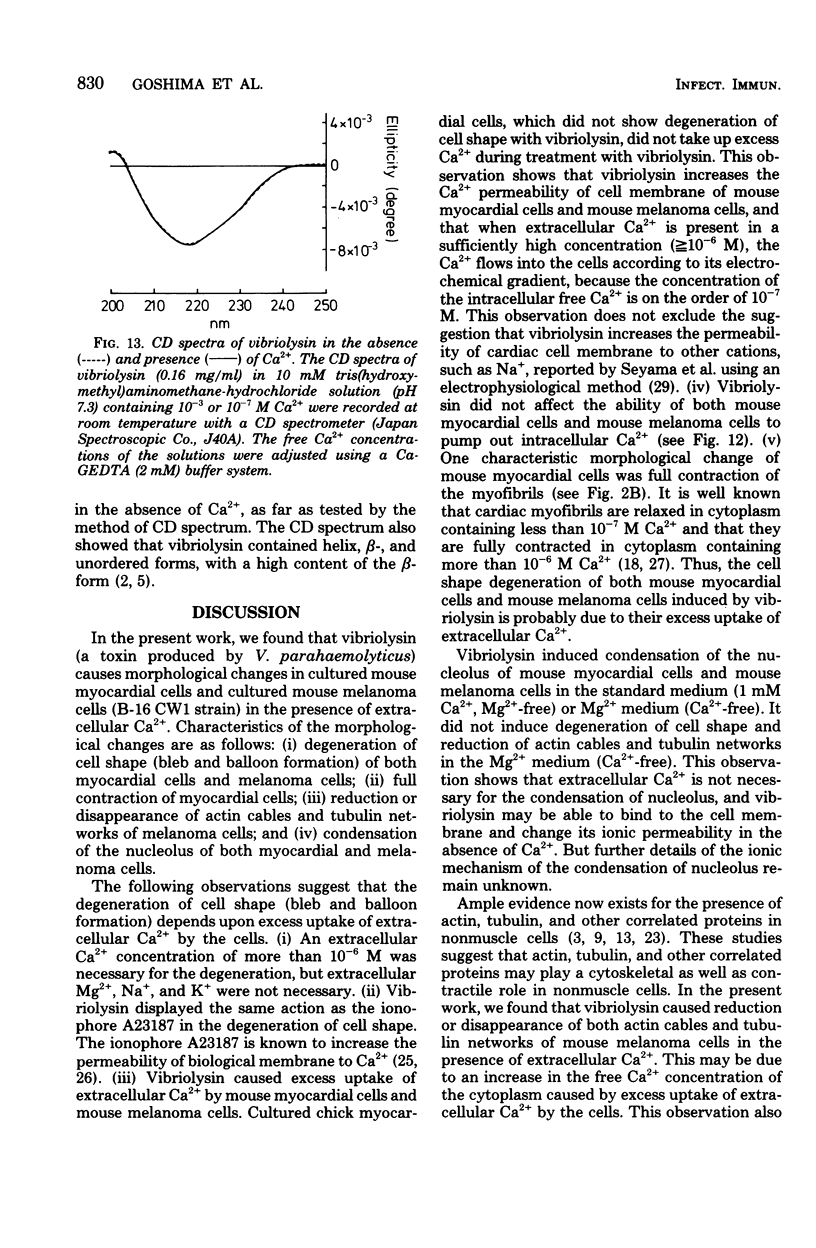
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Goshima K. Arrhythmic movements of myocardial cells in culture and their improvement with antiarrhythmic drugs. J Mol Cell Cardiol. 1976 Mar;8(3):217–238. doi: 10.1016/0022-2828(76)90038-9. [DOI] [PubMed] [Google Scholar]
- Goshima K., Honda T., Hirata M., Kikuchi K., Takeda Y. Stopping of the spontaneous beating of mouse and rat myocardial cells in vitro by a toxin from Vibrio parahaemolyticus. J Mol Cell Cardiol. 1977 Mar;9(3):191–213. doi: 10.1016/0022-2828(77)90029-3. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of motility in nonmuscle cells. J Cell Biol. 1977 Jul;74(1):1–15. doi: 10.1083/jcb.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Goshima K., Takeda Y., Sugino Y., Miwatani T. Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect Immun. 1976 Jan;13(1):163–171. doi: 10.1128/iai.13.1.163-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer G. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1973;35:55–86. doi: 10.1146/annurev.ph.35.030173.000415. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaribe K., Hatano S. Inducation of antibody against actin from myxomycete plasmodium and its properties. Biochemistry. 1975 Jul;14(13):3024–3029. doi: 10.1021/bi00684a035. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Seyama I., Irisawa H., Honda T., Takeda Y., Miwatani T. Effect of hemolysin produced by Vibrio parahaemolyticus on membrane conductance and mechanical tension of rabbit myocardium. Jpn J Physiol. 1977;27(1):43–56. doi: 10.2170/jjphysiol.27.43. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E. High-resolution preparative SDS-polyacrylamide gel electrophoresis: fluorescent visualization and electrophoretic elution-concentration of protein bands. Anal Biochem. 1975 May 12;65(1-2):369–379. doi: 10.1016/0003-2697(75)90521-7. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ogiso Y., Miwatani T. Effect of zinc ion on the hemolytic activity of thermostable direct hemolysin from Vibrio parahaemolyticus, streptolysin O, and Triton X-100. Infect Immun. 1977 Aug;17(2):239–243. doi: 10.1128/iai.17.2.239-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Takeda T., Honda T., Miwatani T. Inactivation of the biological activities of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside Gt1. Infect Immun. 1976 Jul;14(1):1–5. doi: 10.1128/iai.14.1.1-5.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]