Abstract
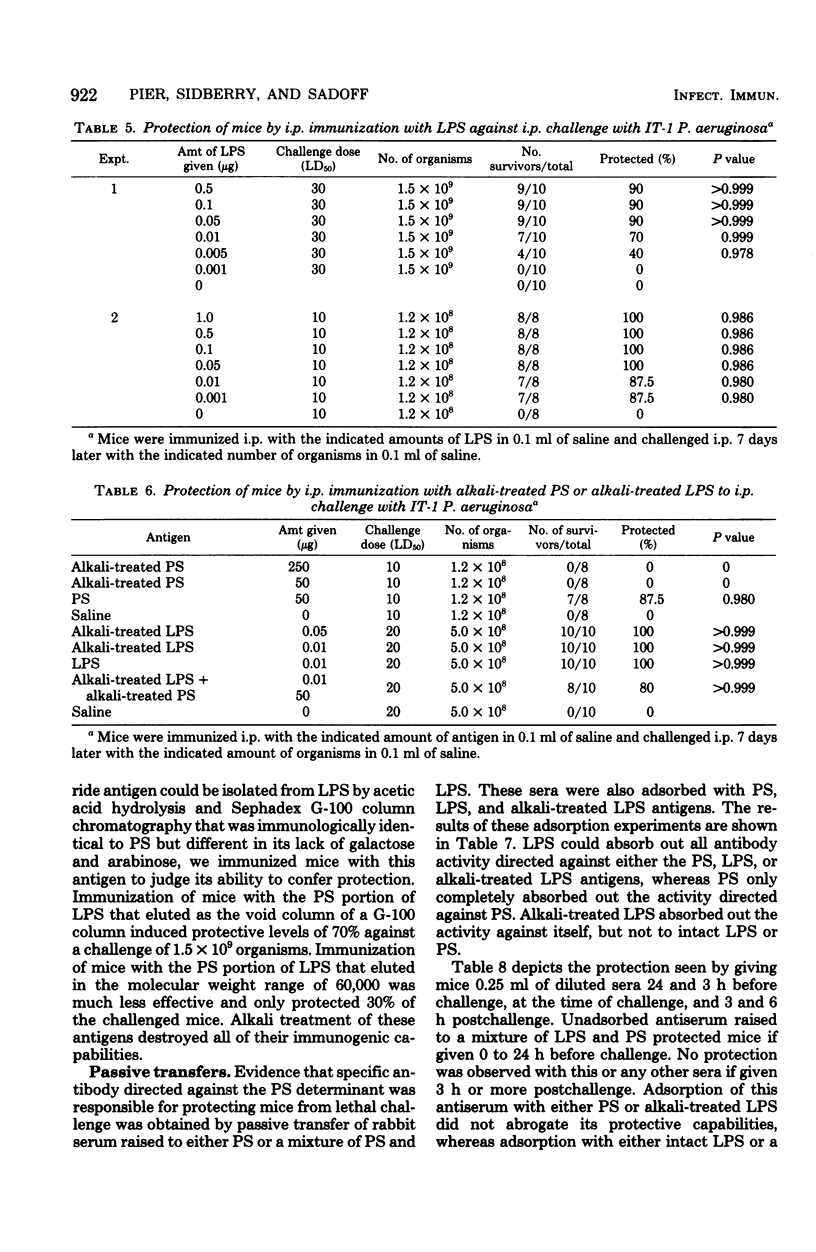
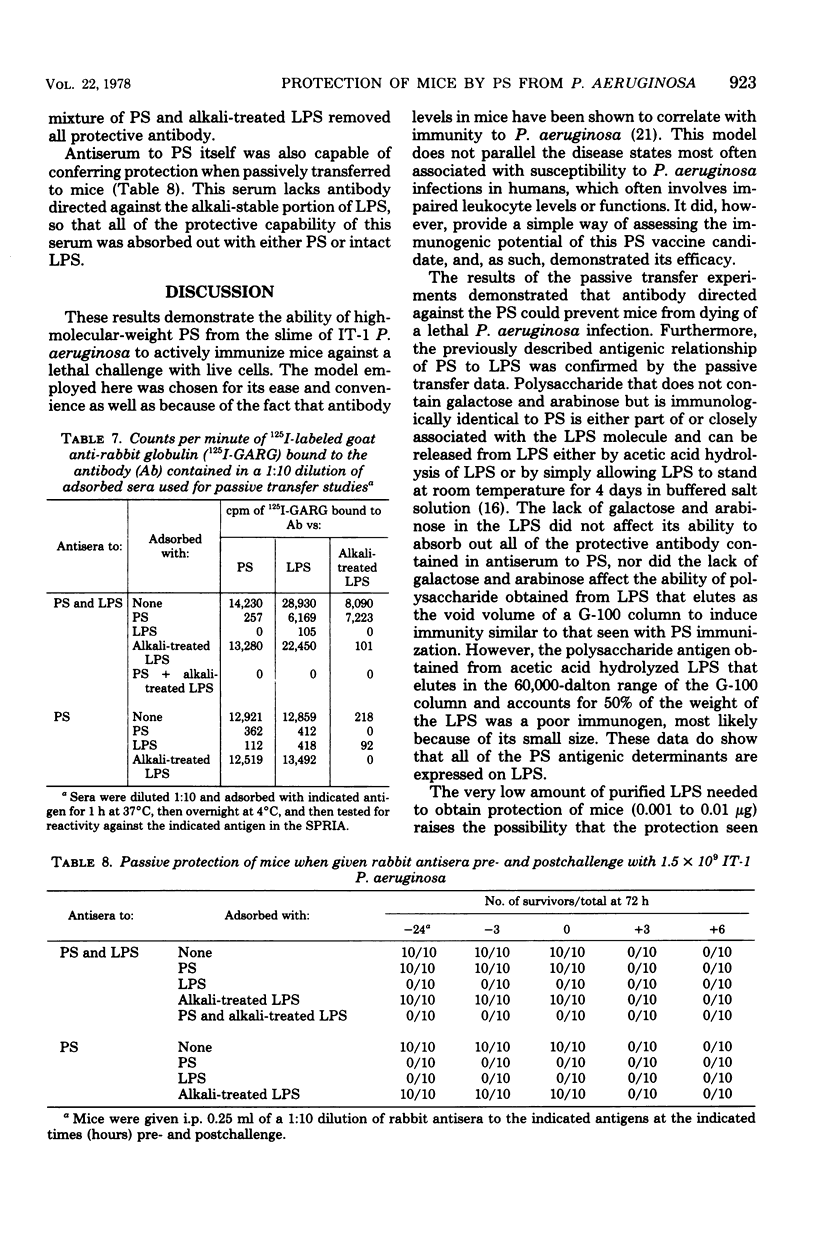
A high-molecular-weight alkali-labile polysaccharide (PS) isolated from the slime of immunotype 1 Pseudomonas aeruginosa was tested for its ability to protect mice from lethal challenge with the live, homologous organism. Intraperitoneal (i.p.) injection of 10 to 25 microgram of the PS protected 60 to 70% of the mice against challenge with up to 50 50% lethal dose units. Although single immunization of mice with up to 250 microgram of PS effected protective levels of only 70%, two successive immunizations provided 100% protection. Subcutaneous and intravenous immunization with PS also provided protection to i.p. challenges with immunotype 1 P. aeruginosa, but not to i.p. challenge with immunotype 4 P. aeruginosa. Although lipopolysaccharide (LPS) was found to be more immunogenic than PS in out studies, contamination of the alkali-labile PS with LPS did not account for the protection seen. Alkali treatment (0.1 N NaOH, 37 degrees C, 2 h) of the PS destroyed its protective effectiveness, while similarly treated LPS retained its capacity for inducing immunity in mice. Adsorption and passive protection studies with sera raised to either PS or a mixture of PS and LPS indicated that antibody directed to the alkali-labile PS antigen was capable of contributing to the protection of mice against challenge with P. aeruginosa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Brown W., Walker H., Mason A. D., Jr, Moncrief J. A. Studies on the isolation of an infection-protective antigen from Psudomonas aeruginosa. Surg Gynecol Obstet. 1966 Nov;123(5):965–977. [PubMed] [Google Scholar]
- Alexander J. W., Fisher M. W., MacMillan B. G., Altemeier W. A. Prevention of invasive pseudomonas infection in burns with a new vaccine. Arch Surg. 1969 Aug;99(2):249–256. doi: 10.1001/archsurg.1969.01340140121018. [DOI] [PubMed] [Google Scholar]
- Austrian R. Prevention of pneumococcal infection by immunization with capsular polysaccharides of Streptococcus pneumoniae: current status of polyvalent vaccines. J Infect Dis. 1977 Aug;136 (Suppl):S38–S42. doi: 10.1093/infdis/136.supplement.s38. [DOI] [PubMed] [Google Scholar]
- Bennett J. V. Nosocomial infections due to Pseudomonas. J Infect Dis. 1974 Nov;130 (Suppl)(0):S4–S7. doi: 10.1093/infdis/130.supplement.s4. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med. 1969 Jun 1;129(6):1385–1395. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Groves E. H. A Clinical Lecture ON A CASE OF BACILLUS PYOCYANEUS PYAEMIA SUCCESSFULLY TREATED BY VACCINE: Delivered at the Bristol General Hospital. Br Med J. 1909 May 15;1(2524):1169–1170. doi: 10.1136/bmj.1.2524.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S., Regan W., Watson D., Haskell T. H. Isolation and characterization of antigenic components of a new heptavalent Pseudomonas vaccine. Nat New Biol. 1971 Feb 17;229(7):209–210. doi: 10.1038/newbio229209a0. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M. Direct evidence for the presence of lipopolysaccharide components in Pseudomonas ribosomal vaccine. Infect Immun. 1977 Aug;17(2):471–473. doi: 10.1128/iai.17.2.471-473.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates A., Zand P. Specificity of the protective response induced by the slime layer of Pseudomonas aeruginosa. J Hyg (Lond) 1974 Aug;73(1):75–84. doi: 10.1017/s002217240002386x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miler J. M., Spilsbury J. F., Jones R. J., Roe E. A., Lowbury E. J. A new polyvalent Pseudomonas vaccine. J Med Microbiol. 1977 Feb;10(1):19–27. doi: 10.1099/00222615-10-1-19. [DOI] [PubMed] [Google Scholar]
- Millican R. C., Evans G., Markley K. Susceptibility of burned mice to Pseudomonas aeruginosa and protection by vaccination. Ann Surg. 1966 Apr;163(4):603–610. doi: 10.1097/00000658-196604000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E. Preliminary investigations of Pseudomonas aeruginosa vaccine in patients with leukemia and cystic fibrosis. J Infect Dis. 1974 Nov;130 (Suppl)(0):S159–S162. doi: 10.1093/infdis/130.supplement.s159. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Schimpff S. C., Greene W. H., Young V. M., Wiernik P. H. Significance of Pseudomonas aeruginosa in the patient with leukemia or lymphoma. J Infect Dis. 1974 Nov;130 (Suppl)(0):S24–S31. doi: 10.1093/infdis/130.supplement.s24. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972 Sep;3(3):291–347. doi: 10.3109/10408367209151698. [DOI] [PubMed] [Google Scholar]
- Young L. S., Meyer R. D., Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973 Oct;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



