Primary central nervous system lymphoma (PCNSL) represents a rare subtype of non-Hodgkin lymphoma (NHL) restricted to the CNS. In adults, PCNSL is associated with a poor prognosis.1 Patients with predisposing conditions such as immunodeficiency have an increased risk of PCNSL.2 In children, very little is known about PCNSL. The International Primary CNS Lymphoma Collaborative Group (IPCG) reported a retrospective collection of 29 PCNSL patients who had been treated with various treatment strategies.3 Another retrospective review from North America identified 12 PCNSL patients,4 and the recent retrospective mono-center experience of the Seoul Children Hospital reported 6 patients treated with relapse-free survival achieved in 5.5 Taking these cases together with earlier case reports,2,4,6–10 far less than 100 cases of PCNSL in children and adolescents have been published so far.
In the current study of the population-based NHL-BFM data (Berlin-Frankfurt-Muenster), the frequency, clinical characteristics, diagnostic difficulties, treatment and outcome of 17 pediatric PCNSL patients are discussed. All patients were uniformly diagnosed and treated according to NHL-BFM protocols. The study aims to add relevant information to the ongoing discussion concerning optimal treatment. Also, the description of the prolonged and difficult diagnosis and differential diagnosis procedures in the current report might raise awareness of pediatric patients with PCNSL.
Children and adolescents diagnosed with any subtype of NHL were registered with the NHL-BFM-center after patients and/or guardians gave informed consent. Standard staging investigations included examination of cerebrospinal fluid (CSF), cranial computed tomography (CT) and/or cranial magnetic resonance imaging (MRI). CNS disease was diagnosed in patients with intracerebral/intraspinal mass(es) (ICM) and/or cranial nerve palsy (CNP) not caused by an extradural mass and/or blasts in the CSF. Epidural NHL was not considered as CNS disease. NHL was classified according to WHO classification and centrally reviewed by NHL-BFM reference pathologists, except for Patients 4 and 12. CSF slides were centrally reviewed by 3 of the authors (WW, AR, BB). Patients were treated according to the NHL-BFM90,11 NHL-BFM9512 or B-NHL BFM0413 protocols. Institutional review board approvals were obtained before patients were included in the study.
For statistical analyses, differences between subgroups were examined by χ2 test or Fisher’s exact test. Probability of survival (pOS) was calculated from diagnosis to first event (death, progression/relapse/non-response, second malignancy) or to last follow up using the Kaplan-Meier method. Differences were compared using the log rank test, and the standard error (SE) was calculated according to Greenwood. Statistical analysis was performed using the SAS program v.9.13 (SAS Institute Inc., Cary, NC, USA).
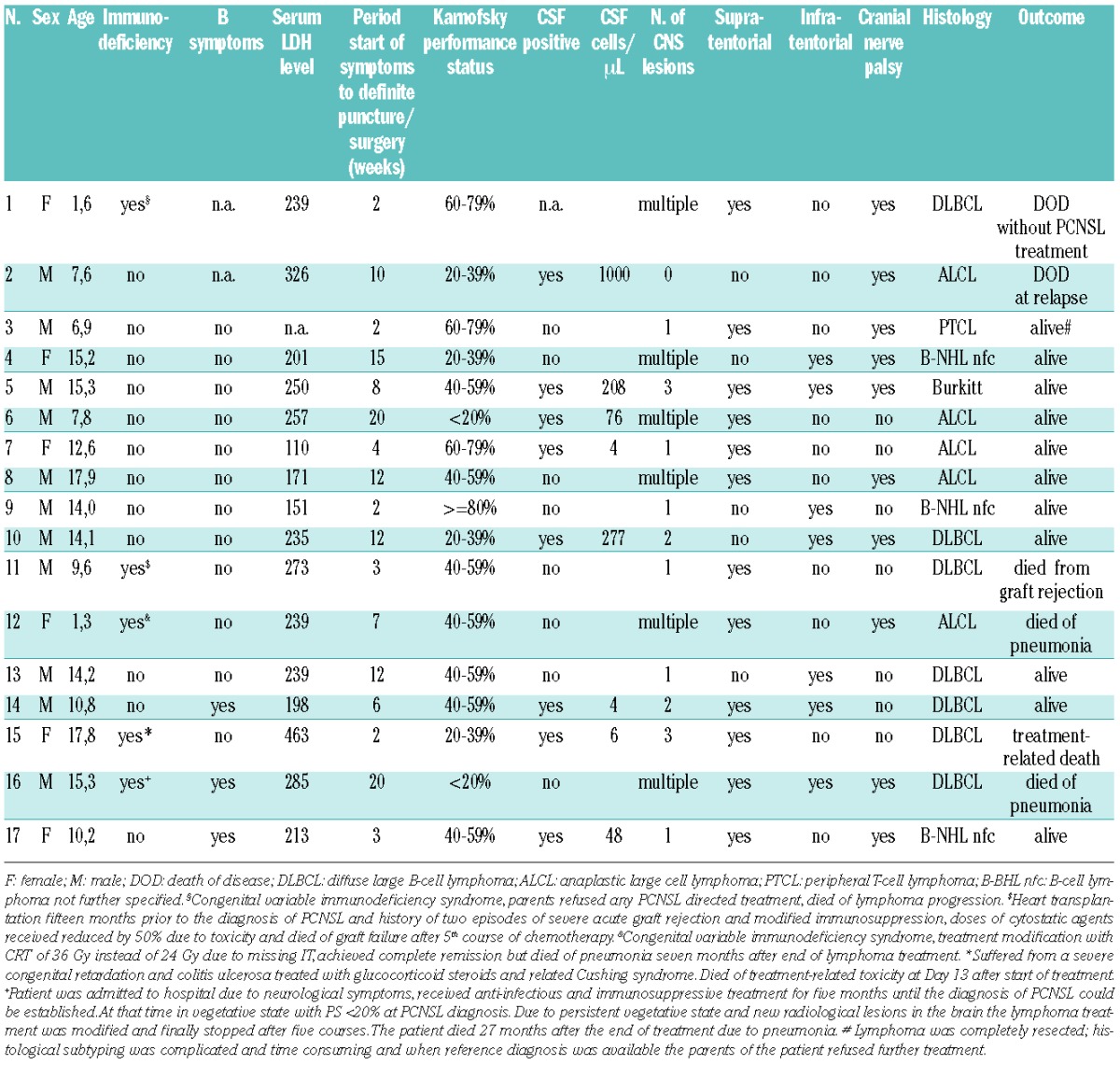
Among 3740 pediatric or adolescent NHL patients registered between 1990 and 2011, 17 patients with PCNSL were identified: 12 immunocompetent and 5 immunocompromised. Median age was 13.3 years (range 1.3–17.9 years). The histological diagnosis was peripheral T-cell lymphoma (PTCL) in one patient, anaplastic large cell lymphoma (ALCL) in 5 patients, and mature aggressive B-cell lymphoma (B-NHL) in 11 patients (Figure 1). Epstein-Barr encoding region (EBER) in situ hybridization to identify Epstein-Barr virus (EBV) antigens in the lymphoma tissue was performed in 5 patients (Patients 1, 5, 14, 15 and 16), and was positive in one (Patient 1) and negative in 4 samples. In all but one patient (Patient 2), CT/MRI showed solid CNS lymphoma manifestation(s) including one patient (Patient 13) with one large intraspinal manifestation. Six patients (38%) had multiple intracerebral lesions. One patient was diagnosed with primary leptomeningeal disease with 1000 lymphoma cells/μL CSF and cranial nerve palsy (Patient 2).
Figure 1.
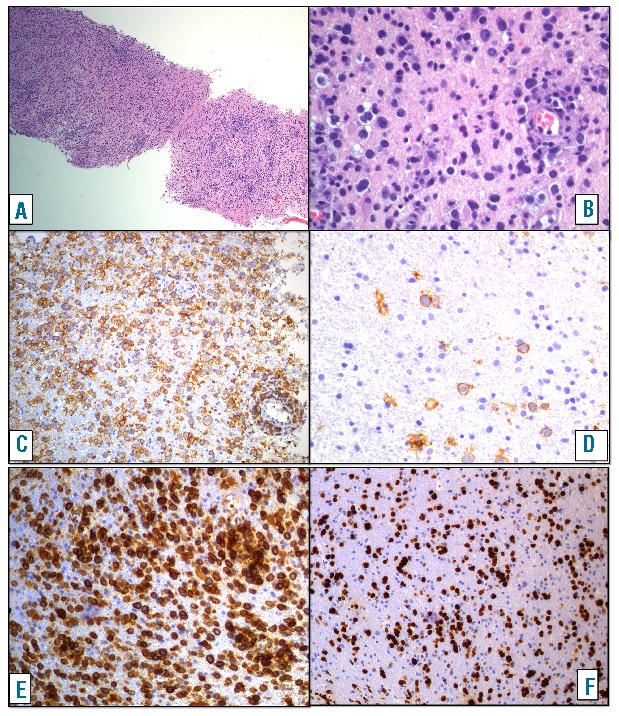
Pathological and immunohistochemical findings of PCNSL of diffuse large B-cell subtype in Patient 16. (A) At low magnification a diffuse infiltration of the CNS-tissue by mononuclear cells can be observed (Hematoxylin-Eosin magnification ×50). (B) At high magnification large blastic lymphoid cells are seen in between glial cells and glial stroma (Hematoxylin-Eosin magnification ×400). The large cells express the B-cell marker CD20. (C and D) Magnification magnification ×200. Areas with dense infiltration (C) and areas with only scattered blasts (D) are present within the same biopsy. The large cells express BCL2 (E) (magnification ×200) and show a high proliferation index (F) KI67 (magnification ×100). Provided by Ilske Oschlies, Kiel, Germany.
Presenting symptoms were often symptoms of increased intracranial pressure with headache (53%), nausea (53%), and vomiting (47%) or ataxia (41%), and symptoms of cranial nerve disorders (59%). Median interval between onset of symptoms and the diagnostic procedure that led to the diagnosis of PCNSL was eight weeks (range 2–20 weeks). Seven patients were treated for meningitis/encephalitis and one patient was treated for multiple sclerosis. Non-response to this anti-infectious treatment resulted in the tumor biopsy that allowed PCNSL diagnosis in these 8 patients. At the time of PCNSL diagnosis, 7 patients presented with severe neurological complications such as hemiparesis (n=1), seizures (n=3), incomplete paraparesis (n=2), and somnolence requiring immediate trepanation (n=1). Five patients suffered from underlying immunodeficiency or immunomodulation (Table 1).
Table 1.
Characteristics of 17 pediatric patients with primary central nervous system lymphoma of the NHL-BFM-group.
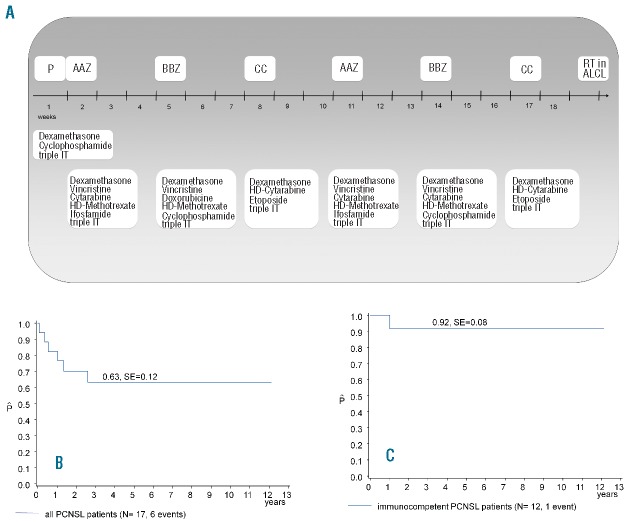
Patients were treated according to NHL-BFM protocols with pre-phase followed by six 5-day courses of chemotherapy (summarized in Figure 2A).11–13 ALCL patients received cranial radiotherapy (CRT) of 24 Gy after chemotherapy.
Figure 2.
(A) Outline of the treatment schema. (B) Probability of survival for all 17 patients with primary central nervous system lymphoma registered in the NHL-BFM center between 1990 and 2011. (C) Probability of survival for 12 immunocompetent patients with primary central nervous system lymphoma registered in the NHL-BFM center between 1990 and 2011. P: Pre-phase; IT: intrathecal treatment; HD: high dose; RT: cranial radiotherapy; ALCL: anaplastic large cell lymphoma.
With a median follow up of 7.5 years (range 2.4–12.1 years), the 3-year probability of overall survival (OS) of all 17 PCNSL patients was 63+12% and 92+8% for the 12 immunocompetent patients (Figure 2). All 5 patients with underlying diseases died 0, 1, 4, 16 and 31 months after start of treatment (Table 1). Among the 12 immunocompetent patients, one event was reported in an ALCL patient who suffered leptomeningeal relapse nine weeks after end of treatment and died of lymphoma progression (Patient 2).
Concerning neurological outcome and late effects, 2 patients suffered from reduced visual acuity due to atrophy of the optical nerve related to prolonged increased intracranial pressure (Patients 6 and 10). One patient suffered from epilepsy after end of treatment (Patient 6), and one patient suffered from panhypopituitarism after surgical lymphoma resection localized in the suprasellar midline (Patient 5). In the remaining 8 patients alive, no relevant neurological deficit was reported 6, 21, 29, 41, 48, 80, 84 and 84 months after start of treatment of PCNSL.
Primary central nervous system lymphoma represents a rare NHL-subtype in adults and children and is associated with a poor prognosis. The current PCNSL series of the NHL-BFM-group represents the largest series of uniformly diagnosed and treated pediatric PCNSL patients. Patients’ characteristics and histological diagnoses of the NHL-BFM cohort are similar to earlier reports except for the initial serum LDH levels, which are normal for the majority of NHL-BFM patients. This is in contrast to the IPCG cohort with elevated LDH levels 53% of 17 cases with available data.3 The detailed chart review of the NHL-BFM PCNSL patients documents for the first time the complicated medical histories until a definite diagnosis in pediatric PCNSL patients can be made. The onset of seizures leads to the shortest intervals between first symptoms and a definite diagnosis. The diagnosis of PCNSL is complicated, especially in adolescents with non-specific symptoms. Although a rare disease, PCNSL should be considered in healthy children and adolescents with non-specific neurological symptoms. The performance status (PS) at diagnosis has been reported as prognostic factor.3 The current NHL-BFM data cannot support an increase or decrease of treatment intensity according to general condition at diagnosis. Any other evaluation of reported prognostic parameters, such as age, LDH, or cortical versus deep brain lesions, could not be tested in the NH-BFM series as only one immunocompetent patient suffered relapse.
All 5 NHL-BFM patients with severe underlying diseases died due to toxicity or complications of the underlying disease (n=4) and lymphoma progression without PCNSL treatment (n=1). Abla reported 4 cases of PCNSL with immunodeficiency of whom one died of infectious complications after treatment.4 The IPCG cohort included 3 patients with immunodeficiency but outcome is not specified.3 In addition, individual cases of pediatric PCNSL patients with immunodeficiency were reported.2,7 In conclusion, the number of pediatric PCNSL patients with immunodeficiency is limited and the individual medical course variable. However, the NHL-BFM-group will critically discuss treatment recommendations when consulted for PCNSL patients with immunodeficiency taking into account the experiences and guidelines for NHL-treatment in patients with chromosomal instability syndromes.14
In the NHL-BFM series, only one out of the 16 patients treated for PCNSL suffered relapse. In spite of the small number of patients, disease control of PCNSL treated according to NHL-BFM protocols for CNS-positive NHL seems comparable to that of systemic NHL with CNS involvement.15 The relapse rate in the current NHL-BFM series is remarkably lower than in the IPCG cohort with 10 relapses in 29 patients (34%), all suffering from isolated CNS relapse.3 The 2-year progression-free survival of 61% and the 3-year OS of 82% for the IPCG cohort might indicate insufficient front-line treatment, at least for a subset of patients. In contrast, the intensive NHL-BFM treatment might reduce the rate of relapse but also selects for resistant disease at relapse. This hypothesis is supported by a recently reported series of 6 pediatric PCNSL patients treated with intensive protocols LMB-FAB96 in 5 patients and CCG-106B in one patient, and the observation of only one relapse but resistant disease at the time of relapse.5
In the current series, only ALCL patients, but not B-NHL patients, received cranial radiotherapy per protocol. Interestingly, the only relapse occurred in one of the 5 ALCL patients with none in the B-NHL patients. This supports the conclusion of the above-mentioned report that stated that PCNSL of B-NHL subtype can be successfully treated without irradiation.5
Primary central nervous system lymphoma is also a rare and aggressive lymphoma subtype in adults, with 32–77% 5-year survival. Whether the differences in outcome between PCNSL in children and adults are related to treatment regimens, to differences in the lymphoma biology or to differences in co-morbidities remains open to debate.
In conclusion, the rare subtype of PCNSL in pediatric patients can be treated successfully with protocols designed for pediatric NHL patients suffering from CNS-positive systemic NHL. However, the treatment of pediatric PCNSL patients with immunodeficiency or other severe underlying diseases remains challenging.
Acknowledgments
The authors would like to thank the expert work of Ulrike Meyer, Bettina Paul and Sabine Mann (data management) and Edelgard Odenwald and Gabriele Buck (cytomorphology).
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013;9(6):317–27. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez MM, Delgado PI, Petito CK. Epstein-Barr virus-associated primary central nervous system lymphoma in a child with the acquired immunodeficiency syndrome. A case report and review of the literature. Arch Pathol Lab Med. 1997;121(12):1287–91. [PubMed] [Google Scholar]
- 3.Abla O, Weitzman S, Blay JY, O’Neill BP, Abrey LE, Neuwelt E, et al. Primary CNS lymphoma in children and adolescents: a descriptive analysis from the International Primary CNS Lymphoma Collaborative Group (IPCG). Clin Cancer Res. 2011;17(2):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abla O, Sandlund JT, Sung L, Brock P, Corbett R, Kirov I, et al. A case series of pediatric primary central nervous system lymphoma: favorable outcome without cranial irradiation. Pediatr Blood Cancer. 2006;47(7):880–5. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JH, Kang HJ, Kim H, Lee JW, Park JD, Park KD, et al. Successful treatment of primary central nervous system lymphoma without irradiation in children: single center experience. J Korean Med Sci. 2012;27(11):1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kai Y, Kuratsu J, Ushio Y. Primary malignant lymphoma of the brain in childhood. Neurol Med Chir (Tokyo). 1998;38(4):232–7. [DOI] [PubMed] [Google Scholar]
- 7.Zhu JQ, Hao NX, Bao WQ, Wu XR. Multiple calcified primary central nervous system lymphoma with immunodeficiency in a child. World J Pediatr. 2011;7(3):277–9. [DOI] [PubMed] [Google Scholar]
- 8.Quadri SA, Sobani ZA, Enam SA, Enam K, Ashraf MS. Primary central nervous system lymphoma causing multiple spinal cord compression and carcinomatous meningitis in a 6-year-old: a case report. J Pediatr Hematol Oncol. 2011;33(4):312–5. [DOI] [PubMed] [Google Scholar]
- 9.Makino K, Nakamura H, Yano S, Kuratsu JI. Pediatric primary CNS lymphoma: longterm survival after treatment with radiation monotherapy. Acta Neurochir (Wien). 2007;149(3):295–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 10.Havlioglu N, Manepalli A, Galindo L, Sotelo-Avila C, Grosso L. Primary Ki-1 (anaplastic large cell) lymphoma of the brain and spinal cord. Am J Clin Pathol. 1995;103(4):496–9. [DOI] [PubMed] [Google Scholar]
- 11.Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94(10):3294–306. [PubMed] [Google Scholar]
- 12.Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105(3):948–58. [DOI] [PubMed] [Google Scholar]
- 13.Lisfeld J, Burkhardt B, Zimmermann M, Meinhardt A, Hoefer V, Woessman W, et al. CNS disease in paediaric patients with B-cell Non-Hodgkin lymphoma and Burkitt leukemia - therapy and outcome in the B-NHL BFM 04 study. Hematological Oncology. 2013;31(Suppl 1):122. [Google Scholar]
- 14.Bienemann K, Burkhardt B, Modlich S, Meyer U, Moricke A, Bienemann K, et al. Promising therapy results for lymphoid malignancies in children with chromosomal breakage syndromes (Ataxia teleangiectasia or Nijmegen-breakage syndrome): a retrospective survey. Br J Haematol. 2011;155(4):468–76. [DOI] [PubMed] [Google Scholar]
- 15.Salzburg J, Burkhardt B, Zimmermann M, Wachowski O, Woessmann W, Oschlies I, et al. Prevalence, clinical pattern, and outcome of CNS involvement in childhood and adolescent non-Hodgkin’s lymphoma differ by non-Hodgkin’s lymphoma subtype: a Berlin-Frankfurt-Munster Group Report. J Clin Oncol. 2007;25(25):3915–22. [DOI] [PubMed] [Google Scholar]