According to the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, myeloproliferative neoplasms (MPN) include chronic myeloid leukemia and the so-called Philadelphia-negative myeloproliferative neoplasms, i.e. essential thrombocythemia, polycythemia vera and primary myelofibrosis.1 Myeloproliferative features are also observed in a group of disorders classified as myelodysplastic/myeloproliferative neoplasms (MDS/MPN).1,2 This category includes clonal myeloid neoplasms that at the time of initial presentation have some features that support a diagnosis of myelodysplastic syndrome (MDS), and other findings more consistent with MPN. MDS/MPN include chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia (aCML), juvenile myelomonocytic leukemia (JMML), and MDS/MPN, unclassifiable (MDS/MPN, U). Of these latter unclassifiable conditions, the best characterized is the provisional entity defined as refractory anemia with ring sideroblasts associated with marked thrombocytosis (RARS-T).
The genetic basis of MPN and MDS/MPN has been broadly defined in the last ten years3,4 and is summarized in Table 1.
Table 1.
Genetic basis of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms.*
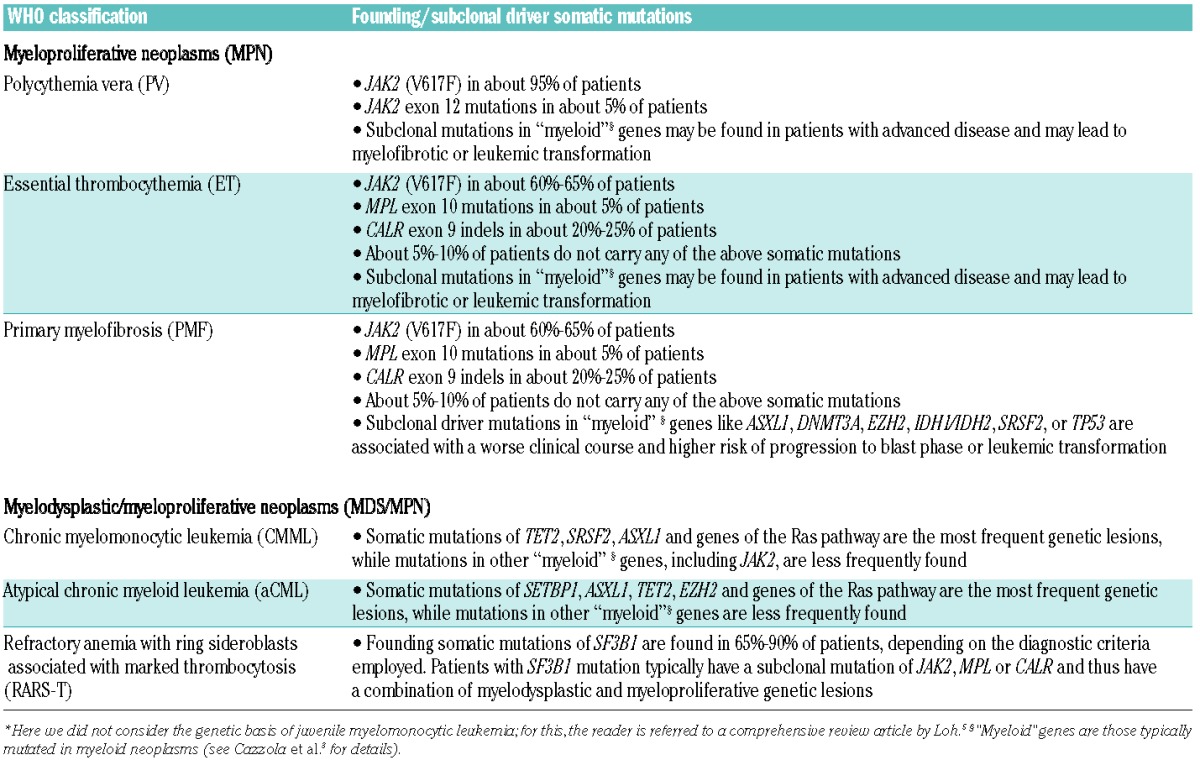
The unique JAK2 (V617F) mutation is found not only in about 80% of all patients with a myeloproliferative neoplasm, but also in a fraction of patients with MDS/MPN, including CMML and RARS-T.6,7 In CMML, the mutation is detected in about 10% of cases and is significantly associated with myeloproliferative features, including high leukocyte and monocyte counts and splenomegaly.6 RARS-T is characterized by anemia with dysplastic, ineffective erythroid proliferation and bone marrow ring sideroblasts, associated with high platelet count and proliferation of large atypical megakaryocytes. JAK2 (V617F) is detectable in about 50%–60% of patients.7,8 Occasional patients with mutations in MPL or JAK2 exon12 have been reported.9 The analysis of the mutant allele burden suggests that the disease may result from a combination of SF3B1 mutation, which usually occurs as an early event and is responsible for myelodysplastic features and ring sideroblasts, and JAK2 or MPL mutation, emerging as a subclone and conferring the myeloproliferative phenotype.10 Interestingly, occasional patients with typical RARS were reported to acquire JAK2 (V617F) and develop abnormal megakaryocyte proliferation and thromobcytosis.9
Somatic mutations of CALR, the gene encoding calreticulin, were detected in 2013 through studies of whole exome sequencing and targeted resequencing in patients with essential thrombocythemia, primary myelofibrosis and RARS-T.11,12 These somatic mutations are mutually exclusive with mutations in both JAK2 and MPL. All CALR mutations are insertions or deletions resulting in a frameshift, and cluster in the last exon (exon 9) of the gene. They generate a novel C-terminus of the mutated protein and likely impair the calcium-binding activity of calreticulin; exon 9 indels also cancel the endoplasmic reticulum retention motif (terminal KDEL amino acid sequence) of the protein. The available evidence suggests that both impaired calcium-binding activity and cellular dislocation within mutant megakaryocytes may play a role in the abnormal platelet production that characterizes patients carrying a somatic mutation of calreticulin.4 CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis.13 The observation that outside essential thrombocythemia and primary myelofibrosis somatic CALR mutations are found only in patients with RARS-T defines a strict relationship between mutant CALR and thrombocytosis phenotype, and reinforces the opinion that calreticulin mutations primarily affect the biology of megakaryocytes.
Patients with essential thrombocythemia carrying a CALR exon 9 mutation have very high platelet counts but a relatively low risk of thrombosis, at least lower than that of patients with JAK2 (V617F).14,15 In a study performed by investigators of the Associazione Italiana per la Ricerca sul Cancro (AIRC) Gruppo Italiano Malattie Mieloproliferative (AGIMM) on 617 patients with primary myelofibrosis, we found that median overall survival was 17.7 years in CALR-mutant, 9.2 years in JAK2-mutant, 9.1 years in MPL-mutant, and 3.2 years in triple-negative patients.16 These observations indicate that driver mutations define distinct disease entities within myeloproliferative neoplasms. Although more than 50 different CALR indels have been described, a 52-bp deletion (type 1 mutation) and a 5-bp insertion (type 2) are found in more than 80% of all CALR-mutant patients. Interestingly, the frequency of type 1 mutation is significantly higher in primary myelofibrosis than in essential thrombocythemia, suggesting a specific role of this mutation in myelofibrotic transformation.16,17
Haematologica has recently published a study by Andrikovics et al.18 on myeloproliferative neoplasms with calreticulin mutations. This study confirmed that calreticulin mutations are found in about one-fourth of patients with essential thrombocythemia or primary myelofibrosis and are associated with distinct clinical characteristics. In particular, the study showed that patients with primary myelofibrosis carrying a CALR indel have a better overall survival compared to those with JAK2 (V617F) or triple-negative cases.
In this issue of Haematologica, Li et al.19 report on a study conducted in 357 Chinese with primary myelofibrosis. They detected CALR mutations in 21% of patients, confirming previously reported frequencies in Caucasian patients (Table 1). The difference was, however, that 27% of Chinese patients were triple-negative; a percentage that is much higher than those previously reported in Caucasian patients.4,16 It should be noted that the same group of Chinese investigators had previously reported a high frequency (20%) of triple-negative patients in essential thrombocythemia.20 The reasons for these discrepancies may include differences in the diagnostic criteria or molecular approaches adopted, and ethnic differences. Ethnic differences would imply that currently unknown mutant genes are responsible for essential thrombocythemia or primary myelofibrosis in a considerable proportion of patients of Chinese descent. Another significant difference between the AGIMM study16 and that carried out by Li et al.19 regards the type of calreticulin mutations detected. In the AGIMM study on Caucasian patients with CALR-mutant primary myelofibrosis, 72% had the 52-bp deletion (type 1 mutation), 16% had the 5-bp insertion (type 2 mutation), and 17 (12%) carried other less frequent indels.16 A recent French study also showed over-representation of type-1 CALR mutation (70%) and under-representation of type-2 CALR mutation (13%) in primary myelofibrosis as compared with essential thrombocythemia.17 In the study by Li et al.,19 by contrast, type-1 mutation was found 32% and type-2 mutation in 64% of the PMF patients studied.
In summary, the identification of calreticulin mutations has thrown light on the genomic landscape of myeloproliferative neoplasms, creating the basis for a molecular classification of these disorders. Additional investigations are now needed to define how somatic mutations, gene expression, demographic data, clinical variables and patient outcome are interconnected. A specific area of research concerning somatic mutations of calreticulin is the potential different biological and clinical effects of type-1 and type-2 mutations.
Footnotes
Funding: the studies on myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms conducted at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, and Department of Molecular Medicine, University of Pavia, Pavia, Italy, are supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (Special Program Molecular Clinical Oncology 5 per Mille, project no. 1005, to MC).
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC, 2008. [Google Scholar]
- 2.Cazzola M, Malcovati L, Invernizzi R. Myelodysplastic/myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011;2011:264–72. [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood. 2013;122(25): 4021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123(24):3714–9. [DOI] [PubMed] [Google Scholar]
- 5.Loh ML. Childhood myelodysplastic syndrome: focus on the approach to diagnosis and treatment of juvenile myelomonocytic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010(1):357–62. [DOI] [PubMed] [Google Scholar]
- 6.Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–36. [DOI] [PubMed] [Google Scholar]
- 7.Malcovati L, Della Porta MG, Pietra D, Boveri E, Pellagatti A, Galli A, et al. Molecular and clinical features of refractory anemia with ringed sideroblasts associated with marked thrombocytosis. Blood. 2009;114(17):3538–45. [DOI] [PubMed] [Google Scholar]
- 8.Szpurka H, Tiu R, Murugesan G, Aboudola S, Hsi ED, Theil KS, et al. Refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), another myeloproliferative condition characterized by JAK2 V617F mutation. Blood. 2006;108(7):2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broseus J, Florensa L, Zipperer E, Schnittger S, Malcovati L, Richebourg S, et al. Clinical features and course of refractory anemia with ring sideroblasts associated with marked thrombocytosis. Haematologica. 2012;97(7):1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malcovati L, Papaemmanuil E, Bowen DT, Boultwood J, Della Porta MG, Pascutto C, et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118(24):6239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. [DOI] [PubMed] [Google Scholar]
- 12.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rumi E, Harutyunyan AS, Pietra D, Milosevic JD, Casetti IC, Bellini M, et al. CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood. 2014;123(15):2416–9. [DOI] [PubMed] [Google Scholar]
- 14.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. [DOI] [PubMed] [Google Scholar]
- 15.Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–5. [DOI] [PubMed] [Google Scholar]
- 16.Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124(7):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabagnols X, Defour JP, Ugo V, Ianotto JC, Mossuz P, Mondet J, et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia. 2014. September 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Andrikovics H, Krahling T, Balassa K, Halm G, Bors A, Koszarska M, et al. Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica. 2014;99(7):1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Xu J, Wang J, Gale RP, Xu Z, Cui Y, et al. Calreticulin mutations in Chinese with primary myelofibrosis. Haematologica; 2014:99(11):1697–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu R, Xuan M, Zhou Y, Sun T, Bai J, Cao Z, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia. 2014;28(9):1912–4. [DOI] [PubMed] [Google Scholar]


