Abstract
We tested 357 Chinese with primary myelofibrosis for mutations in CALR, JAK2 and MPL. CALR mutations were detected in 76 subjects (21%). There were 24 (32%) type-1 (L367fs*46) and 49 (64%) type-2 (K385fs*47) mutations. Seventy-two of 168 subjects (43%) without a JAK2 or MPL mutation had a CALR mutation. Subjects with a type-2 CALR mutation had lower hemoglobin concentrations (P=0.001), lower WBC counts (P<0.001), a higher percentage of blood blasts (P=0.009), and higher conventional (P<0.001) and Chinese-adjusted Dynamic International Prognostic Scoring System (P<0.001) scores compared with subjects with JAK2 mutations. Subjects with a type-2 CALR mutation were also likely to have abnormal platelet levels (<100 × 109/L, P=0.01 or >450 × 109/L, P=0.042) and no splenomegaly (P=0.004). Type-2 CALR mutation or no detectable mutation was an independent high-risk factor for survival in multivariate analyses. These data suggest the ratio between type-1 and type-2 mutations is reversed in Chinese with primary myelofibrosis compared with populations of subjects with primary myelofibrosis of predominately European descent. The unfavorable prognostic impact of CALR mutations in Chinese with primary myelofibrosis is only seen in those with type-2 mutations. These data underscore the need to evaluate the prognostic impact of genetic mutations in different populations.
Introduction
Calreticulin (CALR) mutations occur in approximately 60–90% of subjects with primary myelofibrosis (PMF) without mutations in JAK2 or MPL.1–4 Typically, these CALR mutations involve exon 9 and are somatic insertions or deletions (indels). Type-1 (L367fs*46) and type-2 (K385fs*47) CALR mutations are the most frequent. CALR mutations are associated with younger age, more severe anemia, higher WBC and platelet counts, lower DIPSS-plus scores, and better survival compared to subjects with JAK2 mutations.3 Some recent data suggest CALR mutations may only have a favorable prognostic impact in subjects with type-1 mutations.5
We previously reported important differences in clinical and laboratory features in Chinese with PMF compared with PMF in persons of predominately European descent. These differences led us to revise the Dynamic International Prognostic Scoring System (DIPSS) prognostic staging system that we termed DIPSS-Chinese.6 Our data are consistent with the notion that PMF in Chinese develops on a different genetic background than in persons of predominately European descent and this has phenotypic consequences.
This observation led us to analyze frequency, clinical correlates and the prognostic impact of CALR mutations in Chinese with PMF.
Methods
A bone marrow sample was collected at diagnosis or referral from 357 consecutive subjects with PMF who had given informed consent according to the Declaration of Helsinki. Histological material was re-reviewed by a blinded pathologist and diagnosis was based on World Health Organization (WHO) criteria.7 DIPSS, DIPSS-Plus and modified DIPSS scores for Chinese with PMF (DIPSS-Chinese) were calculated as previously described.6,8,9 Evaluable cytogenetic data were available for 194 subjects and these were further categorized using the DIPSS-plus scoring system. Follow-up data were available for 311 subjects. Subjects were treated as reported.6 Last follow up was date of last contact, date of death or 10 January 2014. Median follow up of survivors was 28 months (range 1–385). The study was approved by the Ethical Committee of the Institute of Hematology at the Chinese Academy of Medical Sciences, according to the guidelines of the Declaration of Helsinki. JAK2 and MPL mutations were tested at diagnosis as described.10–12 The minimal detection limit for JAK2 and MPL mutations is a 2.5% mutation burden. Oligonucleotide primers targeting exon 9 of CALR were used to amplify a 375 bp product: (Forward 5′-GTGGGGCGTAACAAAGGTGA-3′ and Reverse 5′-AGAGACATTATTTGGCGCGG-3′). PCR products were purified and sequenced bi-directionally. The minimal detection limit for CALR mutations is a 5% mutational burden. Mutations were identified using Mutation Surveyor Software (Applied Biosystems Genetic Analyzers). Correlations between sample groups and clinical and laboratory data were calculated with the χ2 test for qualitative variables with discrete categories and the Mann-Whitney U-test or Kruskal-Wallis analysis of variance for continuous variables. Survival distributions were estimated by the Kaplan-Meier method and were compared between subgroups using the log rank test. Cox proportional hazards regression model was used to assess the correlation between variables and survival. Two-tailed P≤0.05 was considered significant.
Results and Discussion
CALR mutations were detected in 76 subjects (21%): JAK2V617F mutations in 178 (50%) and MPL mutation in 11 (3%). Ninety-six subjects (27%) had no detectable mutation in CALR, JAK2 or MPL and are referred to as subjects with no mutation. There were 24 (32%) type-1 and 49 (64%) type-2 CALR mutations (Online Supplementary Figure S1). Seventy-two of 168 subjects (43%) without a JAK2 or MPL mutation had a CALR mutation. Four subjects with CALR and JAK2 mutations are excluded from subsequent analyses. Online Supplementary Table S1 lists base-line clinical and laboratory variables of the 353 study subjects (except for 4 subjects with concomitant CALR and JAK2 mutations) categorized by mutation profile.
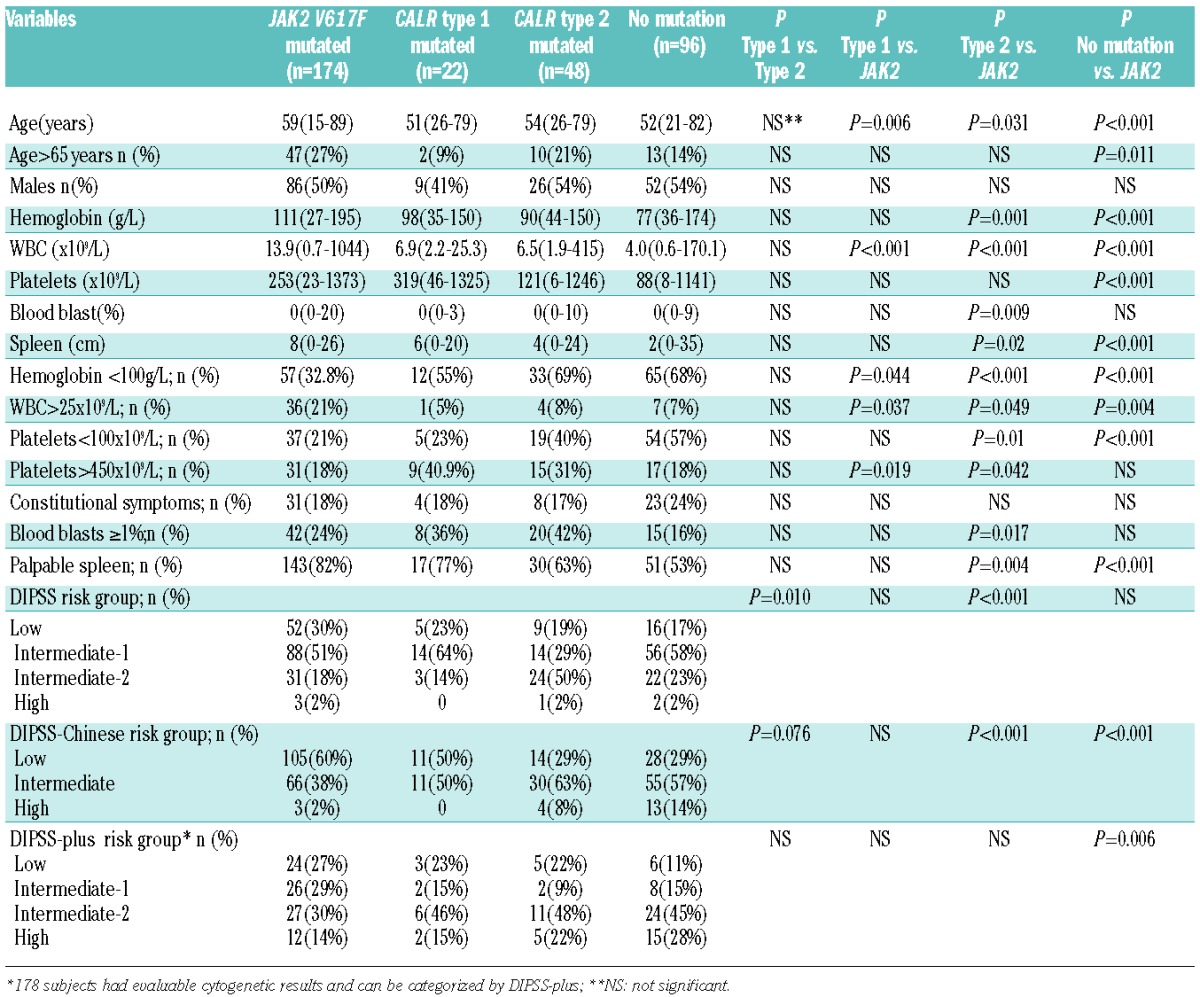
Subjects with type-1 and type-2 CALR mutations were compared to those with JAK2 mutations (Table 1). Subjects with type-1 CALR mutations were younger (P=0.006), had lower WBC counts (P<0.001), more frequent hemoglobin concentrations below 100 g/L (P=0.044), and more frequent platelet counts over 450 × 109/L (P=0.019). Subjects with type-2 CALR mutations were younger (P<0.001), had lower hemoglobin concentrations (P=0.001), lower WBC counts (P<0.001), a higher percentage of blood blasts (P=0.009), and higher DIPSS (P<0.001) and DIPSS-Chinese (P<0.001) scores. They were also likely to have abnormal platelet counts (<100 × 109/L, P=0.01 or >450 × 109/L, P=0.042) and no splenomegaly (P=0.004).
Table 1.
Clinical and laboratory features of 340 subjects with PMF and type-1 or -2 CALR or JAK2 mutations or no detectable mutation.
In univariate survival analysis, subjects with type-2 CALR mutation and those with no detectable mutation had significantly shorter survival compared with subjects with JAK2 mutations (Figure 1A). There was no significant difference in survival between subjects with type-1 CALR mutation and those with JAK2 mutations (Online Supplementary Figure S2A). Subjects with type-2 CALR mutations or no detectable mutation had comparable survival rates (Online Supplementary Figure S2B). In global survival analysis, subjects with type-2 CALR mutations or no detectable mutation had shorter survival compared to those with JAK2, MPL or type-1 CALR or other less common CALR mutations (Figure 1B). In multivariate analysis adjusted for DIPSS-Chinese, type-2 CALR mutation or no detectable mutation was independently correlated to risk of death (HR: 2.15; 95%CI: 1.32–3.51; P=0.002).
Figure 1.
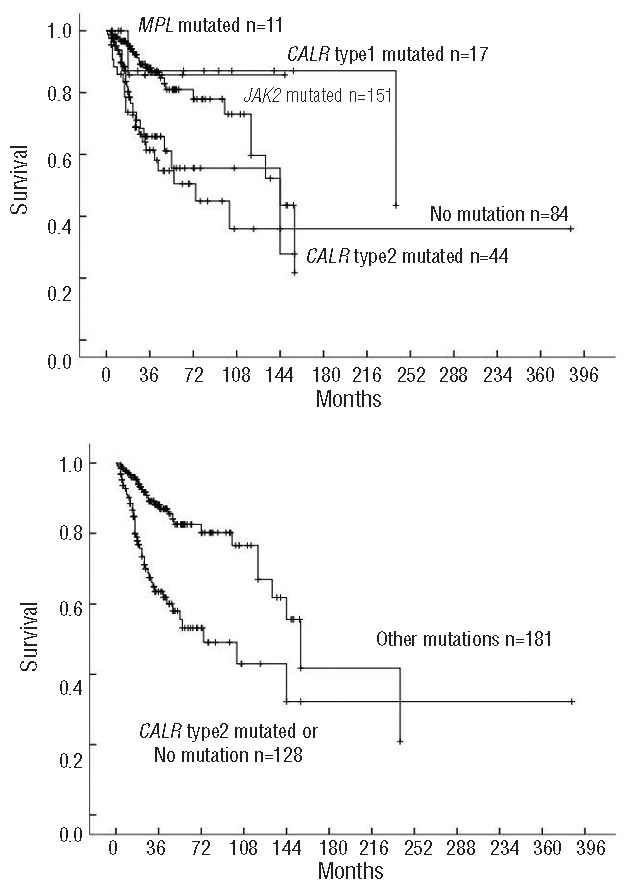
Survival of 309 subjects with PMF classified by CALR, JAK2, MPL or no detectable mutation. (A) CALR type-1 vs. -2 vs. JAK2 vs. MPL vs. no detectable mutation; (P<0.001); (B) CALR type-2 or no detectable mutation vs. JAK2, MPL, CALR type-1 and infrequent CALR mutation (referred to other mutations, P<0.001).
Our data indicate approximately 40% of Chinese with PMF and no detectable mutations in JAK2 or MPL have a CALR mutation. Type-1 and type-2 were the most common CALR mutations. This frequency is substantially lower than that reported in subjects with PMF of predominantly European descent. What remains unknown is how this parallels the different frequencies of JAK2 (49% vs. 58%) and MPL (3% vs. 8%) mutations. Although some of these differences may reflect the different sensitivities of the respective assays used, the different genetic backgrounds on which PMF develops may also be important.6
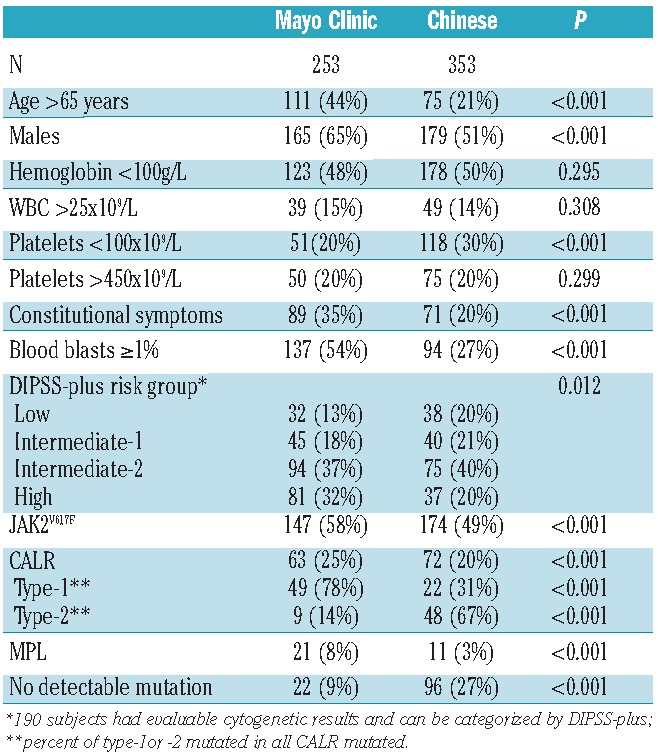
To study this issue further, we compared clinical features of our 353 Chinese subjects with PMF to a cohort reported from the Mayo Clinic3 (Table 2). We also noted differences in the impact of CALR mutations, especially type-2, on clinical features and survival of Chinese versus that of subjects of predominantly European descent with PMF. Chinese with type-2 CALR mutations had adverse clinical and laboratory features, including lower hemoglobin concentrations, a higher percentage of blood blasts, abnormal platelet levels, and less splenomegaly (splenomegaly is a favorable prognostic variable in Chinese populations; see below). These features resulted in higher conventional and Chinese DIPSS scores in subjects with type-2 CALR mutations. Subjects with no detectable mutation were also likely to be clustered in the higher risk group. Subjects with type-2 CALR mutations and those with no detectable mutation had significantly shorter survival than subjects with JAK2 mutations in univariate analysis. In contrast, we found less or no significant difference between subjects with type-1 CALR mutations versus those with JAK2 mutations in clinical or laboratory variables or survival. These data explain the differences in survival between Chinese with CALR mutations and subjects of predominantly European descent who had adverse type-1 and type-2 CALR mutations.1,3
Table 2.
Comparison of features between Chinese subjects and subjects reported from the Mayo Clinic.3
In a previous study, we had reported that splenomegaly at diagnosis correlated with longer survival in Chinese with PMF.6 The opposite is the case in subjects with PMF of predominantly European descent. Consequently, we analyzed the correlation between CALR mutations and splenomegaly in our subjects. Those with type-2 CALR mutations and those with no detectable JAK2 mutation were less likely to have splenomegaly than those with JAK2 mutations; they also had shorter survival. However, subjects with type-1 CALR and those with JAK2 mutations had similar frequencies of splenomegaly and similar survival rates. There was no significant difference in frequency of splenomegaly or survival between subjects with type-2 CALR mutations and those with no detectable mutation. Type-2 CALR mutations or no detectable mutation was an independent unfavorable prognostic factor in multivariate analysis.
In summary, our study shows CALR mutations occur in Chinese with PMF who lack mutations in JAK2 or MPL. However, this frequency of CALR mutations and the ratio of CALR mutation types differ from those reported in subjects with PMF of predominantly European descent. These data raise the possibility that one or more undiscovered mutations may be found in the 48% of Chinese with PMF without mutations in JAK2 and MPL and we are currently using exomic- and whole genome sequencing in our mutation-negative population to explore this. In Chinese with PMF, and in contrast to subjects of predominately European descent, the unfavorable prognostic impact of CALR mutations is limited to those with type-2. Type-2 CALR mutation or no detectable mutation in CALR, JAK2 or MPL is an independent high-risk molecular signature In Chinese with PMF. Consequently, screening of Chinese with PMF for CALR mutations may be useful for diagnosis and estimating survival.13
Footnotes
Funding
Supported, in part, by National Natural Science Funds (n. 81370611, n. 81270585), Tianjin Key Natural Science Funds (12JCZDJC23900) and National Public Health Grand Research Foundation (n. 201202017). RPG acknowledges support from the NIHR Biomedical Research Centre funding scheme.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. [DOI] [PubMed] [Google Scholar]
- 2.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014. January 9 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014. February 5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Lasho TL, Finke C, Belachew AA, Wassie EA, Ketterling RP, et al. Type1 vs type2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014. February 26 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Gale RP, Zhang Y, Qin TJ, Chen HS, Zhang PH, et al. Unique features of primary myelofibrosis in Chinese. Blood. 2012;119(11):2469–73. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010; 115(9):1703–8. [DOI] [PubMed] [Google Scholar]
- 9.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS-Plus: a refined Dynamic International Prognostic Scoring System (DIPSS) for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count and transfusion status. J Clin Oncol. 2011;29(4):392–7. [DOI] [PubMed] [Google Scholar]
- 10.Kralvovics R, Passamonti F, Buser As, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352(17):1779–90. [DOI] [PubMed] [Google Scholar]
- 11.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–6. [DOI] [PubMed] [Google Scholar]
- 12.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–9. [DOI] [PubMed] [Google Scholar]
- 13.Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia. 2014. January 20 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


