High leukocyte counts are often observed in sickle cell anemia (SCA) and are associated with increased clinical severity. In vivo animal model studies indicate that the adhesion of leukocytes, particularly neutrophils, to the vessel wall may promote subsequent secondary red blood cell (RBC) recruitment, in turn triggering the initiation of the vaso-occlusive process.1–3 While circulating neutrophil-platelet aggregates are well documented in inflammatory conditions such as sepsis, atherosclerosis and SCA,4–6 in which they are thought to augment tissue injury,1,6 the formation of leukocyte-RBC aggregates in humans has not been well studied. Circulating RBC-mononuclear cell aggregates have been reported in sickle cell disease (SCD), while the capture of SCD RBC by adherent leukocytes has been observed in vitro.7–9 However, the existence of circulating neutrophil-RBC aggregates has, to our knowledge, yet to be described in humans, even in inflammatory settings.
A total of 28 SCA patients in steady state, receiving HU (SCAHU; 15–30 mg/kg/day) or not, were recruited to the study (Table 1). Healthy controls (HbAA) were age- and gender-matched, where possible. This study was approved by the Ethics Committee of the National Heart, Lung, and Blood Institute, NIH, in accordance with the Declaration of Helsinki. All subjects provided their informed consent (clinicalstudies.info.nih.gov identifier 03-H-0015). Multispectral imaging flow cytometry (Amnis® ImageStreamX MKII; Amnis Corporation, Seattle, WA, USA) was used to observe neutrophil aggregates in peripheral blood samples. For this, granulocytes were isolated from samples by Ficoll sedimentation and neutrophil-RBC aggregates were labeled for cytometry (anti-CD11a-APC, anti-CD11b-APC-Cy7, anti-CD66b-V450, anti-CD71 FITC, anti-CD235a PE, and respective isotype controls; BD Biosciences, San Jose, CA, USA, and Caltag Invitrogen, Camarillo, CA, USA). CD66b-positive neutrophils were acquired as single cells and aggregates (~6000 events); images (40x objective) were taken and data were analyzed using IDEAS 6.0 software.
Table 1.
Clinical characteristics of patients participating in the study (USA population).
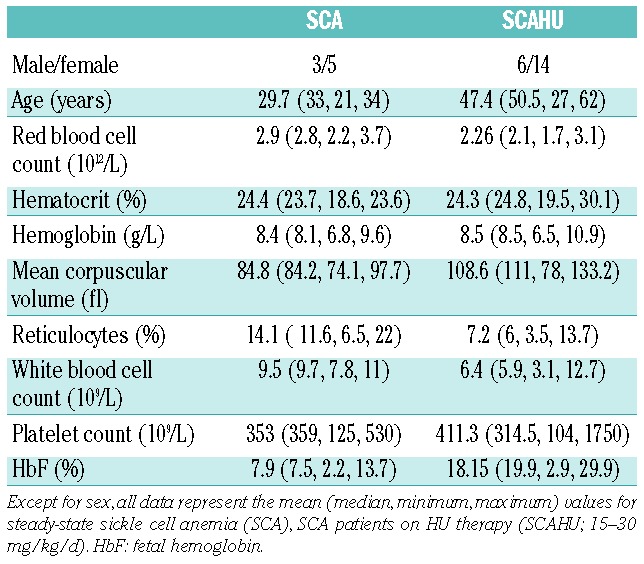
We found that the formation of neutrophil-RBC (CD66b+/CD235a+) aggregates was significantly augmented in SCA patients (Figure 1A and C) compared to healthy individuals. As there was no significant difference in the percentage of neutrophils aggregated to RBC between SCA patient (10.3±1.5%; n=9) and SCAHU patient (12.4±2.3%; n=14; P>0.05) groups, for subsequent analyses, SCA data were grouped together (SCA and SCAHU). To our knowledge, whilst circulating platelet-RBC and mononuclear cell-RBC aggregates8,10 have been previously described in SCD, this is the first description of the formation of neutrophil-RBC aggregates in blood from human SCA subjects.
Figure 1.
The formation of circulating neutrophil-RBC heterocellular aggregates is augmented in SCA patients. (A) Percentage of neutrophils (CD66b+) aggregated to red blood cells (RBCs) in peripheral blood from healthy individuals (Control, n=11) and SCA patients on/off HU (SCA, n=23), as detected by imaging flow cytometry. **P<0.01, compared to control group (Mann-Whitney test). (B) Percentage of neutrophils (CD66b+) aggregated to immature (CD235a+/CD71+) and mature (CD235a+/CD71−) RBCs in the peripheral blood of healthy individuals (Control, n=11) and SCA patients on/off HU (SCA, n=23), as detected by imaging flow cytometry; ***P<0.001, compared to respective control group (Mann-Whitney test). (C) Representative brightfield (BF) and fluorescent images, acquired by imaging flow cytometry; CD66b+ neutrophils (purple) aggregated to immature RBCs (CD235a+ - yellow; CD71+ - green) or mature RBCs (CD235a+ - yellow; CD71−); Final image: merged CD66b+/CD235a+/CD71+. Cells are from representative samples of peripheral blood from control, SCA and SCAHU patients. (D) Correlation of reticulocyte counts, (E) Fetal hemoglobin (HbF) levels and (F) platelet counts with percentages of neutrophil-reticulocyte aggregates (CD235a+/CD71+) in SCA (on/off HU) patients (n=17); Spearman’s non-parametric correlation test.
Immature RBCs, or reticulocytes (CD235a+CD71+) rather than mature RBCs (CD235a+CD71−) were the dominant type of RBC involved in SCA neutrophil-RBC aggregates (Figure 1B and C). Although reticulocyte counts are quite high in SCA (Table 1), reticulocyte numbers in these patients did not correlate with the percentage of neutrophil-reticulocyte aggregates (Figure 1D), suggesting that the presence of reticulocytosis per se is not a determining factor for neutrophil-reticulocyte aggregate formation in SCA, but rather alterations in these reticulocytes may cause these interactions. In contrast, the number of neutrophil-RBC aggregates, especially neutrophil-reticulocyte aggregates, correlated inversely with the level of fetal hemoglobin (HbF) (Figure 1E) which is known to inhibit polymerization of HbS. This decrease in HbS polymerization may prevent some of the alterations in RBC that lead to aggregate formation.
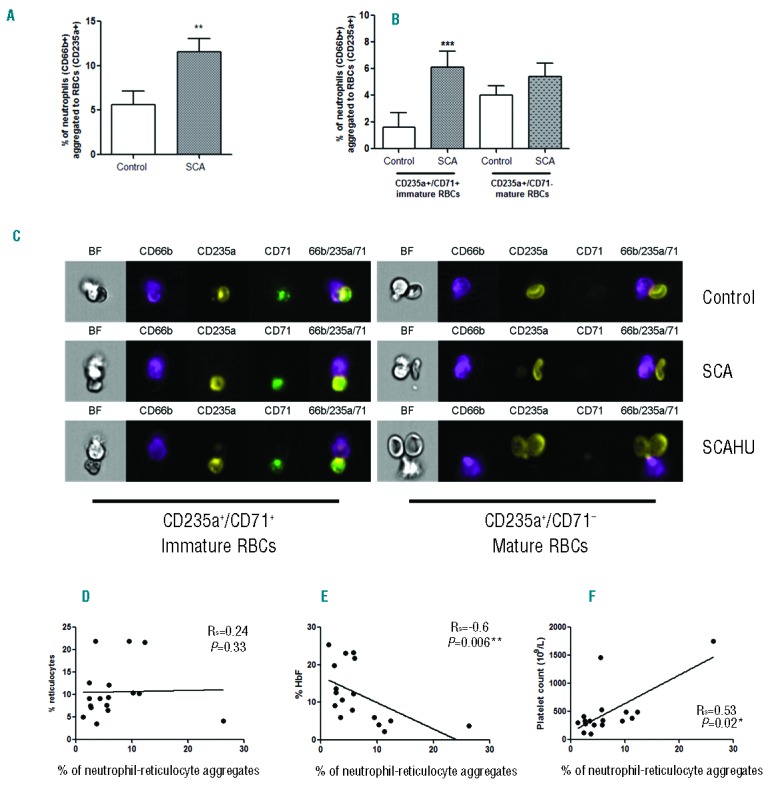
The leukocyte surface expressions of the adhesion molecule components CD11a (Mac-1 integrin subunit) and CD11b (LFA-1 integrin subunit) and the CD66b activation marker were verified on SCA and control individual neutrophil-RBC aggregates (representative images shown in Figure 2A); however, no significant differences in the surface expressions of these molecules were observed on the neutrophils of the control and SCA groups (data not shown). In contrast, function-inhibiting antibody experiments indicated a participation of the neutrophil Mac-1 integrin in neutrophil-RBC aggregates (Figure 2B and C). Integrins can exist in low- and high-affinity states11 and, based on our data, it is probable that changes in integrin affinity, or avidity, rather than expression are responsible for the increased heterocellular leukocyte aggregates observed in SCA. Function-blocking assays also indicated the participation of the VLA-4 integrin on reticulocytes (CD235a+CD71+) (Figure 2B) and of ICAM-4 expressed on mature RBCs (CD235a+CD71−) (Figure 2C) in SCA neutrophil-RBC aggregate formation, in agreement with previous studies investigating leukocyte interactions.1 A non-specific IgG1 antibody also reduced the incidence of aggregates, albeit to a lesser degree, compared to baseline, consistent with reports that intravenous IgG administration in sickle mice reduces neutrophil and erythrocyte interactions and improves microcirculatory blood flow.2
Figure 2.
Inhibition of Mac-1, VLA-4 and ICAM-4 function reverses the formation of neutrophil-RBC heterocellular aggregates. (A) Representative brightfield (BF) and fluorescent images, acquired by imaging flow cytometry, of CD66b+ neutrophils (purple) expressing CD11a (LFA-1 subunit) and CD11b (Mac-1 subunit) and aggregated to immature RBCs (CD235a+ - yellow color; CD71+ - green) or mature RBCs (CD235a+ - yellow; CD71−); Final image: merged CD66b/CD235a/CD71. Aggregates from the peripheral blood of control, SCA and SCAHU patients. Effects of the incubation of granulocyte suspensions (12 min, 37°C) with a non-specific antibody (IgG1), or adhesion-molecule blocking antibodies/peptides against the CD11a (LFA-1), CD11b (Mac-1), CD49d (VLA-4), ICAM-4 and BCAM molecules, on the percentage of neutrophils aggregated to (B) immature RBCs (CD235+CD71+) and (C) mature RBCs (CD235+CD71−) in samples from SCA patients (n=13). Anti-CD11a [clone 38; 10 μg/mL] and anti-CD11b [clone ICRF44; 10 μg/mL] were from AbD Serotec, Raleigh, NC, USA; anti-CD49d [clone 2B4, 10 μg/mL], and anti-BCAM [25 μg/mL] were from R&D Biosystems, Minneapolis, MN, USA; ICAM-4 blocking peptide (SC-27685, 200 μg/mL) was from Santa Cruz Biotech, Santa Cruz, CA, USA. Non-specific IgG1 (10 μg/mL) was from R&D Biosystems. *P<0.05, **P<0.01 (Friedman/Dunn’s), compared to non-specific IgG1 antibody.
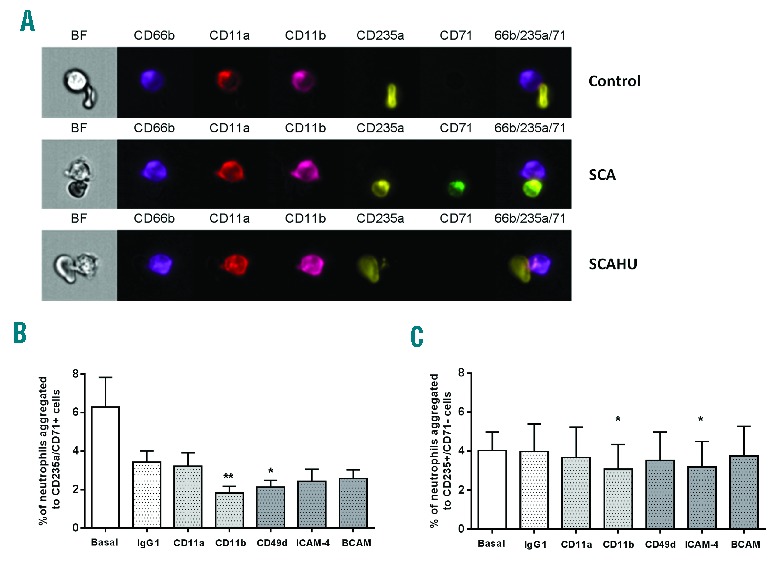
Intriguingly, we observed a positive correlation between platelet counts and neutrophil-reticulocyte aggregates in SCA (Figure 1F) and we therefore investigated a possible role for platelets in aggregate formation. Figure 3A–C demonstrates that the incubation of SCA granulocyte suspensions with a P-selectin-blocking antibody significantly reduced the number of total neutrophil-RBC aggregates, as well as neutrophil-reticulocyte aggregates. Furthermore, analysis by flow cytometry of fluorescently-labeled SCA neutrophil-RBC aggregates that had been concomitantly labeled with anti-CD62P demonstrated that activated platelets participated in the formation of approximately 20% of total neutrophil-RBC aggregates (Figure 3D) and 50% of neutrophil-reticulocyte aggregates (Figure 3E), while 20% of all neutrophil-mature RBC aggregates were CD62P-positive (Figure 3F), indicating the formation of ternary complexes. The corresponding images of these aggregates demonstrated a prominent role of platelets in these heterocellular complexes, apparently forming a bridge between the neutrophil and the erythrocyte/reticulocyte in the majority of aggregates (Figure 3G). In other images, platelets were observed adhered just to neutrophils, which in turn formed heterocellular interactions with RBCs, consistent with a previous in vitro report demonstrating that platelet-bound monocytes are more likely to interact with reticulocytes.7
Figure 3.
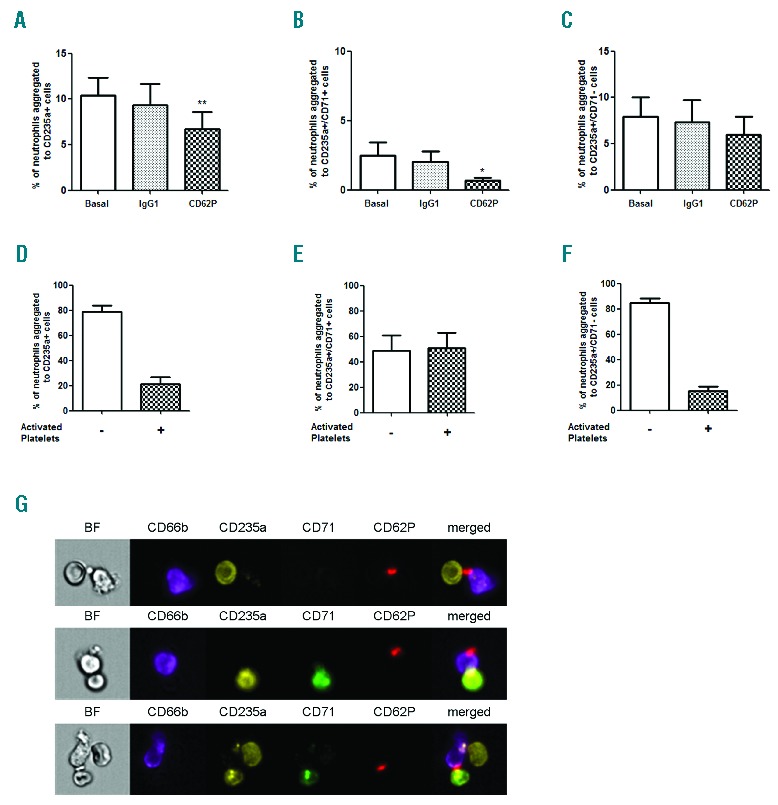
Involvement of platelets in SCA neutrophil-RBC interactions. Percentages of neutrophils from steady-state SCA (on/off HU) individuals aggregated to (A) RBCs (CD235a+), (B) reticulocytes (CD235a+/CD71+) and (C) mature RBCs (CD235a+/CD71−) under basal conditions and after incubation of granulocyte suspension with a non-specific IgG antibody or P-selectin function-blocking antibody (Anti-CD62P; clone 9E1, 2 μg/mL) (n=7); *P<0.05, **P<0.01, compared to basal (Friedman/Dunn’s). Percentages of neutrophils aggregated to (D) RBCs (CD235a+), (E) reticulocytes (CD235a+/CD71+) and (F) mature RBCs (CD235a+/CD71−), and demonstrating the presence (+) or not (−) of activated (P-selectin-positive) platelets in these aggregates when analyzed by flow cytometry (SCA on/off HU; n=7). (G) Representative images of the involvement of platelets (CD62P+; red) in the aggregates of neutrophils (CD66b+; purple) with RBC (CD235a+; yellow) expressing, or not, the transferrin receptor (CD71+; green). BF: brightfield mode and respective channels of fluorescence.
It was somewhat surprising that, while fetal hemoglobin levels correlated negatively with neutrophil-RBC aggregate formation in SCA, no significant alteration in the incidence of neutrophil-RBC aggregates was found in the blood of SCA patients on therapy with HU, compared to SCA patients not on HU. Additional experiments carried out in another population of steady-state SCA patients in Brazil demonstrated a similar lack of difference in neutrophil-RBC aggregate formation in aged-matched patients on and off HU therapy (data not shown). These results might reflect confounding factors by indication, whereby patients with the most severe clinical symptoms of SCA are selectively prescribed HU therapy, while those patients with milder symptoms are often not treated with HU. Alternatively, a lack of significant alterations with HU treatment in some platelet-related parameters has been observed in some recent studies.12,13 Importantly, data provided in Table 1 indicate that, in our study population, the platelet counts of the patients that were on HU were not significantly different to those of patients not on HU. Given the prominent role that platelets appear to play in the formation of these aggregates it may be that the number of platelets and neutrophils and their activation state may be more crucial to the formation of neutrophil-RBC aggregates in the circulation than red cell fetal hemoglobin content. Longitudinal studies would be required to determine whether HU therapy has no real effect on the formation of these aggregates.
In summary, we report a significant capacity for neutrophils to form heterocellular aggregates with red cells, particularly reticulocytes, in the circulation of SCA individuals; furthermore, a key role for platelets in the formation of these aggregates is suggested. Although our in vitro data do not establish whether these complexes circulate in such high numbers in vivo or contribute directly to vaso-occlusion, it is feasible to conclude, based on in vivo data from sickle mouse models,1,3,6 that these aggregates may be formed in the circulation of SCA individuals and at sites of low flow conditions. Given the evidence for the significant destructive effects of such heterocellular aggregates and the initiating role that RBC-neutrophil interactions may have in the vaso-occlusive process, we suggest that the formation of these heterocellular aggregates may contribute to vascular inflammation and occlusion with potentially damaging consequences. Thus, approaches to reduce the activity of platelets (such as the potent oral P-selectin-blocking agent and small molecule selectin inhibitor14,15 that are currently undergoing clinical trials) and to reduce leukocyte Mac-1 integrin activation could be valuable resources for reducing heterocellular interactions.
Acknowledgments
The authors thank Laurel Mendelsohn for assistance with laboratory techniques and Dr. Kleber Fertrin for assistance with data analysis. The authors also thank the patients who provided blood samples for this study.
Footnotes
Funding: this study was funded by grants from the Fundação de Amparo a Pesquisa de Sao Paulo –Brazil (FAPESP, grants 2012/21702-0, 2010/18386-4 and 10/17320-0), CNPq-Brazil and the National Heart, Lung and Blood Institute Division of Intramural Research (1 ZIA HL006013-03).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004; 103(6):2397–400. [DOI] [PubMed] [Google Scholar]
- 3.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA. 2002;99(5):3047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May AE, Langer H, Seizer P, Bigalke B, Lindemann S, Gawaz M. Platelet-leukocyte interactions in inflammation and atherothrombosis. Semin Thromb Hemost. 2007;33(2):123–7. [DOI] [PubMed] [Google Scholar]
- 5.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25(11):843–51. [DOI] [PubMed] [Google Scholar]
- 6.Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Art Thromb Vascular Biol. 2010;30(12):2392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brittain JE, Knoll CM, Ataga KI, Orringer EP, Parise LV. Fibronectin bridges monocytes and reticulocytes via integrin alpha4beta1. Br J Haematol. 2008;141(6):872–81. [DOI] [PubMed] [Google Scholar]
- 8.Chaar V, Picot J, Renaud O, Bartolucci P, Nzouakou R, Bachir D, et al. Aggregation of mononuclear and red blood cells through an {alpha}4{beta}1-Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica. 2010;95(11):1841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnegan EM, Turhan A, Golan DE, Barabino GA. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. Am J Hematol. 2007;82(4):266–75. [DOI] [PubMed] [Google Scholar]
- 10.Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K, Cheung A. Platelet activation and platelet-erythrocyte aggregates in patients with sickle cell anemia. J Lab Clin Med. 1997;129(5):507–16. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. [DOI] [PubMed] [Google Scholar]
- 12.Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, et al. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol. 2012;87(3):326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proenca-Ferreira R, Brugnerotto AF, Garrido VT, Dominical VM, Vital DM, Ribeiro Mde F, et al. Endothelial activation by platelets from sickle cell anemia patients. PloS one. 2014;9(2):e89012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutlar A, Ataga KI, McMahon L, Howard J, Galacteros F, Hagar W, et al. A potent oral P-selectin blocking agent improves microcirculatory blood flow and a marker of endothelial cell injury in patients with sickle cell disease. Am J Hematol. 2012;87(5):536–9. [DOI] [PubMed] [Google Scholar]
- 15.Telen MJ, Wun T, McCavit TL, De Castro LM, Krishnamurti L, Lanzkron S, et al. GMI 1070: Reduction In Time To Resolution Of Vaso-Occlusive Crisis and Decreased Opioid Use In a Prospective, Randomized, Multi-Center Double Blind, Adaptive Phase 2 Study In Sickle Cell Disease. Blood. 2013;122(21):Abstract 776. [Google Scholar]


