Abstract
Background
Infections are the most common non-cardiac complication after cardiac surgery, but their incidence across a broad range of operations, as well as the management factors that shape infection risk, remain unknown.
Objectives
This study prospectively examines the frequency of postoperative infections and associated mortality, and modifiable management practices predictive of infections within 65 days from cardiac surgery.
Methods
This study enrolled 5,158 patients and analyzed independently adjudicated infections using a competing risk model (with death as the competing event).
Results
Nearly 5% of patients experienced major infections. Baseline characteristics associated with increased infection risk included chronic lung disease (hazard ratio [HR] 1.66; CI 1.21–2.26), heart failure (HR 1.47; CI 1.11–1.95), and longer surgery (HR 1.31; CI 1.21–1.41). Practices associated with reduced infection risk included prophylaxis with second-generation cephalosporins (HR 0.70; CI 0.52–0.94), whereas postoperative antibiotic duration >48 hours (HR 1.92; CI 1.28–2.88), stress hyperglycemia (HR 1.32; CI 1.01–1.73); intubation time of 24–48 hours (HR 1.49; CI 1.04–2.14); and ventilation >48 hours (HR 2.45; CI 1.66–3.63) were associated with increased risk. HRs for infection were similar with either <24 hours or <48 hours of antibiotic prophylaxis. There was a significant but differential effect of transfusion by surgery type (excluding left ventricular assist device procedures/transplant) (HR 1.13; CI 1.07–1.20). Major infections substantially increased mortality (HR 10.02; CI 6.12, 16.39).
Conclusions
Major infections dramatically affect survival and readmissions. Second-generation cephalosporins were strongly associated with reduced major infection risk, but optimal duration of antibiotic prophylaxis requires further study. Given practice variations, considerable opportunities exist for improving outcomes and preventing readmissions.
Keywords: Cardiac Surgery, Infection, Risk factors
INTRODUCTION
Health care-acquired infections (HAIs), many of which are preventable, are extraordinarily important. An estimated 1.7 million individuals acquire an infection while hospitalized, resulting in 100,000 deaths annually and $6.5 billion in additional health care expenditures (1). This recognition has galvanized quality improvement (QI) efforts involving clinicians and policymakers alike, leading to important progress in several areas, such as catheter-related bloodstream infections in intensive care units (ICUs) (2). Such rigorous QI efforts have not been applied uniformly to cardiac surgical patients, an increasingly vulnerable and elderly population with multiple co-morbidities. Beyond the risk for catheter-related infections that are common in the ICU environment, the surgical setting presents additional risks related to prolonged mechanical ventilation, extensive blood product usage, indwelling catheter drainage, and open cavities.
Studies of patients undergoing cardiac surgery typically focus on a subset of infections (most notably, deep sternal site infections), primarily address coronary artery bypass grafting (CABG) rather than the broad range of commonly performed cardiac surgical procedures, capture events only during the in-hospital perioperative period, and rely on voluntary reporting (3,4). Moreover, although the literature has examined the relationship between several management practices and postoperative infection risk (2,5,6), many questions about the development of effective preventive strategies remain unanswered. Such information is especially timely, given the decision by the U.S. Centers for Medicare & Medicaid Services (CMS) to withhold reimbursement for care related to some preventable complications, including several HAIs. In addition, CMS has endorsed performance measures developed by the Surgical Care Improvement Project (SCIP), which include choice of antibiotics and control of early postoperative blood glucose level (7).
The Cardiothoracic Surgical Trials Network (CTSN) has addressed these issues by conducting a unique prospective multi-institutional cohort study to investigate frequency of postoperative infections, their microbiology, and associated mortality and identify modifiable management practices associated with postoperative infections within 65 days from index surgery.
METHODS
Participants
The study population includes all patients at the 10 CTSN core site with a clinical indication for cardiac surgery, without an active systemic infection, and at age ≥18 years. The study received Institutional Review Board approval, and all patients provided informed consent.
Design
For this study, we assumed that the 60-day incidence of major infections was approximately 4–5% (3,4). We targeted a minimum sample size of 5,000 patients to obtain at least 200 patients with major infections. This sample size was based not on explicit statistical criteria, but on acquiring an adequate number of events (at least 10 per variable) to ensure stability of coefficient estimates in our models (8,9). Patients were followed for up to 65 days after surgery with 2 planned post-discharge assessments at 30 and 60 days after surgery; the last date of follow-up was November 29, 2010. Data were transmitted electronically to the data coordinating center, which conducted electronic monitoring and sent monitors to the sites to review data quality. An independent event adjudication committee (EAC) consisting of 3 infectious disease physicians reviewed all major infections and organisms. The final date of event adjudication was in June 2011.
Endpoints, Patient and Clinical Characteristics
The primary endpoint was major infection within 65 days of the index cardiac surgery. The 10 major infections included were: deep incisional surgical site infection occurring at the primary chest incision; deep incisional surgical site infection (SSI) occurring at a secondary incision site (e.g., saphenous harvest and groin cannulation sites); mediastinitis; infectious myocarditis or pericarditis; endocarditis; cardiac device infection; pneumonia; empyema; Clostridium difficile colitis; and bloodstream infection. Secondary endpoints included the following minor infections: primary and secondary superficial incisional surgical site infections; symptomatic urinary tract infections; and asymptomatic bacteriuria. Infections were classified based on definitions from the Centers for Disease Control and Prevention (CDC) and the National Healthcare Safety Network surveillance (E Appendix 1) (10). Other secondary endpoints included all-cause mortality, reoperation, and hospital readmission.
We collected data on patient characteristics (demographics, baseline laboratory values, co-morbidities), surgery-related factors (such as prior intra-aortic balloon pump, ventricular assist device (VAD) therapy, and surgery time), and management practices (such as antimicrobial prophylaxis, glycemic control, and line management).
Statistical Analyses
We used time to event analysis to assess the association of patient- and procedure-related variables and process of care variables on occurrence of postoperative infection. Crude risk ratios describe univariate associations between these variables and first major infection. To account for the effect of mortality on infection risk in the multivariable analysis, we fitted competing risk models with death and infection as competing events (11). We fit multivariable models for infection in 2 stages. First, we used proportional hazards regression to select a set of patient- and procedure-related risk factors associated (at p<0.05) with time to onset of major infection, considering mortality as a competing risk. Second, we assessed the additional contribution of management practices utilized prior to the first infection. One exception is postoperative transfusions, where the timing was not always available. At each stage, removal of statistically non-significant variables, refitting, and retesting was continued until all variables in the model had a p-value of 0.05 or less. All patient- and procedure-specific variables selected in the first stage were kept in the second-stage model. We also analyzed the association of type and duration of antibiotics and infection type, adjusting for baseline characteristics. Interactions were tested between surgery type and management practices included in the second stage model. For the analysis of mortality we used proportional hazard models, with infection treated as a time-dependent variable. We used a stepwise selection process; the final model included only variables that had a p-value <0.05. All models were tested for the assumption of proportional hazards. All analyses utilized SAS statistical software (SAS® v9.2; Cary, NC), and R 2.15.
RESULTS
Patients
Between February and October 2010, 10 academic cardiac surgery programs enrolled 5,158 patients, with a mean age of 64±13 years and a median body mass index 28.2 (25.1, 32.3) kg/m2 (Table 1). Diabetes mellitus was present in 1,169 (23%) patients, heart failure in 1,505 (29%), chronic lung disease in 746 (14%), and 958 (19%) had prior cardiac surgery. The most frequently performed procedures were isolated CABG (1,677;33%), isolated valve (1,878;36%), and combined CABG and valve surgery (692;13%), with 3,806 (74%) of all procedures being elective. Median cardiopulmonary bypass time was 105 (78.0, 140.0) minutes.
Table 1.
Patient and Operative Characteristics and Unadjusted Risk Ratios (Major Infection)*
| Infected (N = 237) | Not Infected (N = 4,921) | Overall (N = 5158) | Unadjusted HR | P-value† | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 65.6 (13.8) | 64.3 (13.2) | 64.4 (13.2) | 1.01 | 0.13 |
| Male | 157 (66.2) | 3293 (66.9) | 3450 (66.9) | 0.97 | 0.81 |
| White | 181 (76.4) | 4141 (84.2) | 4322 (83.9) | 0.61 | 0.001 |
| BMI | 28.5 (24.4, 33.2) | 28.2 (25.1, 32.2) | 28.2 (25.1, 32.3) | 1.01 | 0.34 |
| Insurance | 0.11 | ||||
| Medicaid | 14 (5.9) | 219 (4.5) | 233 (4.5) | 1.90 | |
| Medicare | 103 (43.5) | 1825 (37.2) | 1928 (37.5) | 1.69 | |
| Government (Other) | 20 (8.4) | 606 (12.3) | 626 (12.2) | 1.00 (ref) | |
| Private | 87 (36.7) | 2012 (41.0) | 2099 (40.8) | 1.30 | |
| None/Self | 13 (5.5) | 247 (5.0) | 260 (5.1) | 1.58 | |
| Baseline Laboratories | |||||
| WBC, x103/ml | 7.2 (5.8, 8.7) | 6.9 (5.7, 8.4) | 7.0 (5.7, 8.4) | 1.03 | 0.11 |
| Creatinine, mg/dL | 1.1 (0.9, 1.4) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.18 | <.001 |
| Hemoglobin, g/dL | 12.5 (11.0, 13.9) | 13.4 (12.1, 14.6) | 13.4 (12.0, 14.5) | 0.80 | <.001 |
| Cardiac morbidity | |||||
| Heart failure | 110 (46.4) | 1395 (28.4) | 1505 (29.2) | 2.16 | <.001 |
| Ejection fraction | 50.0 (35.0, 60.0) | 55.0 (49.0, 60.0) | 55.0 (48.0, 60.0) | 0.97 | <.001 |
| Previous cardiac surgery | 74 (31.2) | 884 (18.0) | 958 (18.6) | 2.03 | <.001 |
| Noncardiac morbidity | |||||
| Diabetes‡ | 66 (27.9) | 1103 (22.4) | 1169 (22.7) | 1.33 | 0.05 |
| COPD | <.001 | ||||
| None | 178 (75.1) | 4234 (86.0) | 4412 (85.5) | 1.00 (ref) | |
| Mild or moderate | 47 (19.8) | 597 (12.1) | 644 (12.5) | 1.84 | |
| Severe | 12 (5.1) | 90 (1.8) | 102 (2.0) | 3.04 | |
| Operative | |||||
| Surgery time, hours | 4.8 (3.9, 6.4) | 4.2 (3.3, 5.2) | 4.2 (3.3, 5.2) | 1.38 | <.001 |
| Bypass time, minutes§ | 129.0 (94.0, 180.0) | 104.0 (77.0, 138.0) | 105.0 (78.0, 140.0) | 1.01 | <.001 |
| Sternotomy | 218 (92.0) | 4451 (90.5) | 4669 (90.5) | 1.20 | 0.45 |
| Surgery Type | 0.01 | ||||
| Elective | 155 (65.4) | 3651 (74.2) | 3806 (73.8) | 1.00 (ref) | |
| Urgent | 72 (30.4) | 1142 (23.2) | 1214 (23.5) | 1.47 | |
| Emergent | 10 (4.2) | 128 (2.6) | 138 (2.7) | 1.82 | |
| Procedure | <.001 | ||||
| Isolated CABG | 54 (22.8) | 1623 (33.0) | 1677 (32.5) | 1.00 (ref) | |
| Isolated valve | 72 (30.4) | 1806 (36.7) | 1878 (36.4) | 1.20 | |
| CABG + valve | 39 (16.5) | 653 (13.3) | 692 (13.4) | 1.79 | |
| LVAD/Tx | 29 (12.2) | 93 (1.9) | 122 (2.4) | 8.07 | |
| Thoracic aortic | 23 (9.7) | 405 (8.2) | 428 (8.3) | 1.72 | |
| Other|| | 20 (8.4) | 341 (6.9) | 361 (7.0) | 1.75 | |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVAD/Tx, left ventricular assist device or transplant surgery; SD, standard deviation.
Continuous variables are expressed as median (IQR) and categorical variables as count (%).
Based on Cox proportional hazards model where outcome is time to infection and predictor is baseline or patient characteristic.
Insulin or oral medications.
91.1% of patients had on-pump surgical procedures.
Other: ventricular septal defect repairs, atrial septal defect repairs, aneurysmectomies, PFO closures, ablations, septal myectomies, excision of cardiac tumors, pericardiectomies, and limited other procedures
Frequency and Characteristics of Infections
A total of 237 patients (4.6%) experienced 301 major infections (rate/patient month: 0.028; Table E-1 for risk by procedure type), most commonly pneumonia, bloodstream infections, and C. difficile colitis (Table 2). Median time to first major infection was 13 days [6,25]; 134 (45%) occurred after hospital discharge, predominantly deep SSIs, endocarditis and device-related percutaneous site infection. Pneumonia and bloodstream infections occurred more commonly during the index hospitalization, while C. difficile colitis occurred equally before and after discharge. C. difficile infections were the primary post-operative infection for 40 (80%) patients. The incidence of C. difficile infections did not vary with age in this older population, but left ventricular assist device (LVAD)/transplant patients had a higher risk than other cardiac surgery patients. Eight percent of patients experienced minor infections, the most prevalent being symptomatic urinary tract infections (174;3.4%) and superficial incision site infection (137;2.7%).
Table 2.
Frequency, Type and Timing of Infection
| Type of Infection | # of Events | # of Patients | % of Patients (N=5158) | Days from surgery to first infection
|
||
|---|---|---|---|---|---|---|
| Median | Min | Max | ||||
| Pneumonia | 125 | 123 | 2.38 | 8 | 1 | 62 |
| Bloodstream Infection | 59 | 56 | 1.09 | 15 | 0 | 65 |
| C. Difficile Colitis | 52 | 50 | 0.97 | 17 | 3 | 63 |
| Deep Incision Surg site infection (chest)* | 26 | 26 | 0.56 | 20.5 | 5 | 54 |
| Mediastinitis | 12 | 12 | 0.23 | 24.5 | 6 | 60 |
| Deep Incision Surg site infection (groin)* | 10 | 10 | 0.21 | 26 | 6 | 49 |
| Myocarditis or pericarditis | 5 | 4 | 0.08 | 16 | 14 | 27 |
| Empyema | 4 | 3 | 0.06 | 56 | 13 | 63 |
| Endocarditis | 3 | 3 | 0.06 | 25 | 25 | 51 |
| Device-related percut site infection | 3 | 3 | 0.06 | 54 | 9 | 62 |
| Pocket infection† | 2 | 2 | 2.33 | 38.5 | 15 | 62 |
Denominator for patients with a deep SSI is patients having a sternotomy (N=4669).
Denominator for pocket infection is patients who had LVAD placed, replaced, or removed for heart transplant (N=86).
Positive microbial isolates were identified for 230 (76%) of major infections; 85 (32%) were Gram-positive, and 123 (47%) were Gram-negative. Table 3 depicts the organism distribution for each infection type.
Table 3.
Organisms
| Pneumonia | % | Endocarditis | % |
|---|---|---|---|
| Gram Positive Bacteria | 12.6 | Gram Positive Bacteria | 100 |
|
| |||
| Staphylococcus Aureus | 9.5 | Staphylococcus Aureus | 66.7 |
|
| |||
| Meth Resistant (44%) | Meth Resistant (50%) | ||
|
| |||
| Streptococcus sp | 3.2 | Staphylococcus Hominis | 33.3 |
|
|
|
||
| Gram Negative Bacteria | 82.1 | Empyema | |
|
| |||
| Enterobacteriaceae | 43.2 | Gram Positive Bacteria | 60 |
|
| |||
| Pseudomonas | 15.8 | Staphylococcus Aureus | 60 |
|
| |||
| Other Health Care GNR* | 13.7 | Meth Resistant (67%) | |
|
| |||
| Serratia Marcesens | 6.3 | Gram Negative Bacteria | 20 |
|
| |||
| H. Influenzae | 3.2 | Pseudomonas | 20 |
|
| |||
| Other | 5.3 | Other | 20 |
|
|
|
||
| BSI | SSI | ||
|
| |||
| Gram Positive Bacteria | 47.5 | Gram Positive Bacteria | 62.9 |
|
| |||
| Staphylococcus Aureus | 13.1 | Staphylococcus Aureus | 40 |
|
| |||
| Meth Resistant (38%) | Meth Resistant (50%) | ||
|
| |||
| Staphylococcus Epi | 9.8 | Staphylococcus Epi | 14.3 |
|
| |||
| Meth Resistant (50%) | Meth Resistant (80%) | ||
|
| |||
| Enterococcus | 11.5 | Enterococcus | 5.7 |
|
| |||
| Fungi (Candida) | 9.8 | Fungi (Candida) | 2.9 |
|
| |||
| Streptococcus sp | 1.6 | Gram Negative Bacteria | 28.6 |
|
| |||
| Staph Hominis (Coag neg) | 1.6 | Enterobacteriaceae | 17.1 |
|
| |||
| Gram Negative Bacteria | 47.5 | Pseudomonas | 5.7 |
|
| |||
| Enterobacteriaceae | 29.5 | Other Health Care GNR* | 2.9 |
|
| |||
| Serratia Marcesens | 8.2 | Other (Unidentified) | 2.9 |
|
| |||
| Other Health Care GNR* | 4.9 | Other | 8.6 |
|
|
|
||
| Pseudomonas | 3.3 | Myocarditis/Pericarditis | |
|
| |||
| Anaerobe (Bact. Fragilis) | 1.6 | Gram Positive Bacteria | 100 |
|
| |||
| Other | 4.9 | Streptococcus sp | 50 |
|
| |||
| Mediastinitis | Enterococcus | 25 | |
|
| |||
| Gram Positive Bacteria | 61.5 | Fungi (Candida) | 25 |
|
|
|
||
| Staphylococcus Aureus | 46.2 | Pocket Infection | |
|
| |||
| Meth Resistant (33%) | Gram Positive Bacteria | 100 | |
|
| |||
| Staphylococcus Epi | 7.7 | Staphylococcus Aureus | 66.7 |
|
| |||
| Meth Resistant (100%) | Meth Resistant (50%) | ||
|
| |||
| Fungi (Candida) | 7.7 | Fungi (Candida) | 33.3 |
|
|
|
||
| Gram Negative Bacteria | 38.5 | Cardiac Device | |
|
| |||
| Enterobacteriaceae | 15.4 | Gram Positive Bacteria | 50 |
|
| |||
| Pseudomonas | 7.7 | Staphylococcus Epi | 50 |
|
| |||
| Other Health Care GNR* | 7.7 | Meth Resistant (0%) | |
|
| |||
| Other (Unidentified) | 7.7 | Gram Negative Bacteria | 50 |
|
| |||
| C. difficile colitis | Pseudomonas | 50 | |
|
| |||
| Clostridium difficile | 100 | ||
Abbreviations: Meth, methicillin; sp, species; BSI, bloodstream infections; Epi, epidermidis, SSI, surgical site infections.
Other healthcare GNRs: Acinetobacter, Stenotrophomonas, Achromobacter, and Burkholderia
Risk Factors for Major Infection
Patient and operative procedure characteristics associated with increased risk of major infections were chronic lung disease, heart failure, elevated creatinine, use of corticosteroids, LVAD, and transplant surgery, leaving an open sternum for secondary closure and longer surgery time (Table 4). Higher hemoglobin levels were protective.
Table 4.
Baseline and Procedure Characteristics Associated With Infection
| Baseline Variable | HR (95% CI) | P Value |
|---|---|---|
| COPD (yes/no) | 1.66 (1.21, 2.26) | 0.002 |
| Heart failure (yes/no) | 1.47 (1.11, 1.95) | 0.007 |
| Corticosteroids (yes/no) | 1.91 (1.19, 3.05) | 0.007 |
| Creatinine, mg/dL | 1.15 (1.08, 1.22) | <.001 |
| Hemoglobin, g/dL | 0.90 (0.84, 0.97) | 0.008 |
| LVAD/Tx (yes/no) | 2.89 (1.86, 4.50) | <.001 |
| Open sternum (yes/no) | 6.35 (2.62, 15.38) | <.001 |
| Duration of surgery (hours) | 1.31 (1.21, 1.41) | <.001 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVAD/Tx, left ventricular assist device or transplant surgery.
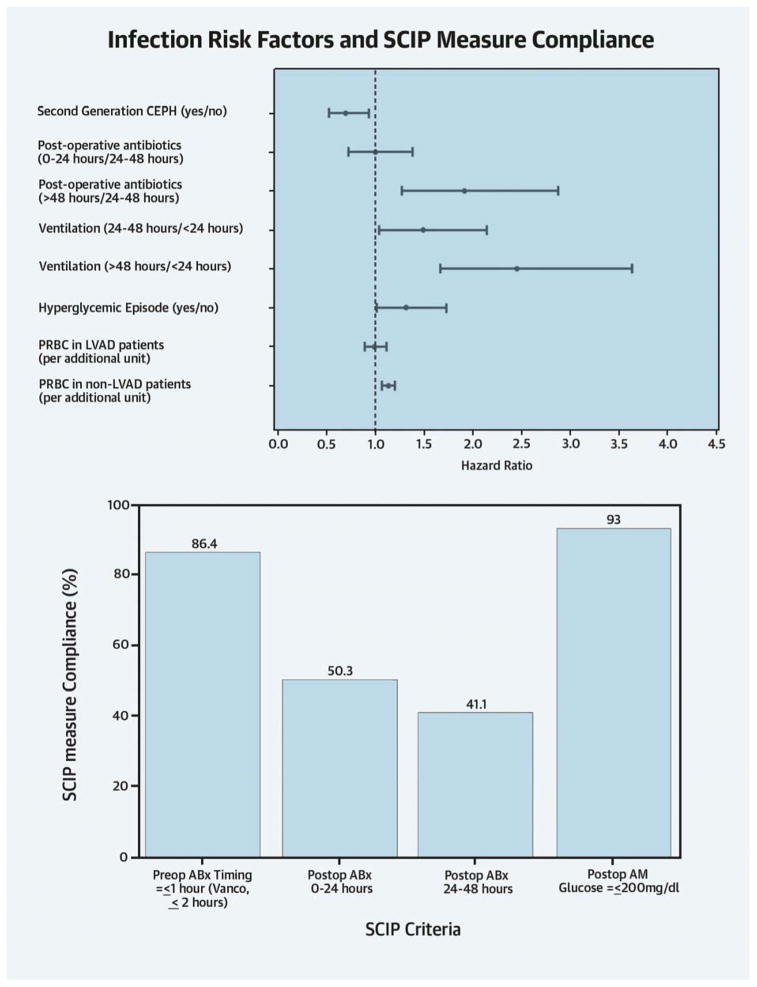
There was substantial variation in management practices; Table 5 shows frequency of use and their association with infection (univariate analysis). Close to 50% (2,427) of patients received nasal decontamination, predominantly with mupirocin, with variation not at the individual physician level, but rather at the institutional level. Nearly all patients received appropriate surgical site hair removal or did not require hair removal (a SCIP measure). Most patients (4,148;82%) were scrubbed with chlorhexidine preparations, and 11% (542) of patients had more than 1 central line simultaneously placed prior to first infection. Eighty-six percent (4,422) of patients received prophylactic antibiotics according to the SCIP measure (<1 hour prior to incision, and <2 hours if receiving vancomycin), but virtually all patients undergoing longer procedures (i.e., after 6 hours) were redosed intra-operatively. Preoperative antibiotics administered included second-generation cephalosporins (± vancomycin; 2,245;44%), first-generation cephalosporins (± vancomycin; 1,859;36%), and vancomycin alone (967;19%). Distribution of postoperative antibiotics resembled pre-operative use; however, only 41% (2,126) of patients received the recommended prophylactic antibiotics for 48 hours after surgery end time. The first postoperative glucose level was ≤200 mg/dL in 93% (4,779) of patients, another SCIP measure. Moreover, 43% (2,228) of patients had a hyperglycemic episode in the first 48 hours after surgery but prior to first infection. Slightly more than 20% (1,070) of patients received mechanical ventilation for over 24 hours after surgery prior to first infection. There was no variation in use of intra-operative urinary catheters, head of bed elevation, and secretion management postoperatively.
Table 5.
Management Practices and Unadjusted Risk Ratios (Major Infection)
| Variable | No. (%) | Infection Rate | Unadjusted HR* | 95% CI |
|---|---|---|---|---|
| Nasal decontamination (yes/no) | 2427 (47.1) | 0.023 | 0.80 | 0.61–1.03 |
| Hair removal (Males only) | ||||
| Shaving | 108 (3.1) | 0.013 | 0.60 | 0.19–1.87 |
| Clipping, depilatory cream, no hair removal | 3342 (96.9) | 0.028 | 1.00 (ref) | |
| Scrubbing surgical site | ||||
| Chlorhexidine preparations | 4148 (81.6) | 0.029 | 1.36 | 0.94–1.97 |
| Iodophors, alcohol, soap and water, other | 933 (18.4) | 0.021 | 1.00 (ref) | |
| Central lines | ||||
| More than 1§ (vs. ≤1 line) | 542 (10.5) | 0.051 | 2.06 | 1.49–2.85 |
| Femoral§ (vs. no femoral line) | 37 (0.72) | 0.166 | 5.63 | 2.78–11.39 |
| Appropriate timing of preoperative antibiotics† (yes/no) | 4422 (86.4) | 0.028 | 0.97 | 0.67–1.40 |
| Intraoperative antibiotic re-dosed after 6 hrs (yes/no) | 5034 (97.6) | 0.026 | 0.41 | 0.23–0.71 |
| Type of perioperative antibiotics | ||||
| 2nd generation cephalosporins | 2405 (46.6) | 0.020 | 0.64 | 0.49–0.83 |
| 1st generation cephalosporins, vancomycin, other | 2753 (53.4) | 0.034 | 1.00 (ref) | |
| Postoperative antibiotic duration§ | ||||
| 0–24 hours | 2599 (50.4) | 0.020 | 1.14 | 0.83–1.55 |
| 24–48 hours | 2126 (41.2) | 0.020 | 1.00 (ref) | |
| >48 hours | 433 (8.4) | 0.113 | 5.90 | 4.25–8.19 |
| Packed Red Blood Cells, unit (median, IQR)‡ | 3 (2, 5) | 0.043 | 1.29 | 1.24–1.33 |
| Venue of urinary catheter insertion | ||||
| Bedside/Other hospital | 65 (1.3) | 0.089 | 1.00 (ref) | |
| Operating room | 5075 (98.7) | 0.027 | 0.28 | 0.15–0.52 |
| Nasogastric tube used (yes/no) | 3749 (72.7) | 0.023 | 1.20 | 0.89–1.62 |
| Glucose management (1st 48 hours after surgery) § | ||||
| Hyperglycemic episode (>180 mg/dl) | 2228 (43.3) | 0.037 | 1.78 | 1.35–2.33 |
| No Hyperglycemic episode (≤180 mg/dl) | 2923 (56.8) | 0.020 | 1.00 (ref) | |
| Mechanical ventilation§ | ||||
| ≤ 24 hours | 4084 (79.2) | 0.015 | 1.00 (ref) | |
| 24–48 hours | 683 (13.3) | 0.049 | 2.69 | 1.92–3.76 |
| > 48 hours | 387 (7.5) | 0.117 | 7.74 | 5.77–10.39 |
| Elevation of head of bed (yes/no) | 5139 (99.7) | 0.027 | 0.35 | 0.09–1.42 |
| Routine aspiration of secretions (yes/no) | 4955 (96.1) | 0.028 | 3.21 | 1.03–10.02 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, interquartile range.
HR for glucose management adjusted for diabetes
Within 1 hour prior to surgical incision (2 hours if receiving vancomycin)
48.1% patients received packed red blood cell transfusion
Frequency (%) not accounting for timing of infection:
More than 1 central line: 556 (10.8)
Femoral line: 38 (0.74)
Postoperative Antibiotic Duration: 0–24 hours: 2596 (50.3); 24–48 hours: 2120 (41.1); > 48 hours: 442 (8.6)
Glucose Management: Hyperglycemia: 2229 (43.3); no Hyperglycemia: 2925 (56.8)
Mechanical Ventilation: ≤24 hours: 4067 (78.9); 24–48 hours: 670 (13.0); >48 hours: 417 (8.1)
Table 6 identifies management practices associated with risk of major infection, adjusted for baseline risk factors. Perioperative prophylaxis with second generation cephalosporins (cefuroxime, cefoxitin, ± concomitant vancomycin) was associated with a 30% reduction in major infection (HR 0.70;CI 0.52–0.94). Second-generation cephalosporins were associated with decreasing both the risk of a broad range of Gram positive (HR 0.43;CI 0.24–0.76) and Gram negative (HR 0.51;CI 0.32–0.83) infections, without significantly affecting the risk of C. difficile colitis. HRs between shorter (0–24 hours) and longer duration (24–48 hours) of antibiotic prophylaxis were similar, but prophylaxis over 48 hours was associated with a near doubling of major infection risk (HR 1.92;CI 1.28–2.88). Prolonged prophylaxis (>48 hours) was associated with a six-fold increased risk for C. difficile colitis (HR 6.31;CI 2.86–14.0). There was a significant but differential effect of transfusion by surgery type (p=0.03); the risk of infection was unchanged for LVAD/transplant patients (HR 0.99;CI 0.89, 1.11), but red blood cells (RBCs) were associated with increased risk for all other surgical patients (HR 1.13;CI 1.07–1.20). Having a hyperglycemic episode was associated with a 30% increased infection risk (HR 1.32;CI 1.01–1.73). Compared with postoperative ventilation less than 24 hours, intubation time of 24–48 hours was associated with a 50% (HR 1.49;CI 1.04–2.14) increased risk of major infection, and ventilation exceeding 48 hours with a more than a 2-fold higher risk (HR 2.45;CI 1.66–3.63). Although significant in the univariate analysis, femoral lines, and multiple central lines were not significant in the multivariable analysis, mainly because they were highly correlated with ventilator time (e.g., patients having ventilation >48 hours were more than 2.5 times more likely to have multiple lines compared to ventilation ≤24 hours). Thus, ventilator time subsumed the fraction of the risk imparted by multiple central lines and remained in the model.
Table 6.
Process of Care Variables Associated with Infection
| Process of Care Variables* | HR (95% CI) | P Value |
|---|---|---|
| Second generation CEPH (yes/no) | 0.70 (0.52, 0.94) | 0.02 |
| Post-operative antibiotics (vs 24–48 hrs) | ||
| 0–24 hours | 1.00 (0.72, 1.38) | 0.98 |
| > 48 hours | 1.92 (1.28, 2.88) | 0.002 |
| Ventilation (vs. ≤24 hours) | ||
| 24–48 hours | 1.49 (1.04, 2.14) | 0.03 |
| >48 hours | 2.45 (1.66, 3.63) | <0.001 |
| Hyperglycemia (yes/no) | 1.32 (1.01, 1.73) | 0.04 |
| PRBC (unit) | 0.03 | |
| LVAD/Tx | 0.99 (0.89, 1.11) | |
| No LVAD/Tx | 1.13 (1.07, 1.20) | |
Abbreviations: CI, confidence interval; CEPH, cephalosporin; HR, hazard ratio; PRBC, packed red blood cells; LVAD/Tx, left ventricular assist device or transplant surgery.
Model adjusted for baseline risk factors depicted in Table 4
Mortality and Readmissions
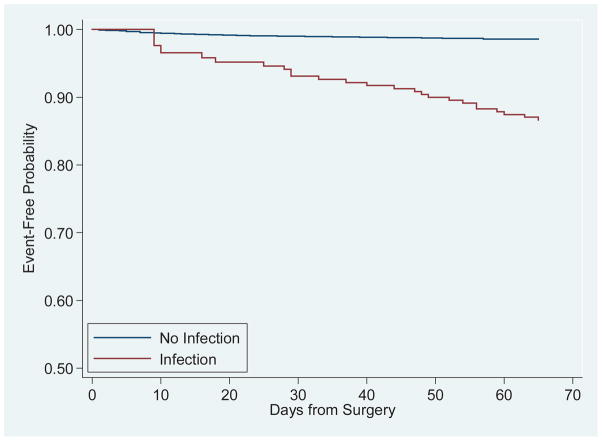
Figure 1 depicts survival stratified by presence of a major infection. Major infection had a substantial effect on survival (HR 10.02;CI 6.12–16.39), as also did higher creatinine (HR 1.17;CI 1.06–1.29), heart failure (HR 2.01;CI 1.34–3.00), diabetes (HR 1.65;CI 1.08–2.51) and older age (HR 1.04; CI 1.02–1.06) (E-Table 2). Mortality risk for men was half that of women (HR 0.49;CI 0.33–0.72). The 65-day mortality rate for infected patients was 5% and 0.7% for non-infected patients. The 30-day readmission rate was 14%, while the overall readmission rate in this cohort was 19%. Infections accounted for 16% of all readmissions.
Figure 1. Mantel Byar Survival Curve.
97 deaths occurred over the 65-day post-surgery follow-up period.
DISCUSSION
The findings from this prospective cohort study of more than 5,000 patients challenge the common perception that SSIs, particularly deep sternal site infections, are the most important infections acquired by patients undergoing cardiac surgery. Instead, we found that pneumonia, bloodstream infections, and C. difficile colitis accounted for 79% of all major postoperative infections. Moreover, these major infections occurred later than expected, with 45% becoming evident only after hospital discharge. These findings are particularly important given the prevalence of infections in this population (12% of patients experienced infections, with 4.6% being major), the high mortality risk (10-fold) for major infections, and their impact on readmissions.
To assess QI strategies, we examined baseline predictors for the full range of cardiac surgery procedures, including the nature of the procedure itself. Patient- and procedure-related factors associated with infection risk are similar to those found in studies of infections after CABG and in ICU patients (3,12). Contrary to previous observations, this study did not find that obesity, diabetes, or urgent surgery were independent risk factors for infection in the multivariable analysis (4,12).
Several management practices were associated with postoperative infection risk, including the type and duration of antibiotic prophylaxis. The Society of Thoracic Surgeons (STS) guidelines recommend using a first-generation cephalosporin, and if patients are beta-lactam or penicillin allergic, using vancomycin with additional Gram negative coverage (13,14). In this study, second-generation cephalosporins (± vancomycin) were more commonly used than first-generation antibiotics and were strongly associated with reduced infection risk. This is consistent with our finding that Gram-negative organisms, which are better treated by second-generation cephalosporins, were the largest category of isolates (47%) among infected patients.
Interestingly, we also observed that this class of antimicrobials was associated with improved prevention of Gram positive infections, with S. aureus as the most common isolate observed. As per STS and SCIP recommendations, 86% of patients received prophylactic antibiotics an hour before skin incision (except for vancomycin). The median time for antibiotic administration in patients receiving “out-of-window” care was 74 minutes. This small difference in start time may explain the lack of difference in occurrence of infection. Both STS and SCIP recommend 48 hours of prophylactic antibiotics post-surgery (14), which was met in only 41% of patients. We found no difference in HRs for short-duration (24 hours) versus longer duration (48 hours) of antibiotic prophylaxis, whereas >48 hours was associated with increased risk. Two recent meta-analyses favored prophylaxis prolongation up to 48 hours postoperatively in preventing SSIs, but no definitive conclusions could be drawn given differences in outcome definitions, antibiotic regimens and likely bias in the published trials (15,16). A randomized trial is needed to evaluate the optimal duration of prophylaxis in preventing not only SSIs, but a broader range of infections, such as C. difficile, which increases substantially with longer antimicrobial prophylaxis and raises concerns about the ever-present threat of microbial resistance.
RBCs were transfused in 48% of patients. The infection risk associated with RBC transfusion was dose-dependent, with a 13% increase in infection risk for each additional unit (except for LVAD/transplant patients). This argues for decreasing the amount of blood transfused, but the risks and benefits of transfusion must be weighed against the risks of anemia. Several practices may reduce the need for transfusion, including cell salvage, small priming volumes, vacuum-assisted venous return with rapid autologous priming, ultrafiltration, and pre-operative measures to elevate hematocrit (6).
Recent CDC data document a 54% decrease in bloodstream infections in ICUs of teaching hospitals, but as the second most common infection in our study, they remain high in cardiac surgery patients (17). Efforts to decrease such infection include full barrier precautions for central lines, avoiding the femoral site and removing unnecessary central venous catheters. In our univariate analysis, multiple central lines, and femoral lines increased major infection risk, supporting the practice of minimizing the duration of their use. But multiple lines correlated strongly with prolonged ventilator time, which remained in the multivariable model. Our study quantified the risk for longer ventilation times; even a modest prolongation of ventilation (24 versus 48 hours) was associated with a 50% increase in risk of infection, arguing for terminating mechanical ventilation as soon as possible.
Stress hyperglycemia, defined as one or more blood sugar measurements above 180 mg/dl during the first 48 hours postoperatively, was associated with a 30% risk of major infection. Hyperglycemia has been found in the literature to be a predictor of mortality and major morbidity, and averting hyperglycemia (as assessed by the first 6.00 am blood sugar after surgery) has been the focus of national QI efforts (18). Yet, a substantial number of our patients still experienced hyperglycemic episodes as well as hypoglycemic episodes, which argues for the adoption of novel approaches, such as glycemic control management systems that track blood sugars and provide the therapeutic guidance that would reduce blood sugar variations through software-based algorithms.
Controversy surrounds the value of routine screening for S. aureus nasal carriage and nasal decontamination in surgical patients, with the strongest evidence for its use in patients undergoing cardiac surgery or receiving an implant (19). In our study, only half of the CTSN sites used nasal decontamination, and this practice was not associated with reduced infection risk. Further trials are needed to substantiate the benefits in cardiac surgery.
Our study has potential limitations. Its purpose was to quantify the burden of all serious infections in the post-operative period and identify a constellation of management practices associated with reduced infection risk, which does not easily lend itself to a randomized design and limits our ability to measure the independent impact of specific interventions for specific infections. We were careful to take into consideration the timing of management practices and incorporated in the analysis only those practices that were used prior to the onset of infection (except transfusions, the timing of which was sometimes unavailable). Finally, infection and mortality events occurred later than anticipated, and a longer follow-up period may have identified further events.
In this unique prospective cohort study of infection after all types of cardiac surgery, major infections were common and dramatically increased mortality. Prolonged ventilation and transfusion were strongly associated with adverse outcomes, while the use of second-generation cephalosporins was associated with improved outcomes. Given the variation in practices, our findings offer opportunities for improving patient outcomes and speak to policy incentives to avert infections, including reimbursement penalties for preventable infections, public reporting of adherence to SCIP measures, and incorporation of hospital-specific infection rates in ranking systems to inform consumer choice (21,22). Interestingly, our study demonstrated reasonably high adherence to SCIP measures and STS guidelines, but deviation from several measures seemed to have little impact on infection outcomes. Others have made similar observations using administrative datasets (23). These findings suggest that – in a dynamic environment of evolving interventions, patients and offending microbes – we need to create an infrastructure for frequent re-evaluation of the incidence of health care-acquired infections, effectiveness of management practices, and adequacy of management guidelines. This is especially critical in an era that emphasizes early postoperative discharge and reduction of preventable readmissions, which are both heavily influenced by infection.
Supplementary Material
Central Illustration. Infection Risk Factors and SCIP measure Compliance.
SCIP, Surgical Care Improvement Project; CEPH, cephalosporin; PRBC, packed red blood cells; LVAD/Tx, left ventricular assist device or transplant surgery; ABx is antibiotics; hyperglycemic episode is >180 mg/dl; vanco is vancomycin. Figure 1 was adjusted for baseline patient and procedure risk factors.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEGE 1
Within 2 months after cardiac surgery, 5% of patients experienced major infections, nearly half of which were not identified until after hospital discharge. The most frequent were pneumonia (41%), bloodstream infections (20%), and C. difficile colitis (17%).
COMPETENCY IN MEDICAL KNOWLEGE 2
Patients whose cardiac surgery was complicated by major infection complications suffered a mortality rate 10 times greater than those who did not.
COMPETENCY IN PATIENT CARE
Administration of prophylactic antibiotic medication for more than 48 hours, mechanical ventilation for more than 48 hours, stress-induced hyperglycemia, and transfusions of blood products are associated with an increased risk of infection after cardiac surgery.
TRANSLATIONAL OUTLOOK
Prospective clinical trials are needed to define the optimum type and duration of antibiotic prophylaxis and thresholds for transfusion of blood products and assess the efficacy of these strategies to reduce the risk of infection in patients undergoing cardiac surgery.
Acknowledgments
Funding Sources: The National Heart, Lung, and Blood Institute, Bethesda, MD, the Canadian Institutes of Health Research, Ottawa, ON and the National Institute of Neurological Diseases and Stroke, Bethesda, MD (Grant no. 7U01 HL088942).
Abbreviations
- CABG
coronary artery bypass grafting
- CTSN
Cardiothoracic Surgical Trials Network
- ICU
intensive care unit
- QI
quality improvement
- SSI
surgical site infection
Footnotes
Most infection definitions have been adapted from the CDC/NHSN surveillance definition of health care-associated infection (www.cdc.gov/ncidod/dhqp/nhsn.html).
A culture-negative finding does not meet this criterion.
No disclosures.
Clinical Trial Registration: Clinicaltrials.gov identifier NCT 01089712
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perencevich EN, Pittet D. Preventing catheter-related bloodstream infections. Thinking outside the checklist. JAMA. 2009;301:1285–7. doi: 10.1001/jama.2009.420. [DOI] [PubMed] [Google Scholar]
- 2.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, O’Brien SM, Muhlbaier LH, et al. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112:I358–65. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 4.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg. 2004;77:676–83. doi: 10.1016/S0003-4975(03)01523-6. [DOI] [PubMed] [Google Scholar]
- 5.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 6.Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43:322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 8.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 9.Harrel FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 13.Engelman R, Shahian D, Shemin R, et al. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007;83:1569–76. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Edwards FH, Engelman RM, Houck P, et al. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, Part I: Duration. Ann Thorac Surg. 2006;81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Lador A, Nasir H, Mansur N, et al. Antibiotic prophylaxis in cardiac surgery; systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:541–50. doi: 10.1093/jac/dkr470. [DOI] [PubMed] [Google Scholar]
- 16.Mertz D, Johnstone J, Loeb M. Does duration of perioperative antibiotic prophylaxis matter in cardiac surgery? A systematic review and meta-analysis. Ann of Surg. 2011;254:48–54. doi: 10.1097/SLA.0b013e318214b7e4. [DOI] [PubMed] [Google Scholar]
- 17.Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301:727–36. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 18.Ascione R, Rogers CA, Rajakaruna C, et al. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and non-diabetic patients undergoing Cardiac Surgery. Circulation. 2008;118:113–123. doi: 10.1161/CIRCULATIONAHA.107.706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel RP. Minimizing surgical-site infections. N Engl J Med. 2010;362:75–7. doi: 10.1056/NEJMe0908753. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ. 2013;346:f2743. doi: 10.1136/bmj.f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graves N, McGowan JE., Jr Nosocomial infection, the Deficit Reduction Act, and incentives for hospitals. JAMA. 2008;300:1577–9. doi: 10.1001/jama.300.13.1577. [DOI] [PubMed] [Google Scholar]
- 22.Fry DE. Surgical site infections and the surgical care improvement project (SCIP): evolution of national quality measures. Surg Infect (Larchmt) 2008;9:579–84. doi: 10.1089/sur.2008.9951. [DOI] [PubMed] [Google Scholar]
- 23.Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–85. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.